Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9481
Peer-review started: June 22, 2021
First decision: July 5, 2021
Revised: July 28, 2021
Accepted: August 25, 2021
Article in press: August 25, 2021
Published online: November 6, 2021
Processing time: 129 Days and 4.1 Hours
The novel coronavirus disease 2019 (COVID-19) has spread widely around the world with strong infectivity, rapid mutation and a high mortality rate. Mechanical ventilation has been included in the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8) as an important treatment for severe and critical COVID-19 patients, but its clinical efficacy in COVID-19 patients is various. Therefore, it is necessary to study the influencing factors on the efficacy of mechanical ventilation in severe and critical COVID-19 patients.
The aim of this study was to determine the influencing factors on the efficacy of mechanical ventilation in severe and critical COVID-19 patients.
A total of 27 severe and critical COVID-19 patients were enrolled in this study and treated with mechanical ventilation at the Optical Valley Campus of Hubei Maternal and Child Health Care Hospital (Wuhan, Hubei Province) from February 20, 2020 to April 5, 2020. According to the final treatment outcomes, the patients were divided into the “effective group” and “death group.” The clinical data of the two groups, such as the treatment process and final outcome, were retrospectively analyzed in order to determine the specific curative effects on the two groups and the reasons for the differences in such curative effects, as well as to explore the factors related to death.
This study enrolled 27 severe and critical COVID-19 patients, including 17 males (63.0%) and 10 females (37.0%). Their ages were 74.41 ± 11.73-years-old, and 19 patients (70.4%) were over 70-years-old. Severe COVID-19 patients over 70-years-old who were treated with mechanical ventilation died in 14 cases (82.4%); thus, this was the peak age. A total of 17 patients died of basic disease, 16 of whom had more than two basic diseases. The basic diseases were hypertension, diabetes, and cardiovascular and cerebrovascular diseases. At the same time, 13 patients (76.5%) died from an abnormal increase in blood glucose. Among them, eight had diabetes before contracting COVID-19 and five had a stress-induced increase in blood glucose after contracting COVID-19. Diabetic ketoacidosis occurred in one case. The use of tocilizumab may be a double-edged sword that carries a certain risk in clinical usage. Among the patients who died, 16 (94.1%) went into septic shock at the end. There were significant differences in the degree of infection, cardiac and renal function, and blood glucose between the death group and effective group.
Age, blood glucose, cardiac and renal function, and inflammatory reaction are important indicators of poor prognosis for mechanical ventilation in severe and critical COVID-19 patients.
Core Tip: A total of 27 severe and critical coronavirus disease 2019 (COVID-19) patients were treated with mechanical ventilation and divided into the “effective group” and “death group” according to the final outcomes of the treatment. There were significant differences in the degree of infection, cardiac and renal function, and blood glucose between the death group and effective group. We found that age, blood glucose, cardiac and renal function, and inflammatory reaction are important indicators of poor prognosis for mechanical ventilation in severe and critical COVID-19 patients.
- Citation: Zeng J, Qi XX, Cai WW, Pan YP, Xie Y. Retrospective analysis of influencing factors on the efficacy of mechanical ventilation in severe and critical COVID-19 patients. World J Clin Cases 2021; 9(31): 9481-9490
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9481.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9481
The novel coronavirus disease 2019 (COVID-19) has spread widely around the world with strong infectivity, rapid mutation, and a high mortality rate[1-3]. It has a wide clinical spectrum, including mild respiratory disease, asymptomatic infection, and severe pneumonia with acute respiratory failure and even death. Mechanical ventilation has been included in the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8) as an important treatment for severe and critical COVID-19 patients, but its clinical efficacy is various. Therefore, it is necessary to study the influencing factors on the efficacy of mechanical ventilation in severe and critical COVID-19 patients. From February 19, 2020 to April 5, 2020 at Hubei Maternal and Child Health Hospital in Guanggu District (Wuhan, Hubei Province, China), a total of 27 severe and critical COVID-19 patients were treated with noninvasive or invasive mechanical ventilators, 17 of whom died. We retrospectively analyzed the clinical features of the 27 patients in order to improve our understanding of COVID-19 and provide references for clinical treatment.
A total of 27 patients underwent ventilator treatment at Hubei Maternal and Child Health Hospital from February 19, 2020 to April 5, 2020. The criteria for diagnosis and classification were taken from the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8) issued by the National Health Commission and State Administration of Traditional Chinese Medicine.
