Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10257
Peer-review started: April 20, 2021
First decision: July 15, 2021
Revised: August 9, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: November 26, 2021
Processing time: 216 Days and 7.1 Hours
Kabuki syndrome (KS) is a rare syndrome characterized by multisystem conge
This study reports a de novo KDM6A mutation in a Chinese infant with KS. A 2-month-old Chinese baby was diagnosed with KS, which manifested as hypog
We present a Chinese KS patient with a novel KDM6A frameshift mutation (c.704_705delAG, p. N236Sfs*26) (GRCh37/hg19), broadening the mutation spectrum.
Core Tip: The case report describes a de novo KDM6A mutation in a Chinese patient with Kabuki syndrome (KS). This novel KDM6A frameshift mutation broadens the KS mutation spectrum and knowledge of its clinical manifestations.
- Citation: Guo HX, Li BW, Hu M, Si SY, Feng K. Novel KDM6A mutation in a Chinese infant with Kabuki syndrome: A case report. World J Clin Cases 2021; 9(33): 10257-10264
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10257.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10257
Kabuki syndrome (KS), also termed Kabuki make-up syndrome or Niikawa–Kuroki syndrome, is a congenital anomaly/mental retardation syndrome[1,2] characterized by five main clinical features: a distinctive face, skeletal anomalies, dermatoglyphic abnormalities, mental retardation, and postnatal growth retardation[3]. The incidence of KS is approximately 1 in every 32000 births[4,5], and to date, there have been a number of cases reported in PubMed, showing that KS affects all ethnic populations without preference of gender or race, although it was originally reported in Japan (https://rarediseases.org/rare-diseases/kabuki-syndrome/). Nevertheless, due to misdiagnoses and missed diagnoses, the actual number of KS cases is underestimated. In China, there have been only a few cases reported, while the KS type II cases were even rarer (i.e. KDM6A mutations)[6-9]. The whole-exome sequencing of KS DNA samples has shown that KS development is mainly caused by mutations of KMT2D[10] and KDM6A[11]. It includes KMT2D-associated, autosomal-dominant KS type I (KS-1) and KDM6A-associated, X-linked-dominant KS type II (KS-2) and 56%–70% and 3%–8% of KS patients have mutations in KMT2D and KDM6A, respectively[12,13], whereas 25%–30% are diagnosed clinically without any known gene mutations[14-16].
In this case report, we identified and diagnosed a 2-month-old Chinese male baby with KS. DNA sequencing of his blood revealed a novel KDM6A frameshift mutation (c.704_705delAG, p. N236Sfs*26) (GRCh37/hg19), which clinically led to hypog
A 2-month-old boy was admitted to our hospital due to persistent feeding difficulties, poor weight gain and weak crying for 2 mo.
The patient was the second child of his mother and was born via spontaneous vaginal delivery. The gestational age was 34 wk. Apgar score was 10 points. There were no abnormalities in the placenta and umbilical cord except for oligohydramnios (100 mL). At birth, the infant had the following birth parameters: 31.5 cm head circumference, 2.5 kg body weight, and 46 cm length, placing him in the 25–50th percentile in Chinese newborns. Ten minutes later, he was immediately admitted to the neonatal intensive care unit because of transient respiratory difficulty, and was diagnosed with neonatal hypoglycemia and congenital anal atresia. He was thereafter treated with respiratory support, glucose rehydration, and surgical correction of the anal atresia. Three weeks later, he was discharged from the hospital except feeding difficulty and poor weight gain.
The patient was the second child of Chinese parents who were healthy and non-consanguineous. He was born at 34 weeks’ gestation from a healthy 32-year-old woman via spontaneous vaginal delivery. Prenatal ultrasound imaging showed that the mother had reduced amniotic fluid level since 32 weeks’ gestation and the amniotic fluid index was 7.0 cm. The ultrasound imaging also suggested mild hydronephrosis with dilatation of the upper ureteral diameter (0.6 cm) on the right kidney. There were no other abnormalities identified. The mother had irregular vaginal bleeding 8 h before delivery. His mother did not suffer from fever or use tobacco, alcohol, or illicit drugs during the entire pregnancy.
The infant was born at 34 weeks’ gestation from a healthy 32-year-old woman via spontaneous vaginal delivery and the father was aged 34 years. The parents were healthy and unrelated. The infant had a healthy 4-year-old brother. Family history was unremarkable.
