Published online Nov 16, 2021. doi: 10.12998/wjcc.v9.i32.10033
Peer-review started: July 6, 2021
First decision: July 26, 2021
Revised: August 8, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: November 16, 2021
Processing time: 126 Days and 16.3 Hours
Iatrogenic aortic dissection (IAD) is a rare but fatal complication of interventional treatment for the proximal supra-aortic large vessels. Several cases of IAD after endovascular treatment of subclavian artery have been reported. Nevertheless, the pathogenesis of IAD is still unclear. Here we report a patient with IAD following a balloon expandable stent implanted into the left subclavian artery (LSA).
An 84-year-old man with a history of hypertension was admitted to the Neurology Department of our hospital complaining of dizziness and gait disturbance for more than 1 mo. Computed tomography angiography of the head and neck showed severe stenosis at the proximal LSA and the origin of the left vertebral artery. Magnetic resonance diffusion-weighted imaging of the brain revealed subacute infarctions in cerebellum, occipital lobe and medulla oblongata. He suffered a Stanford type B aortic dissection after the proximal LSA angioplasty with a balloon expandable stent. Thoracic endovascular aortic repair was performed immediately with the chimney technique and he was discharged 20 d later. After exploring the pathogenesis with multimodal imaging analysis, an easily neglected focal intramural hematoma (IMH) in the aorta near the junction of the LSA was found to be the main cause of the IAD. The risk of IAD should be sufficiently evaluated according to the characteristics of aortic arch lesions before the proximal LSA angioplasty.
Focal aortic IMH is a potential risk factor for IAD during a seemingly simple stenting of the proximal LSA.
Core Tip: Iatrogenic aortic dissection (IAD) is a rare complication during angioplasty or stenting for the proximal left subclavian artery (LSA) stenosis. The authors present an 84-year-old patient with posterior circulation stroke who suffered an aortic dissection immediately after proximal LSA stenting and achieved a favorable outcome following active treatment. The easily neglected focal intramural hematoma is a potential risk factor for IAD in this procedure. The characteristics of aortic arch lesions should be sufficiently evaluated with multimodal imaging analysis before the proximal LSA angioplasty to avoid the occurrence of such event.
- Citation: Zhang Y, Wang JW, Jin G, Liang B, Li X, Yang YT, Zhan QL. Focal intramural hematoma as a potential pitfall for iatrogenic aortic dissection during subclavian artery stenting: A case report. World J Clin Cases 2021; 9(32): 10033-10039
- URL: https://www.wjgnet.com/2307-8960/full/v9/i32/10033.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i32.10033
Subclavian artery stenosis can lead to chronic blood insufficiency of the upper limb or vertebrobasilar system and, in severe cases, to subclavian steal syndrome or posterior circulation ischemic stroke. At present, endovascular stenting is one of the most important interventional treatments for symptomatic atherosclerotic stenosis of the subclavian artery with a certain degree of reliability and safety[1]. However, subclavian artery stenting complicated by iatrogenic aortic dissection (IAD) is a rare and frustrating situation. In this case, we report a patient with IAD following implantation of a balloon expandable stent into the left subclavian artery (LSA) due to a focal aortic intramural hematoma (IMH).
An 84-year-old male was admitted to our department due to dizziness and gait disturbance lasting more than 1 mo.
The patient had a history of hypertension for more than 10 years. Except for age, gender and hypertension, the patient had no other predisposing factors for stroke such as trauma, obesity, diabetes, dyslipidemia, heart disease, atrial fibrillation, smoking, alcohol consumption, drug abuse, asymptomatic carotid stenosis and sleep apnea syndrome.
There was no previous stroke or transient ischemic attack in his history.
There was no obvious abnormality in his personal and family history.
The blood pressure of the right upper limb was 128/78 mmHg, while that of the left was 113/66 mmHg. The neurological examination revealed the ataxia of right limb, but had no visual field defects and typical clinical symptoms of Wallenberg’s syndrome such as nystagmus, dysphagia, intersectional paresthesia and Horner’s sign.