Exclusion criteria were as follows: previous medical history combined with organ transplantation and immune system disease, pregnant or lactating women, suffering from mental illness, and not consenting to ventilator treatment.
The clinical data of the 27 COVID-19 patients was collected and studied including gender, age, basic disease, blood oxygen saturation on admission, complications, oxygenation index before ventilation, time and type of ventilator use, whether glucocorticoid and tocilizumab were used in combination, laboratory examination, computed tomography (CT) manifestation of lungs, and direct death factors. The approval of the Ethics Committee of Hubei Maternal and Child Health Hospital was obtained.
Laboratory examination: white blood cell count, lymphocyte count, lymphocyte granulocyte ratio, platelet count, C-reactive protein (CRP), interleukin-6 (IL-6), calcitonin, erythrocyte sedimentation rate, lactic acid, D-dimer, B-type natriuretic peptide (BNP), urea nitrogen, creatinine and fasting blood glucose of all patients were measured on admission, before ventilator use and before death, discharge or transfer. Chest CT examination or bedside chest X-ray examination was administered.
The treatment process and final outcomes of the patients were statistically analyzed. According to the final treatment results, the patients were divided into the “effective group” and “death group.” Then the above observation indexes of the two groups were retroactively tracked, and a retrospective control study was established in order to determine the specific curative effects on the two groups and the reasons for the differences in such curative effects, as well as to explore the factors related to death.
Descriptive statistical analyses were performed using SPSS 25.0 software (IBM, Chicago, IL, United States). Counting data are expressed as a percentage, and the two sample rates were compared using χ2test; measurement data conforming to the normal distribution are expressed as the mean ± SD, and measurement data that are not normally distributed are expressed as the median (interquartile range) from description. Measurement data with the same normal distribution and uniform variance between the two groups were compared using the t-test, and the variance was not uniform by the t’-test; the measurement data between the two groups with normal distribution and uniform variance were the same item in the same group at different times. Analysis of variance was used for intercomparison, and rank-sum test was used for those with uneven variance. The Mann-Whitney U-test or Kruskal-Wallis H-test was used to compare the measurement data of skewed distribution between groups. Spearman’s rank correlation coefficient was used for correlation analysis of measurement data conforming to skewed distribution. P < 0.05 represents a significant difference.
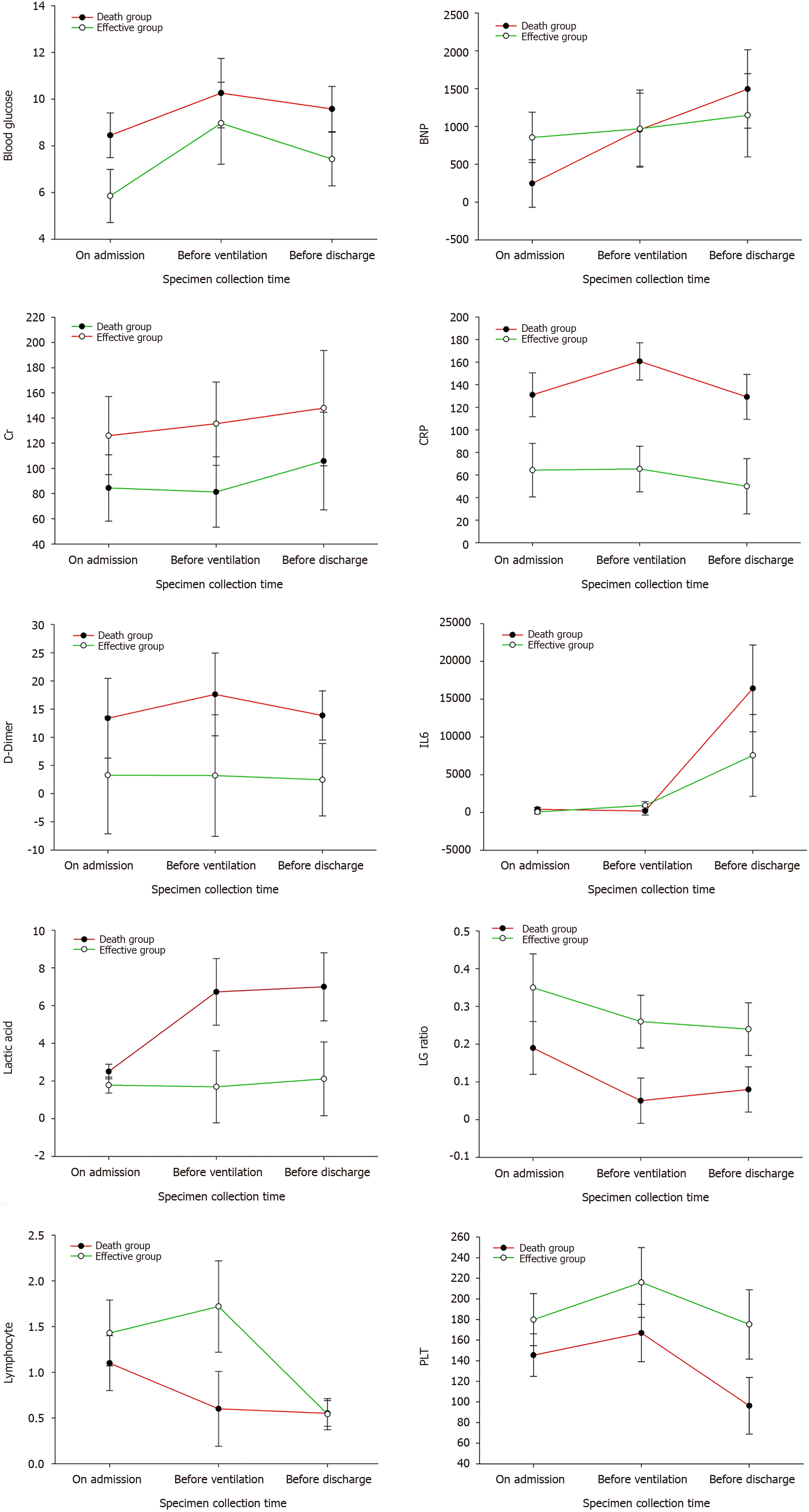