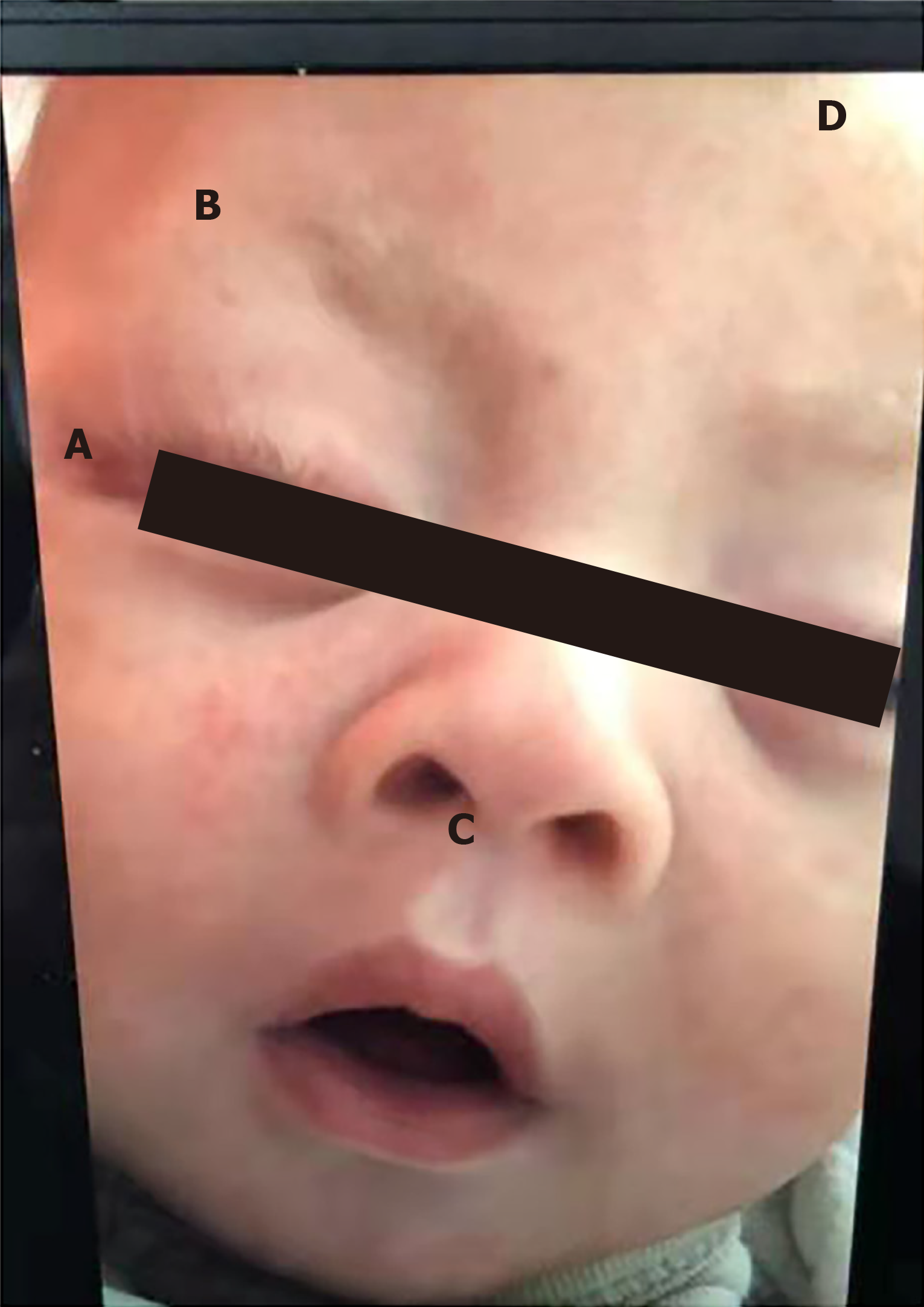
He had severe malnutrition and poor skin elasticity with stable vital signs, but his growth and development level was below the normal range with the 3rd centiles, e.g., his height was 50.0 cm, weight 3.05 kg and head circumference 35.0 cm, and according to the WHO (2006) child growth standards, he was indicated as having postnatal onset of growth retardation. He also had distinctive body features, namely a long palpebral fissure, arched eyebrow, lateral sparse of the eyebrow, long eyelashes, and high-arched palate, but short nasal columella with a broad and depressed nasal tip (Figure 1). His palms had a simian crease. He also showed weak crying, muscle hypotonia, and motor delay and could not lift his head and accomplish a test of audio and visual tracking.