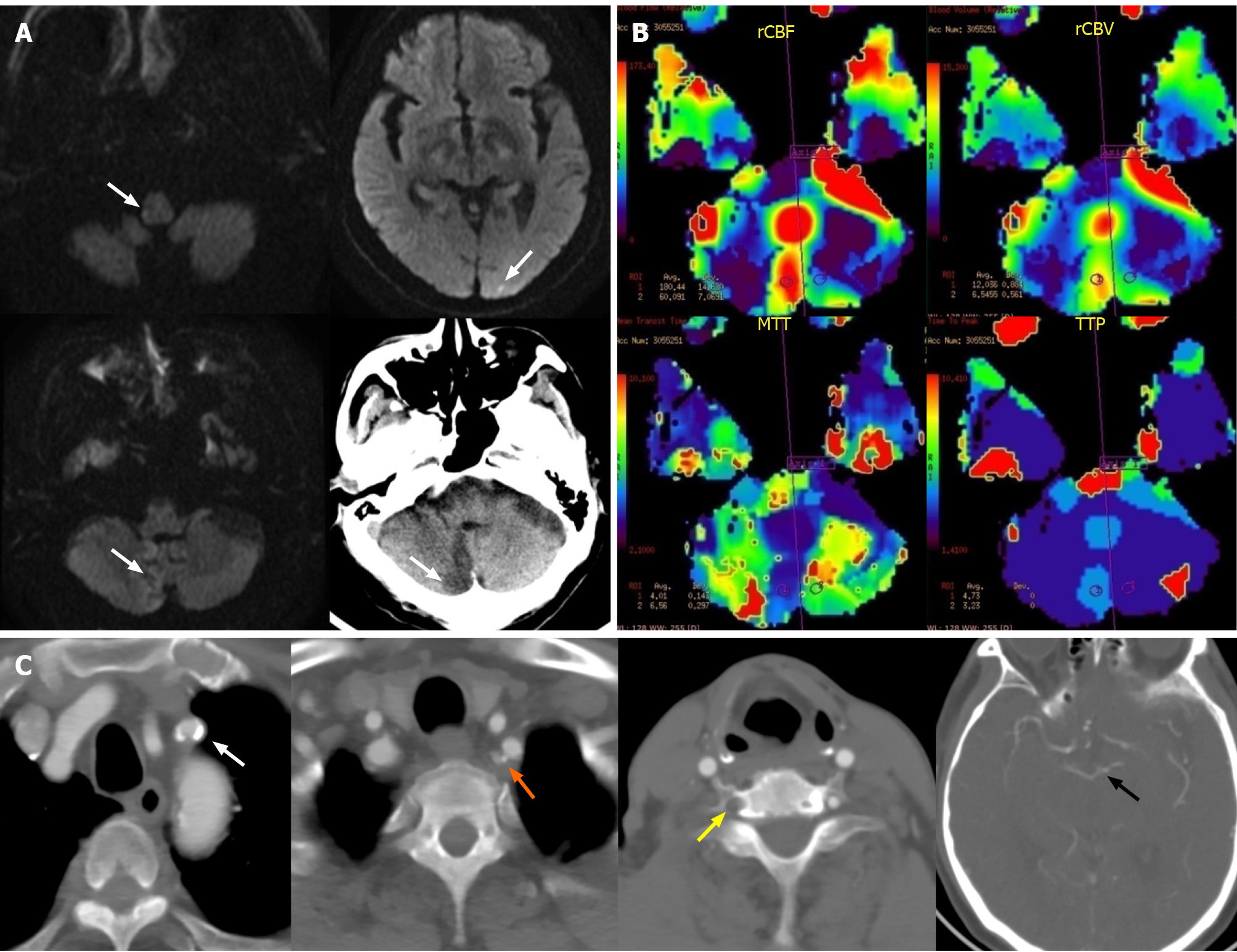
Magnetic resonance (MR) diffusion-weighted imaging and non-contrast computed tomography of the brain revealed subacute infarctions in cerebellar hemisphere, occipital lobe and medulla oblongata after admission (Figure 1A). MR perfusion-weighted imaging also showed that there was no significant difference in relative cerebral blood flow, relative cerebral blood volume, the mean transit time and the time to peak between bilateral cerebellar hemispheres except the infarct core (Figure 1B).