The 27 severe and critical COVID-19 patients were 17 males (63.0%) and 10 females (37.0%). Their ages were 74.41 ± 11.73-years-old, and 19 patients (70.4%) were over 70-years-old. Among them, 15 patients (55.6%) had hypertension, 10 (37%) had diabetes, 10 (37%) had nervous system disease, and 9 (33.3%) had heart disease. The distribution of other combined diseases is shown in Table 1. A total of 23 patients (85.2%) were complicated with two or more basic diseases. The lymphocyte count, platelet count, CRP, IL-6, lactic acid, D-dimer, BNP, creatinine, and fasting blood glucose of all patients were measured on admission, before ventilator use and before discharge (death) (Tables 1 and 2, Figure 1).
| Clinical characters | n (%) | Death group, 17 cases, n (%) | Effective group, 10 cases, n (%) | P value |
| Gender | ||||
| Male | 17 | 11 (64.7) | 6 (35.3) | 0.310 |
| Female | 10 | 6 (60) | 4 (40) | |
| Age (yr) | 74.41 ± 11.73 | 77.94 ± 10.49 | 68.4 ± 11.75 | 0.049a |
| < 60 | 6 (22.2) | 2 (11.8) | 4 (40) | 0.10 |
| 60–69 | 2 (7.4) | 1 (5.9) | 1 (10) | 0.48 |
| 70–79 | 7 (25.9) | 4 (23.5) | 3 (30) | 0.32 |
| > 80 | 12 (44.4) | 10 (58.8) | 2 (20) | 0.049a |
| Underlying disease | ||||
| Hypertension | 15 (55.6) | 8 (47) | 7 (70) | 0.17 |
| Diabetes | 10 (37) | 8 (47) | 2 (20) | 0.13 |
| Cardiopathy | 9 (33.3) | 5 (29.4) | 4 (40) | 0.28 |
| Nervous system disease | 10 (37) | 7 (41.2) | 3 (30) | 0.28 |
| Respiratory disease | 8 (29.6) | 4 (23.5) | 4 (40) | 0.23 |
| Malignant tumor | 6 (22.2) | 4 (23.5) | 2 (20) | 0.36 |
| Digestive system disease | 2 (7.4) | 1 (5.9) | 1 (10) | 0.48 |
| Hematological disease | 1 (3.7) | 1 (5.9) | 0 (0) | 0.63 |
| Chronic renal insufficiency | 4 (14.8) | 2 (11.8) | 2 (20) | 0.35 |
| Rheumatoid arthritis | 1 (3.7) | 1 (5.9) | 0 (0) | 0.63 |
| Decubitus | 1 (3.7) | 1 (5.9) | 0 (0) | 0.63 |
| More than two diseases | 23 (85.2) | 16 (94.1) | 7 (70) | 0.12 |
| Clustering onset | 10 (37) | 8 (47) | 2 (20) | 0.13 |
| Admission classification | ||||
| Severe | 16 (59.3) | 10 (58.8) | 6 (60) | 0.31 |
| Critical | 11 (40.7) | 7 (41.2) | 4 (40) | |
| SpO2 at rest on admission (%) | 85.0 ± 13.46 | 82.82 ± 16.47 | 88.7 ± 4.11 | 0.28 |
| CT on admission | ||||
| Multiple lesions in both lungs | 21 (87.5) | 13 (76.5) | 8 (80) | 0.36 |
| Limited in single lung | 6 (7.4) | 4 (23.5) | 2 (20) | |
| Ventilation mode | ||||
| Noninvasive ventilation | 15 (55.6) | 10 (58.8) | 5 (50) | 0.28 |
| Invasive ventilation | 21 (77.8) | 13 (76.5) | 8 (90) | 0.36 |
| ECMO | 3 (11.1) | 1 (5.9) | 2 (20) | 0.26 |
| Prone position ventilation | 7 (25.9) | 4 (23.5) | 3 (30) | 0.32 |
| Tocilizumab | 14 (51.9) | 9 (52.9) | 5 (50) | 0.31 |
| Glucocorticoid | 15 (55.6) | 12 (70.6) | 3 (30) | 0.04a |
| Oxygenation index before ventilation | 132.64 ± 59.3 | 122.31 ± 56.88 | 149.16 ± 62.32 | 0.270 |
| Days of ventilator treatment | 7.04 ± 6.65 | 4.76 ± 4.63 | 10.9 ± 7.95 | 0.011a |
| Complications | ||||
| Bacterial pneumonia | 24 (88.9) | 14 (82.4) | 10 (100) | 0.23 |
| Anemia | 18 (66.7) | 12 (70.6) | 6 (60) | 0.28 |
| Pneumothorax | 3 (11.1) | 2 (11.8) | 1 (10) | 0.46 |
| Pleural effusion | 5 (18.5) | 4 (23.5) | 1 (10) | 0.29 |
| Arrhythmia | 8 (29.6) | 7 (41.2) | 1 (10) | 0.09 |
| Myocardial ischemia | 12 (44.4) | 10 (58.8) | 2 (20) | 0.049a |
| Heart failure | 10 (37) | 9 (52.9) | 1 (10) | 0.03a |