Routine blood analyses revealed mild anemia (hemoglobin, 98 g/L), blood sugar level was low (2.31 mmol/L; normal range, 3.9–6.1 mmol/L) and his blood ammonia level was high (76 μmol/L; normal range, < 60 μmol/L). The level of insulin-like growth factor 1 was low (< 25 ng/mL) and growth hormone (GH) level was in the normal range. Liver, kidney and thyroid functions and electrolyte level were normal. Laboratory tests of urine and blood samples did not show any amino acid or aliphatic acid metabolic disorders. Furthermore, his chromosome count was normal (46, XY).
Cardiac ultrasound revealed patent foramen ovale and ductus arteriosus, and urological ultrasound indicated mild hydronephrosis and dilatation in the right kidney. Brain magnetic resonance imaging revealed corpus callosum hypoplasia, enlarged ventricles, and white matter dysplasia. Chest X-ray and abdominal ultrasound showed no apparent abnormality. Ophthalmological examination revealed hypoplasia of the optic nerve and retina with hearing loss in both ears (Table 1).
| Organ | Manifestations |
| Eye | (1) Long palpebral fissure, arched eyebrow, long eyelashes; and (2) sparse lateral eyebrows, optic nerve, and retina hypoplasia |
| Ear | Hearing loss |
| Nose | Short columella with depressed nasal tip, wide nasal bridge |
| Oral cavity | High-arched palate |
| Dermatoglyphic | Simian crease |
| Limbs and joints | Joint laxity |
| Head | High forehead and hairline |
| Heart | Patent ductus arteriosus, patent foramen ovale |
| Gastrointestinal | Anal atresia, persistent feeding difficulties |
| Genitourinary | Mild hydronephrosis and dilatation on the right kidney |