Before admission, computed tomography angiography (CTA) of the head and neck had been performed in another hospital, showing total occlusion of the right vertebral artery (VA) and severe stenosis at the proximal LSA and the origin of the left VA. CTA also revealed the bilateral posterior communicating arteries were not opening which means the incomplete Willis circle (Figure 1C).
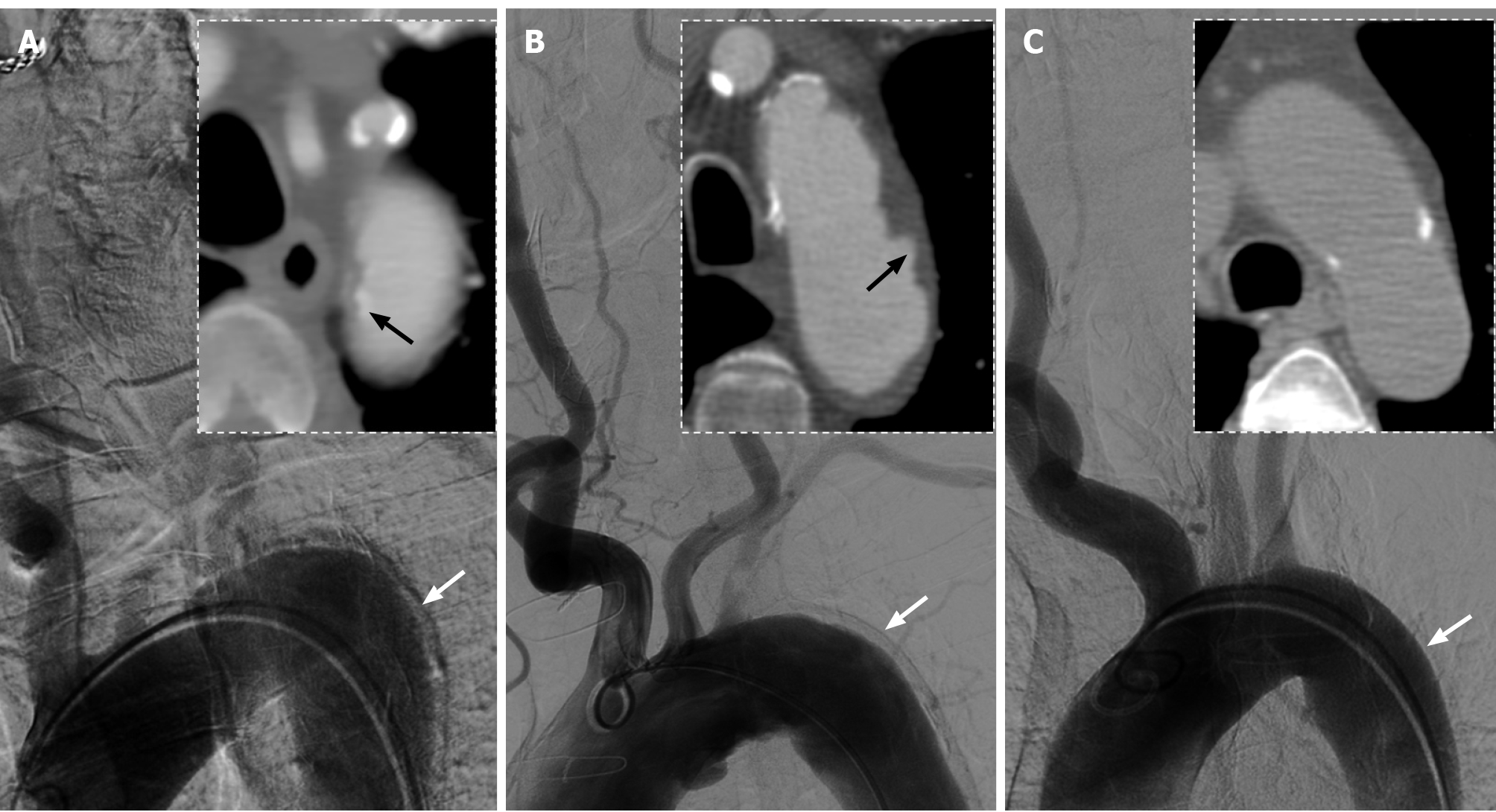
In the review of the interventional procedure, we noticed that a significant motion artifact was displayed on the lateral wall of the descending aorta in the first aortic arch angiogram. Furthermore, the axial views on pre-admission CTA revealed the displacement of aortic intimal calcification near the junction of the LSA (Figure 2A). We also compared the CTA and digital subtraction angiography (DSA) images of two other adult patients and found that the second patient, who had presented with penetrating atherosclerotic ulcers, showed a similar artifact on anteroposterior DSA image (Figure 2B), while the third patient, who had no IMH or ulcers, had no obvious artifact (Figure 2C).