| Hepatic dysfunction | 8 (29.6) | 5 (29.4) | 3 (30) | 0.33 |
| Acute renal dysfunction | 7 (25.9) | 5 (29.4) | 2 (20) | 0.31 |
| Gastrointestinal hemorrhage | 3 (11.1) | 3 (17.6) | 0 (0) | 0.23 |
| Shock | 17 (63) | 16 (94.1) | 1 (10) | 0.004a |
| Abnormal blood glucose | 19 (70.4) | 13 (76.5) | 6 (60) | 0.23 |
| Electrolyte disorder | 10 (37) | 6 (35.3) | 4 (40) | 0.31 |
| Hemoptysis | 1 (3.7) | 0 (0) | 1 (10) | 0.37 |
| Item | Death group, 17 cases | Effective group, 10 cases | ||||
| On admission | Before ventilation | Before death | On admission | Before ventilation | Before discharge | |
| Lactic acid | 2.44 ± 1.07 | 5.82 ± 6.27 | 17.41 ± 7.10b | 2.00 ± 0.12 | 2.00 ± 0.12 | 2.00 ± 0.12 |
| IL-6 | 347.53 ± 922.41a,b | 125.76 ± 139.63a,b | 12099.43 ± 19424.47a,b | 2.36 ± 1.75 | 1.50 ± 1.22 | 1.00 ± 0.89 |
| CRP | 123.20 ± 94.63 | 151.02 ± 82.27 | 129.23 ± 86.94 | 6802.48 ± 9458.94a,b | 854.56 ± 1322.69a,b | 878.56 ± 1251.27a,b |
| Blood glucose | 8.45 ± 4.37 | 10.97 ± 5.91 | 10.38 ± 5.83 | 12.72 ± 9.76 | 126.03 ± 146.14a,b | 135.51 ± 158.42a,b |
| PLT | 151.00 ± 83.35b | 171.13 ± 103.79 | 96.27 ± 97.446 | 14.86 ± 13.02 | 70.27 ± 13.06 | 77.74 ± 15.31 |
| D-Dimer | 13.38 ± 30.23 | 17.18 ± 30.18 | 13.06 ± 17.51 | 4.21 ± 3.70 | 2.30 ± 1.64 | 49.5 ± 41.95 |
| BNP | 250.36 ± 349.23a,b | 753.91 ± 1323.52a,b | 1410.28 ± 1806.89a,b | 1.0 ± 0.12 | 1.40 ± 0.52 | 1.90 ± 0.32 |
| Cr | 81.70 ± 39.82 | 78.75 ± 34.44 | 105.81 ± 55.53b | 65.32 ± 41.76 | 49.94 ± 59.33 | 1.18 ± 2.65 |
| Lymphocyte | 1.04 ± 1.34 | 0.57 ± 0.45 | 0.55 ± 0.50 | 1.46 ± 2.09 | 1.45 ± 1.60 | 1.04 ± 1.09 |
| Lymphocyte/granulocyte | 16.58 ± 29.10 | 4.58 ± 3.87 | 7.15 ± 7.47 | 57.37 ± 42.08 | 273.06 ± 109.09a,b | 79.4 ± 25.15b |
There were 24 cases of bacterial pneumonia (88.9%), 18 cases of anemia (66.7%), 8 cases of arrhythmia (29.6%), 10 cases of heart failure (37%), 7 cases of acute renal failure (25.9%), 3 cases of gastrointestinal bleeding (11.1%), 8 cases of liver function damage (29.6%), 10 cases of electrolyte disorder (37%), 19 cases of abnormal hyperglycemia (70.4%), 3 cases of pneumothorax (11.1%), 5 cases of pleural effusion (18.5%), and 17 cases of shock (63%).
Our study showed that severe COVID-19 patients over 70 years of age who were treated with mechanical ventilation died in 14 cases (82.4%); thus, this was the peak age. A total of 17 patients died of basic disease, 16 of whom had more than two basic diseases. The basic diseases were hypertension, diabetes and cardiovascular and cerebrovascular diseases. At the same time, 13 patients (76.5%) died from an abnormal increase in blood glucose. Among them, eight had diabetes before contracting COVID-19 and five had a stress-induced increase in blood glucose after contracting COVID-19. Diabetic ketoacidosis occurred in one case.