| Metabolic | Persistent hypoglycemia, mild high blood lactic acid levels |
| Immunologic | Immune dysfunction, frequent pulmonary infections |
| Neurologic | Hypotonia, weak crying |
| Neuroimaging | Corpus callosum hypoplasia, enlarge ventricles, and white matter dysplasia |
| Growth delay | Normal growth parameters at birth, postnatal growth retardation, motor delay |
| Intellectual disability | Mental retardation |
| Endocrine system | Low insulin-like growth factor 1 deficiency |
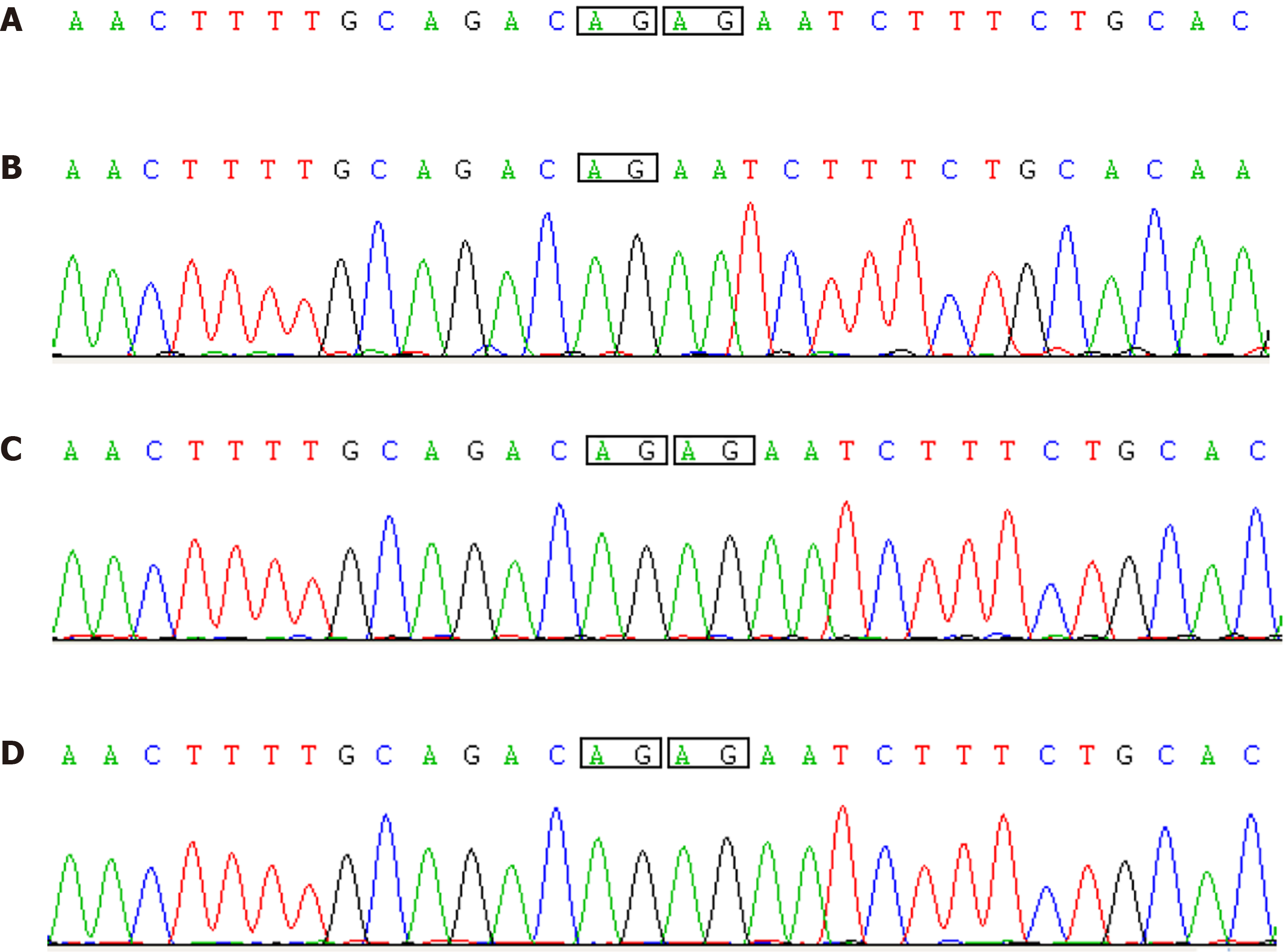
As this infant showed peculiar facial features, multisystem anomalies, persistent feeding difficulties, hypoglycemia, and serious postnatal growth deficiency, KS diagnosis was indicated. Thus, the venous blood samples from both patient and parents were collected for whole-exome sequencing to confirm the diagnosis. Data from the infant’s sample showed a novel KDM6A frameshift mutation (c.704_705
The final diagnosis of the presented case was KS due to a novel KDM6A frameshift mutation (c.704_705delAG, p. N236Sfs*26) (GRCh37/hg19).
There are no curable treatment options for KS currently available. At age 6 mo, the patient’s physical development parameters were as follows: weight 5.0 kg, head circumference 37.5 cm and body length 61.5 cm, (all < 3rd percentiles). The patient was started on GH replacement therapy. At the same time, rehabilitation training was carried out.
The therapeutic effects were unsatisfactory. There was no improvement in growth and development. At age 7 mo, the patient had recurrent respiratory tract infection. He died of pulmonary infection at age 13 mo after failure of treatment and rescuing.