Based on the above analysis, the final diagnosis of the case is IAD due to a focal aortic IMH.
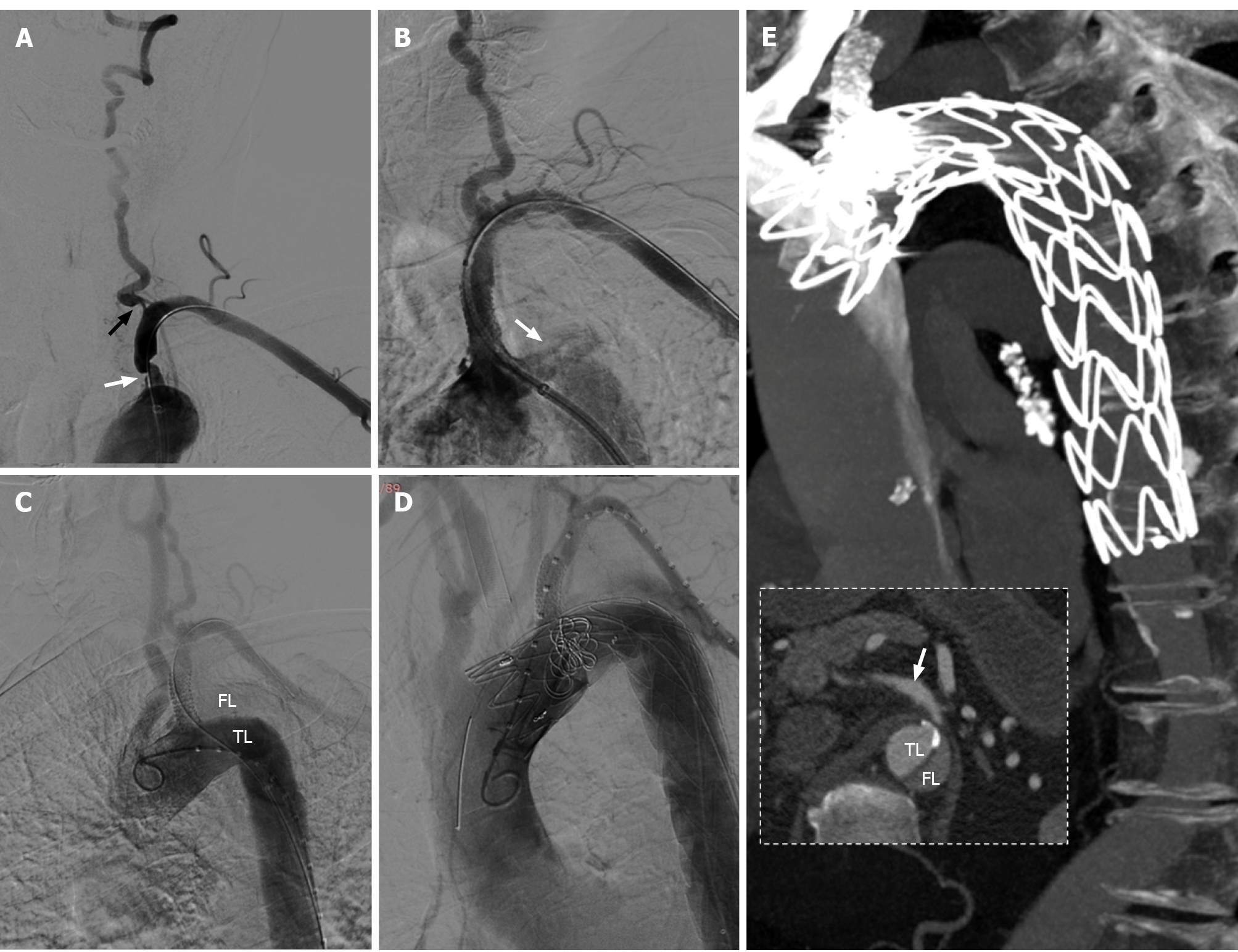
After 4 d of intensive therapy with antiplatelet drugs and statins, endovascular stenting for the stenosis of the LSA and left VA origin was scheduled. The right femoral artery access was established under local anesthesia and DSA showed that the vascular lesions were consistent with those on CTA (Figure 3A). A 9 mm × 25 mm balloon expandable stent (Boston Scientific, Galway, Ireland) was subsequently implanted at a pressure of 10 atm into the proximal LSA. Unfortunately, the patient suffered significant chest pain immediately after the stent was released. Subsequent angiography showed contrast entering the aortic wall near the proximal edge of the stent (Figure 3B) and narrowing of the true lumen of the descending aorta, which was considered a Stanford type B aortic dissection (Figure 3C).
The patient was placed under general anesthesia immediately. Left femoral and brachial artery access was established. Considering the high risk of stroke due to severe stenosis of left VA and malperfusion of LSA, we chose the chimney technique to shorten the procedure time. After measuring vascular diameter and length with a calibrated pigtail catheter, a 32 mm × 20 cm thoracic aorta covered stent-graft system (Medtronic Vascular, Santa Rosa, Calif), and another 8 mm × 50 mm covered stent (Viabahn, Cook Medical, Bloomington, IN) was placed in the