Studies have shown[4-6] that the immune response ability of patients with diabetes is decreased, and there is often immune dysfunction such as the decrease of CD3+ T cells, imbalance of CD4+/CD8+ cells and decrease of natural killer T cell activity, which increase the risk of virus infection. Angiotensin converting enzyme-2 (ACE-2), the functional receptor of severe acute respiratory syndrome coronavirus (SARS-CoV), is also expressed in islets. During infection, the virus may destroy islets and aggravate diabetes mellitus through ACE-2. After COVID-19 infection, blood glucose metabolism is affected in patients with an irregular diet, limited exercise after hypoxia and the use of glucocorticoids. Therefore, blood glucose monitoring should be strengthened throughout the course of the disease, and the hypoglycemic program should be adjusted in time.
SARS-CoV-2 can enter lung cells through ACE-2 receptor-mediated endocytosis and proliferate in large quantities, releasing more viruses by budding or inducing programmed cell death. After being recognized by the pattern recognition receptors on the immune cells of the body, a large number of cytokines are released through signal transduction, activating more immune cells to participate in the elimination of the virus, and forming a cytokine storm. An overactivated immune system will certainly kill a large number of normal lung cells and seriously damage the lung ventilation function, leading to respiratory failure and eventual death by hypoxia. The cytokine storm induced by COVID-19 is mainly related to IL-1B, IL-6, IL-12, interferon gamma, interferon gamma-induced protein, and monocyte chemoattractant protein-1. The expression of IL-6 was higher than that of tumor necrosis factor alpha and IL-1. A high concentration of IL-6 can induce thrombosis, vascular leakage, and various pathological functions related to myocardial dysfunction, leading to tissue hypoxia, hypotension, multiple organ dysfunction and disseminated intravascular coagulation. Therefore, IL-6 in the COVID-19 cytokine storm is generally used as a biomarker for judging the severity and prognosis of the disease[7-10]. In this study, the total number of lymphocytes, white blood cells, platelet count, lactic acid, IL-6, and D-dimer were selected as the observation indexes. Through comprehensive analyses of these indicators in 17 patients on admission, before ventilator use and before death, we can see that the absolute values of the lymphocyte and platelet counts in critical COVID-19 patients showed a progressive decline, IL-6 showed an abnormal increase, and lactic acid increased. According to the pathological changes in COVID-19 patients, the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8) added immunotherapy, tocilizumab, blood purification therapy, etc. In our 17 dead patients, 9 used tocilizumab and 1 used DFPP. We found that after the use of tocilizumab, IL-6 increased abnormally up to 50000 pg/mL and lymphocytes decreased progressively. In one lymphocytic case, the lowest absolute value of B cells was 0, indicating that the cytokine storm was not completely inhibited. At the same time, it was observed that two patients died the following day and three experienced worse symptoms the following day. Thus, the use of tocilizumab may be a double-edged sword which carries a certain risk in clinical usage. After blocking the IL-6 receptor, the abnormal rise in IL-6 aggravated tissue hypoxia, hypotension and multiple forms of organ damage[11-14].