KS, a rare congenital disorder, was first reported in 1981 by two groups of Japanese physicians[4,5]. The estimated prevalence in Japan is approximately 1/32000 versus 1/86000 in Australia and New Zealand or in Europe and America[14,15]. KS cases have also been reported in China and our PubMed search showed that only six KS-2 cases have been so far reported in Mainland Chin [6-9], indicating that our current case is the seventh. The typical KS features include facial abnormality (long palpebral fissures with eversion of the lateral third of the lower eyelid; arched and broad eyebrows; sparse lateral eyebrows; short columella with depressed nasal tip; large, prominent, or cupped ears); postnatal growth retardation; mild to moderate intellectual impairment; scoliosis deformity; short and small fifth finger; susceptibility to infection; visceral deformity; dermatoglyphic abnormalities; blue sclera; hearing impairment; hypotonia; lack of GH; and other abnormalities[16]. KMT2D and KDM6A are two pathogenic genes that have been identified in KS. KMT2D gene mutation leads to KS type I, which is autosomal dominant; KMT2D gene encodes the lysine specific methyltransferase 2D, a methyltransferase that specifically modifies the lysine residue at the fourth acid lysine (H3K4) on histone H3 and catalyzes H3K4 from unme
In our current case, the patient was diagnosed with early-stage disease, possibly because of his serious symptoms that caused his early death. This patient had most of the KS clinical manifestations and the diagnosis was established based on these clinical findings (i.e., preterm at age 34 wk), transient respiratory difficulty at birth, persistent hypoglycemia, and congenital anal atresia in the neonatal period. Moreover, the patient had persistent feeding difficulty, weak crying, hypotonia, and postnatal growth retardation, as well as distinctive facial features, multiple congenital internal malformations and increased infection susceptibility, which are consistent with KS diagnostic criteria [13]. Our current case report confirmed that KS is associated with novel KDM6A frameshift mutation (c.704_705delAG, p. N236Sfs*26) (GRCh37/hg19). Taken together, the data show that KS is genetically heterogeneous. Further studies with a larger number of KS cases will provide a better understanding of KS path
Previous Chinese studies[8,9] have reported that KS patients have typical facial features, including the long palpebral fissures, sparse lateral or notched eyebrows, depressed nasal tip and large ears. However, the microcephaly, cleft lip/palate, and cardiac defects occurred less frequently in Chinese KS patients. Moreover, these studies[8,9] also showed the brain abnormalities, such as thinning of the pituitary and myelination of the cerebral white matter in Chinese KS patients, suggesting a strong association between various brain abnormalities and KS.
It is worth noting that KS is a congenital multiple organ dysplasia and to date, there is no unique and specific perinatal diagnostic methodology. Long et al reported two infants who presented with prenatal hydrops/ascites, who were subsequently diagnosed with KS[22]. Guo showed the final diagnosis KS II of a 3-month-old patient with congenital hydrocephalus and suggested that congenital hydrocephalus was closely associated with KS II[7], while Rosenberg et al[23] collected retrospective data from 49 individuals with KS and over one third had complications of polyhydramnios, and reduced placental weight also complicated KS pregnancies, suggesting that the differential diagnosis for polyhydramnios in the absence of intrauterine growth retardation should include KS. A Chinese study[24] reported that a 24-week-old fetus was diagnosed with KS II using the chromosomal microarray analysis plus growth retardation and cardiovascular and musculoskeletal abnormalities using routine color Doppler ultrasonography. Another previous study[25] retrospectively reviewed 11 patients and showed that prenatal ultrasound was an important tool, while a molecular technique was also important in KS diagnosis. The most frequent ultrasound features observed were cardiac anomalies (49.4%), followed by polyhydramnios or oligohydramnios (28.9%), genitourinary anomalies (26.5%), single umbilical artery (15.7%), intrauterine growth restriction (14.5%) and hydrops fetalis/pleural effusion/ascites (12.0%); 50.6% of which had more than one abnormal antenatal ultrasound finding. These enlighten us that there are no distinct signs in fetuses to suggest the KS diagnosis prenatally. More and more investigators have suggested that prenatal phenotypic heterogeneity is associated with KS. If fetal ultrasound abnormalities show one or more deformities, KS should be considered. We need to complete a relevant gene analysis as soon as possible to realize early diagnosis and early intervention.
This case report identified a de novo frameshift KDM6A mutation localized on chromosome Xp11 (c.704_705delAG, p. N236Sfs*26) (GRCh37/hg19) in a Chinese male infant with KS. After literature review, we believe that his severe clinical manifestations were part of the KS II phenotype spectrum. Our data support the investigation of a genotype–phenotype correlation, which explains the phenotypic variability of KS II. This case provides more information about the mutational spectrum of KS II.