proximal LSA to maintain blood flow to the left VA and axillary artery (Figure 3D). After these procedures were completed, the contrast was still observed to slowly enter the entrance of the aortic dissection, so two 12 mm × 14 cm and four 10 mm × 14 cm coils (Cook Medical, Bloomington, IN) were deployed near the intimal tear for further embolization.
The patient developed bloody pleural effusion and severe anemia after thoracic endovascular aortic repair. After blood transfusion and pleural effusion drainage, the patient was discharged 20 d later. CTA of the thoracic aorta at the 2-wk follow-up showed complete exclusion of the false aortic lumen, and no endoleak had occurred (Figure 3E). At the 3-mo follow-up over telephone interview, the patient reported no more chest pain, and the symptoms of dizziness and ataxia had been significantly relieved.
IAD typically occurs after coronary artery bypass graft, thoracic endovascular aneurysm repair and aortic valve surgery[2]. However, IAD is rarely observed during endovascular treatment of the proximal supra-aortic arch vessels such as the subclavian artery and brachiocephalic trunk. Although several cases which related to IAD after LSA stenting, balloon angioplasty and occlusion recanalization have been reported in the scientific literature, the pathogenesis is still unclear[3-5]. There are opinions suggesting that severe aortic calcification or atherosclerosis may be the cause of IAD after interventional treatment[3,6].
In the present case, CTA showed that the stenosis of the proximal LSA was associated with calcified plaque, but the degree of calcification of the aortic arch was not high. One prominent sign on axial view CTA of the aortic arch was the inward displacement of aortic intimal calcification near the junction of the LSA, which is an important characteristic of both IMH and aortic dissection[7]. CTA also showed no obvious two-lumen flow and crescent-shaped aortic wall thickening. Besides, the patient had no symptoms of chest and back before the intervention procedure, so it was most likely to be considered a focal asymptomatic IMH rather than an aortic dissection. It is noteworthy that IMH may progress to aortic dissection under certain conditions[8]. After the balloon expandable stent was implanted into the proximal LSA in this case, the mechanical squeeze on the IMH may have resulted in intimal layer rupture and subsequent progression to a type B aortic dissection. Furthermore, on anteroposterior aortic arch DSA images, another suspicious sign is an obvious motion artifact on the lateral wall of the descending aorta. We compared the DSA images of two other male patients who had been examined with the same imaging device and found that the artifacts of the descending aorta seemed to be associated with the severity of the aortic wall lesions. However, considering the differences in respiratory rate, heart rate, blood pressure and degree of cooperation among three patients may also affect the occurrence of aortic artifacts, studies with larger sample sizes are needed to further confirm this hypothesis.
Several lessons should be noted in the treatment of this case. First, possible peri-procedural complications such as subclavian artery dissection or rupture, stent thrombosis, stroke and embolism of distal upper limb artery were considered before LSA stenting. Nevertheless, CTA and DSA should be combined to observe the aortic wall near the ostium of the LSA to exclude IMH, penetrating atherosclerotic ulcers or other lesions to comprehensively evaluate the interventional risk of IAD. In addition, the calibrated pigtail catheter was more accurate than the guiding catheter in the measurement of the LSA diameter (8 mm vs 9 mm in this case). A balloon expandable stent with larger diameter can increase compression on the vessel wall, which may lead to a higher risk of IAD. Finally, transradial access could have been considered for angioplasty of the left VA without treatment of the LSA stenosis in this case.
Focal aortic IMH is a potential risk factor for IAD during a seemingly simple stenting of the proximal LSA. Sufficient evaluation of multimodal imaging should be performed before the interventional procedures to avoid the occurrence of such event.
| 1. | Chatterjee S, Nerella N, Chakravarty S, Shani J. Angioplasty alone vs angioplasty and stenting for subclavian artery stenosis--a systematic review and meta-analysis. Am J Ther. 2013;20:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Ram H, Dwarakanath S, Green AE, Steyn J, Hessel EA 2nd. Iatrogenic Aortic Dissection Associated With Cardiac Surgery: A Narrative Review. J Cardiothorac Vasc Anesth. 2021;35:3050-3066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Wang YC, Hwang JJ, Lai LP, Tseng CD. Iatrogenic aortic dissection during left subclavian artery stenting: immediate detection by calcium sign under fluoroscope. Cardiovasc Intervent Radiol. 2011;34 Suppl 2:S36-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Sailer AM, van Ommen VG, Tordoir JH, Schurink GW, van Zwam WH. Iatrogenic type A aortic dissection: conservative treatment after complicated left subclavian artery recanalization. J Vasc Interv Radiol. 2013;24:1923-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Millán X, Azzalini L, Dorval JF. Iatrogenic subclavian artery and aortic dissection with mesenteric ischemia following subclavian artery angioplasty: Endovascular management. Catheter Cardiovasc Interv. 2015;86:E194-E199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Januzzi JL, Sabatine MS, Eagle KA, Evangelista A, Bruckman D, Fattori R, Oh JK, Moore AG, Sechtem U, Llovet A, Gilon D, Pape L, O'Gara PT, Mehta R, Cooper JV, Hagan PG, Armstrong WF, Deeb GM, Suzuki T, Nienaber CA, Isselbacher EM; International Registry of Aortic Dissection Investigators. Iatrogenic aortic dissection. Am J Cardiol. 2002;89:623-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Oderich GS, Kärkkäinen JM, Reed NR, Tenorio ER, Sandri GA. Penetrating Aortic Ulcer and Intramural Hematoma. Cardiovasc Intervent Radiol. 2019;42:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Mussa FF, Horton JD, Moridzadeh R, Nicholson J, Trimarchi S, Eagle KA. Acute Aortic Dissection and Intramural Hematoma: A Systematic Review. JAMA. 2016;316:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 361] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hiremath CS S-Editor: Fan JR L-Editor: Filipodia P-Editor: Liu JH