Digestive tract hemorrhage occurred among the death complications. After COVID-19 infection, the platelet counts abnormally decreased on admission, before ventilator use and before death. The lowest platelet count decreased to six and liver function damage occurred, further aggravating the damage of the coagulation function. At the same time, D-dimer abnormally increased in severe COVID-19 patients, and the risk of thrombosis also increased[15-18]. Patients with severe infection and sepsis may be at risk of falling into a hypercoagulable state which leads to a risk of thrombosis, and those in a low coagulation state may be at risk of bleeding. In addition, anticoagulant monitoring in the treatment of COVID-19 patients receiving extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy is particularly important for reducing bleeding complications and blood product consumption. In early critical patients, mechanical ventilation for more than 48 h is a high-risk factor for the occurrence of stress ulcers in the digestive tract. Thus, it is necessary to strengthen early gastrointestinal nutrition and proton pump inhibitors. In our successful cases, we found that platelets and hemoglobin decreased with timely blood transfusion, timely search for bleeding lesions and timely use of proton pump inhibitors to prevent stress ulcers. The coagulation function and platelet count were detected.
Among the death cases, 16 (94.1%) went into septic shock at the end, which was related to cytokine storm, vascular leakage, insufficient volume, etc. The creatinine value of the effective group was slightly higher than that of the death group, and the creatinine before discharge was better than that on admission and before ventilator use, with the highest creatinine value during hospitalization, indicating that renal failure was not the direct cause of death from COVID-19, but mainly changed after hypoxia and systemic inflammatory reaction[19-21]. However, we detected the COVID-19 virus in the saliva, urine, throat swab and anal swab of a patient treated with ECMO.
Our study found that age, blood glucose, cardiac and renal function, and inflammatory reaction are important indicators of poor prognosis for mechanical ventilation in severe and critical COVID-19 patients.
The clinical efficacy of ventilator treatment in coronavirus disease 2019 (COVID-19) patients is varied. As such, it is necessary to study the influencing factors on the efficacy of mechanical ventilation in severe and critical COVID-19 patients.
Mechanical ventilation has been included in the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8) as an important treatment for severe and critical COVID-19 patients. However, the influencing factors on the efficacy of mechanical ventilation in severe and critical COVID-19 patients are unclear and worth studying.
This study determined the influencing factors on the efficacy of mechanical ventilation in severe and critical COVID-19 patients.
A total of 27 severe and critical COVID-19 patients were enrolled in this study and treated with mechanical ventilation. According to the final treatment outcomes, the patients were divided into the “effective group” and “death group.” The clinical data of the two groups such as treatment process and final outcome were retrospectively analyzed.
The 27 severe and critical COVID-19 patients were 17 males (63.0%) and 10 females (37.0%). Their ages were 74.41 ± 11.73-years-old, and 19 patients (70.4%) were over 70-years-old. Of the patients over 70-years-old treated with mechanical ventilation, 14 died. A total of 17 patients died of basic disease, 16 of whom had more than two basic diseases. The basic diseases were hypertension, diabetes and cardiovascular and cerebrovascular diseases. There were significant differences in the degree of infection, cardiac and renal function, and blood glucose between the death group and effective group.
Age, blood glucose, cardiac and renal function, and inflammatory reaction were important indicators of poor prognosis for mechanical ventilation in severe and critical COVID-19 patients.