We thank the patient’s family who agreed to this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Plougmann JI S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Yu HG
| 1. | Adam MP, Hudgins L. Kabuki syndrome: a review. Clin Genet. 2005;67:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Hannibal MC, Buckingham KJ, Ng SB, Ming JE, Beck AE, McMillin MJ, Gildersleeve HI, Bigham AW, Tabor HK, Mefford HC, Cook J, Yoshiura K, Matsumoto T, Matsumoto N, Miyake N, Tonoki H, Naritomi K, Kaname T, Nagai T, Ohashi H, Kurosawa K, Hou JW, Ohta T, Liang D, Sudo A, Morris CA, Banka S, Black GC, Clayton-Smith J, Nickerson DA, Zackai EH, Shaikh TH, Donnai D, Niikawa N, Shendure J, Bamshad MJ. Spectrum of MLL2 (ALR) mutations in 110 cases of Kabuki syndrome. Am J Med Genet A. 2011;155A:1511-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Niikawa N, Kuroki Y, Kajii T, Matsuura N, Ishikiriyama S, Tonoki H, Ishikawa N, Yamada Y, Fujita M, Umemoto H. Kabuki make-up (Niikawa-Kuroki) syndrome: a study of 62 patients. Am J Med Genet. 1988;31:565-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 319] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Niikawa N, Matsuura N, Fukushima Y, Ohsawa T, Kajii T. Kabuki make-up syndrome: a syndrome of mental retardation, unusual facies, large and protruding ears, and postnatal growth deficiency. J Pediatr. 1981;99:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 368] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Kuroki Y, Suzuki Y, Chyo H, Hata A, Matsui I. A new malformation syndrome of long palpebral fissures, large ears, depressed nasal tip, and skeletal anomalies associated with postnatal dwarfism and mental retardation. J Pediatr. 1981;99:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 310] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Yang P, Tan H, Xia Y, Yu Q, Wei X, Guo R, Peng Y, Chen C, Li H, Mei L, Huang Y, Liang D, Wu L. De novo exonic deletion of KDM6A in a Chinese girl with Kabuki syndrome: A case report and brief literature review. Am J Med Genet A. 2016;170:1613-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Guo Z, Liu F, Li HJ. Novel KDM6A splice-site mutation in kabuki syndrome with congenital hydrocephalus: a case report. BMC Med Genet. 2018;19:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Shangguan H, Su C, Ouyang Q, Cao B, Wang J, Gong C, Chen R. Kabuki syndrome: novel pathogenic variants, new phenotypes and review of literature. Orphanet J Rare Dis. 2019;14:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Li N, Su Z, Xu Y, Liu S, Chen Y, Li X, Shen Y, Hung C, Wang J, Wang X, Bodamer O. The phenotypic spectrum of Kabuki syndrome in patients of Chinese descent: A case series. Am J Med Genet A. 2020;182:640-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ng SB, Bigham AW, Buckingham KJ, Hannibal MC, McMillin MJ, Gildersleeve HI, Beck AE, Tabor HK, Cooper GM, Mefford HC, Lee C, Turner EH, Smith JD, Rieder MJ, Yoshiura K, Matsumoto N, Ohta T, Niikawa N, Nickerson DA, Bamshad MJ, Shendure J. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet. 2010;42:790-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 988] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 11. | Lederer D, Grisart B, Digilio MC, Benoit V, Crespin M, Ghariani SC, Maystadt I, Dallapiccola B, Verellen-Dumoulin C. Deletion of KDM6A, a histone demethylase interacting with MLL2, in three patients with Kabuki syndrome. Am J Hum Genet. 2012;90:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 12. | Bögershausen N, Gatinois V, Riehmer V, Kayserili H, Becker J, Thoenes M, Simsek-Kiper PÖ, Barat-Houari M, Elcioglu NH, Wieczorek D, Tinschert S, Sarrabay G, Strom TM, Fabre A, Baynam G, Sanchez E, Nürnberg G, Altunoglu U, Capri Y, Isidor B, Lacombe D, Corsini C, Cormier-Daire V, Sanlaville D, Giuliano F, Le Quan Sang KH, Kayirangwa H, Nürnberg P, Meitinger T, Boduroglu K, Zoll B, Lyonnet S, Tzschach A, Verloes A, Di Donato N, Touitou I, Netzer C, Li Y, Geneviève D, Yigit G, Wollnik B. Mutation Update for Kabuki Syndrome Genes KMT2D and KDM6A and Further Delineation of X-Linked Kabuki Syndrome Subtype 2. Hum Mutat. 