In this study, we found that age, blood glucose, cardiac and renal function, and inflammatory reaction are important indicators of poor prognosis for mechanical ventilation in severe and critical COVID-19 patients. The use of tocilizumab may be a double-edged sword which carries a certain risk in clinical usage.
| 1. | Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention. Analysis of 54 Mortality Cases of Coronavirus Disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35:e132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Zuin M, Rigatelli G, Zuliani G, Rigatelli A, Mazza A, Roncon L. Arterial hypertension and risk of death in patients with COVID-19 infection: Systematic review and meta-analysis. J Infect. 2020;81:e84-e86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao S, Long D, Yu L. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets. 2020;31:490-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 4. | Li J, Wang X, Chen J, Zhang H, Deng A. Association of Renin-Angiotensin System Inhibitors with Severity or Risk of Death in Patients with Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol. 2020;5:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 446] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 5. | Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in COVID-19. N Engl J Med. 2020;382:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 719] [Cited by in RCA: 724] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 6. | Zeng J, Xie MH, Yang J, Chao SW, Xu EL. Clinical efficacy of Tocilizumab treatment in severe and critical COVID-19 patients. World J Clin Cases. 2020;8:3763-3773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, Gan H, Sun YL, Fu W, Li W, Liang HL, Cao YY, Yan Q, Cao C, Gao HY, Brüggen MC, van de Veen W, Sokolowska M, Akdis M, Akdis CA. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76:428-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 920] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 8. | Pietrobon AJ, Teixeira FME, Sato MN. Immunosenescence and Inflammaging: Risk Factors of Severe COVID-19 in Older People. Front Immunol. 2020;11:579220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Setiati S, Harimurti K, Safitri ED, Ranakusuma RW, Saldi SRF, Azwar MK, Marsigit J, Pitoyo Y, Widyaningsih W. Risk factors and laboratory test results associated with severe illness and mortality in COVID-19 patients: A systematic review. Acta Med Indones. 2020;52:227-245. [PubMed] |
| 10. | Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1,014-1,015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 475] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Liu P, Wang M, Wang J, Chen J, Yuan W, Li M, Xie Z, Dong W, Li H, Zhao Y, Wan L, Chu T, Wang L, Zhang H, Tao T, Ma J. The clinical data from 19 critically ill patients with coronavirus disease 2019: a single-centered, retrospective, observational study. Z Gesundh Wiss. 2020;1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16-e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1186] [Cited by in RCA: 1480] [Article Influence: 246.7] [Reference Citation Analysis (0)] |
| 13. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5573] [Article Influence: 928.8] [Reference Citation Analysis (1)] |
| 14. | Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F, Balleyguier C, Besse B, Marabelle A, Netzer F, Merad M, Robert C, Barlesi F, Gachot B, Stoclin A. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: A case report. Ann Oncol. 2020;31:961-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 15. | Shojaee S, Pourhoseingholi MA, Ashtari S, Vahedian-Azimi A, Asadzadeh-Aghdaei H, Zali MR. Predicting the mortality due to COVID-19 by the next month for Italy, Iran and South Korea: A simulation study. Gastroenterol Hepatol Bed Bench. 2020;13:177-179. [PubMed] |
| 16. | Tatum D, Taghavi S, Houghton A, Stover J, Toraih E, Duchesne J. Neutrophil-to-Lymphocyte Ratio and Outcomes in Louisiana COVID-19 Patients. Shock. 2020;54:652-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1757] [Cited by in RCA: 1835] [Article Influence: 305.8] [Reference Citation Analysis (0)] |
| 18. | Mizumoto K, Chowell G. Estimating Risk for Death from Coronavirus Disease, China, January–February 2020. Emerg Infect Dis. 2020;26:1,251-1,256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 19. | Gao L, Jiang D, Wen XS, Cheng XC, Sun M, He B, You LN, Lei P, Tan XW, Qin S, Cai GQ, Zhang DY. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 20. | Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, Hu M, Li XY, Peng P, Shi HZ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 769] [Cited by in RCA: 924] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 21. | Hu H, Yao N, Qiu Y. Comparing Rapid Scoring Systems in Mortality Prediction of Critically Ill Patients with Novel Coronavirus Disease. Acad Emerg Med. 2020;27:461-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification:
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hansson A, Simadibrata M S-Editor: Yan JP L-Editor: Filipodia P-Editor: Wu RR