2016;37:847-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 13. | Banka S, Lederer D, Benoit V, Jenkins E, Howard E, Bunstone S, Kerr B, McKee S, Lloyd IC, Shears D, Stewart H, White SM, Savarirayan R, Mancini GM, Beysen D, Cohn RD, Grisart B, Maystadt I, Donnai D. Novel KDM6A (UTX) mutations and a clinical and molecular review of the X-linked Kabuki syndrome (KS2). Clin Genet. 2015;87:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Philip N, Meinecke P, David A, Dean J, Ayme S, Clark R, Gross-Kieselstein E, Hosenfeld D, Moncla A, Muller D. Kabuki make-up (Niikawa-Kuroki) syndrome: a study of 16 non-Japanese cases. Clin Dysmorphol. 1992;1:63-77. [PubMed] |
| 15. | Schrander-Stumpel C, Meinecke P, Wilson G, Gillessen-Kaesbach G, Tinschert S, König R, Philip N, Rizzo R, Schrander J, Pfeiffer L. The Kabuki (Niikawa-Kuroki) syndrome: further delineation of the phenotype in 29 non-Japanese patients. Eur J Pediatr. 1994;153:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Adam MP, Banka S, Bjornsson HT, Bodamer O, Chudley AE, Harris J, Kawame H, Lanpher BC, Lindsley AW, Merla G, Miyake N, Okamoto N, Stumpel CT, Niikawa N; Kabuki Syndrome Medical Advisory Board. Kabuki syndrome: international consensus diagnostic criteria. J Med Genet. 2019;56:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 17. | Cocciadiferro D, Augello B, De Nittis P, Zhang J, Mandriani B, Malerba N, Squeo GM, Romano A, Piccinni B, Verri T, Micale L, Pasqualucci L, Merla G. Dissecting KMT2D missense mutations in Kabuki syndrome patients. Hum Mol Genet. 2018;27:3651-3668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Miyake N, Koshimizu E, Okamoto N, Mizuno S, Ogata T, Nagai T, Kosho T, Ohashi H, Kato M, Sasaki G, Mabe H, Watanabe Y, Yoshino M, Matsuishi T, Takanashi J, Shotelersuk V, Tekin M, Ochi N, Kubota M, Ito N, Ihara K, Hara T, Tonoki H, Ohta T, Saito K, Matsuo M, Urano M, Enokizono T, Sato A, Tanaka H, Ogawa A, Fujita T, Hiraki Y, Kitanaka S, Matsubara Y, Makita T, Taguri M, Nakashima M, Tsurusaki Y, Saitsu H, Yoshiura K, Matsumoto N, Niikawa N. MLL2 and KDM6A mutations in patients with Kabuki syndrome. Am J Med Genet A. 2013;161A:2234-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Bögershausen N, Wollnik B. Unmasking Kabuki syndrome. Clin Genet. 2013;83:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Chi YI, Stodola TJ, De Assuncao TM, Leverence EN, Tripathi S, Dsouza NR, Mathison AJ, Basel DG, Volkman BF, Smith BC, Lomberk G, Zimmermann MT, Urrutia R. Molecular mechanics and dynamic simulations of well-known Kabuki syndrome-associated KDM6A variants reveal putative mechanisms of dysfunction. Orphanet J Rare Dis. 2021;16:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Tran N, Broun A, Ge K. Lysine Demethylase KDM6A in Differentiation, Development, and Cancer. Mol Cell Biol. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 22. | Long A, Sinkovskaya ES, Edmondson AC, Zackai E, Schrier Vergano SA. Kabuki syndrome as a cause of non-immune fetal hydrops/ascites. Am J Med Genet A. 2016;170:3333-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Rosenberg CE, Daly T, Hung C, Hsueh I, Lindsley AW, Bodamer O. Prenatal and perinatal history in Kabuki Syndrome. Am J Med Genet A. 2020;182:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Lin CZ, Qi BR, Hu JS, Huang XQ. A fetus with Kabuki syndrome 2 detected by chromosomal microarray analysis. Int J Clin Exp Pathol. 2020;13:302-306. [PubMed] |
| 25. | So PL, Luk HM, Cheung KW, Hui W, Chung MY, Mak ASL, Lok WY, Yu KPT, Cheng SSW, Hau EWL, Ho S, Lam STS, Lo IFM. Prenatal phenotype of Kabuki syndrome: A case series and literature review. Prenat Diagn. 2021;41:1089-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |