Published online Nov 6, 2021. doi: 10.12998/wjcc.v9.i31.9452
Peer-review started: March 27, 2021
First decision: May 11, 2021
Revised: May 20, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: November 6, 2021
Processing time: 210 Days and 8.4 Hours
At present, large-scale studies on the clinical characteristics of sepsis-induced cardiomyopathy (SIC) are lacking.
To investigate the clinical characteristics of SIC.
Based on the analysis of the MIMIC-III public database, we performed a large-scale retrospective study involving sepsis patients who were admitted to the intensive care unit (ICU) and had no concomitant cardiac disease. We used propensity score matching analysis and multivariate logistic regression to ensure the robustness of the results. The primary outcome was hospital mortality, and the secondary outcomes included the number of patients who received mechanical ventilation or renal replacement therapy during their hospital stay, the number of patients administered with vasopressors, the length of ICU stay, and the length of hospital stay.
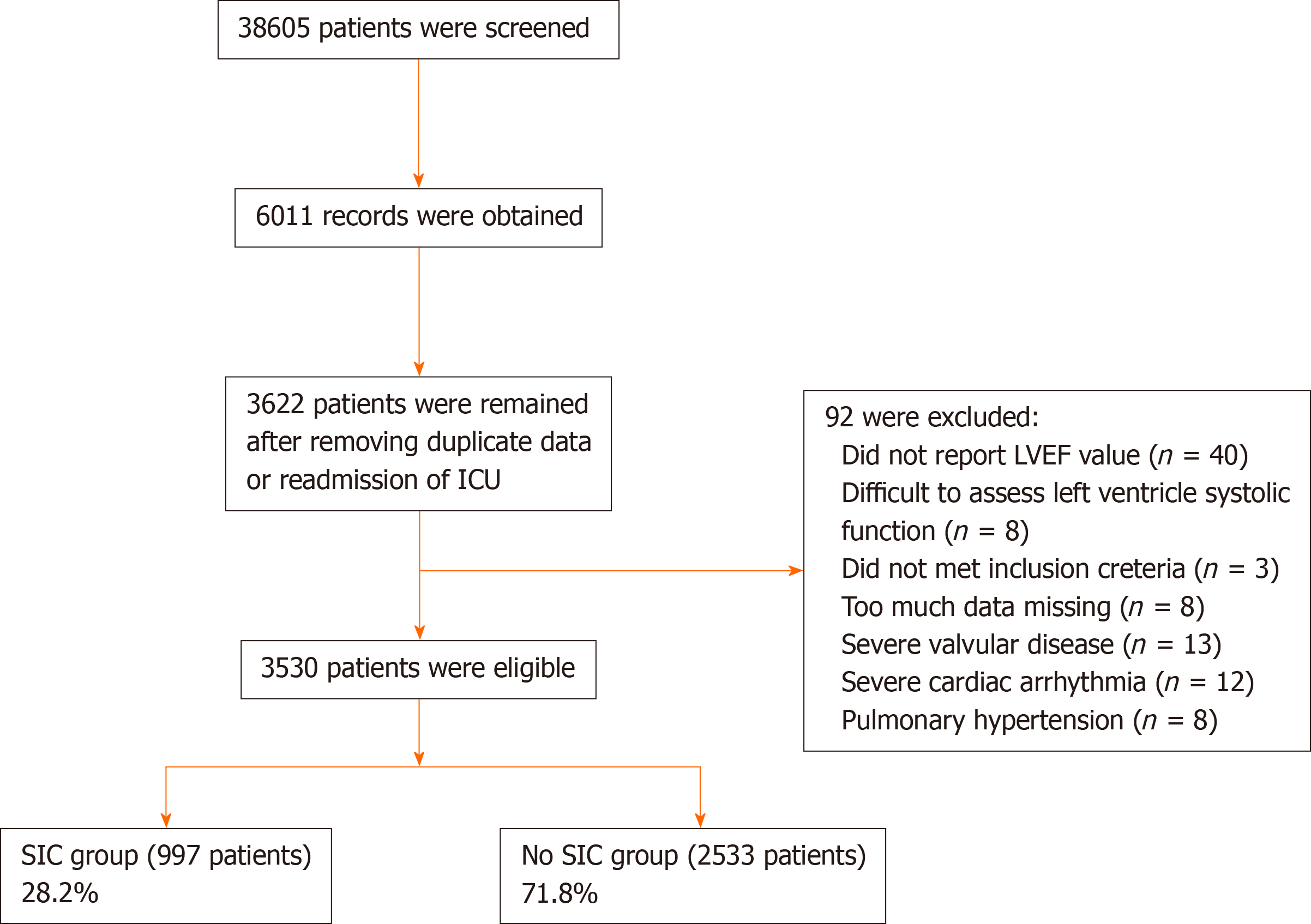
In the present study, after screening 38605 patients, 3530 patients with sepsis were included. A total of 997 patients met the SIC diagnostic criteria, and the incidence of SIC was 28.20% (95% confidence interval [CI]: 26.80%-29.70%). Compared to patients in the non-SIC group, patients in the SIC group were of older age and had a higher Simplified Acute Physiology Score (SAPS)-I score, SAPS-II score, and Elixhauser comorbidity index (ECI). A total of 367 (36.8%) of 997 patients in the SIC group and 818 (32.3%) of 2533 patients in the non-SIC group died in the hospital, which resulted in a significant between-group difference (odds ratios = 1.22, 95%CI: 1.05-1.42; P = 0.011). For the secondary outcomes, more patients in the SIC group received mechanical ventilation and vasopressors. Multivariate logistic regression analysis showed that age, male sex, ECI, hemoglobin level, diabetes, and mechanical ventilation use on the first day of ICU admission were risk factors for SIC.
Compared with non-SIC patients, hospital mortality is higher in SIC patients.
Core Tip: We performed a large-scale, retrospective study to investigate the clinical characteristics of sepsis-induced cardiomyopathy (SIC). Our study showed that the incidence of SIC was 28.20% (95% confidence interval: 26.80%-29.70%). Hospital mortality was higher in SIC patients than in non-SIC patients.
- Citation: Liang YW, Zhu YF, Zhang R, Zhang M, Ye XL, Wei JR. Incidence, prognosis, and risk factors of sepsis-induced cardiomyopathy. World J Clin Cases 2021; 9(31): 9452-9468
- URL: https://www.wjgnet.com/2307-8960/full/v9/i31/9452.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i31.9452
Sepsis-induced cardiomyopathy (SIC) is a complication of sepsis and septic shock that was first described by Parker et al[1,2] in 1984. SIC is characterized by reduced left ventricular ejection fraction (LVEF) and the reduced LVEF could be reversed within 7-10 d in survivors; however, these reversions were less significant in those who died[1,3,4]. The pathologic mechanism of SIC is still unclear, although it is speculated to be related to myocardial inhibitors released by the pathogens and the host, as well as global ischemia after septic distributive shock[5-12].
At present, large-scale studies on the clinical characteristics, such as the incidence, prognosis, and risk factors of SIC, are lacking. A few small studies have been performed to investigate the incidence, risk factors, and mortality of SIC, although these studies demonstrated conflicting results, with the incidence of SIC varying from 13.8%-64%[13-20]. One retrospective cohort study involving 210 adult patients with sepsis or septic shock reported that SIC developed in 13.8% of sepsis patients[14]. Another study screened 67 sepsis patients who had no previous cardiac disease and survived more than 48 h after admission to the intensive care unit (ICU); the results showed that the incidence of SIC within 60 h of ICU admission was 60%[17]. Furthermore, the mortality of patients with SIC varied greatly among the studies, ranging from 24.1%-90%[13-20]. To further investigate the clinical characteristics of SIC, we performed a large-scale, retrospective study.
This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement[21]. This was a single-center, retrospective study based on the third edition of the MIMIC-III database, which was developed and maintained by the Laboratory for Computational Physiology at MIT[22,23]. The MIMIC-III database is a single-center database including longitudinal data on 38605 patients who were admitted to the ICU of Beth Israel Deaconess Medical Center from 2002 to 2011 for a total of 53423 distinct admissions. This study was approved by the ethics committee of Guangzhou Red Cross Hospital (Approval No. AF/SC-107/02.0). As the present study was based on the analysis of MIMIC-III public database, informed consent form was waived.
We screened the discharge diagnosis of patients in MIMIC-III database by ICD-9 and ICD-10 codes. Adult patients (age ≥ 18 years) who had a discharge diagnosis of sepsis, severe sepsis, or septic shock and were admitted to the ICU of Beth Israel Deaconess Medical Center from 2002 to 2011 in the MIMIC-III database were screened for inclusion.
To exclude the effects of concomitant cardiac disease on cardiac function, in the present study, patients who had a discharge diagnosis of any other cardiac disease, such as acute coronary syndrome, chronic heart dysfunction, severe valvular heart disease, severe cardiac arrhythmia, ischemic heart disease, hypertensive heart disease, congenital heart disease, rheumatic heart disease, myocarditis, infective endocarditis, any other cardiomyopathy (such as hypertrophic cardiomyopathy, dilated cardiomyopathy, restrictive cardiomyopathy, ischemic cardiomyopathy, and stress-induced cardiomyopathy), echocardiographic manifestation of intracardiac thrombus, mass, vegetation, pulmonary hypertension, or echocardiographic evidence of severe basal septal hypertrophy with an outflow gradient, were under age 18, had severe hypoxemia, were pregnant, or had no echocardiography examination were excluded.
The definitions and diagnostic criteria for sepsis, severe sepsis, and septic shock were unchanged between 2002 and 2011, according to the Surviving Sepsis Campaign Guidelines[24-26].
Due to the lack of a gold standard and unified consensus for the diagnosis of SIC at present, referring to the inclusion standard of previous studies[13-20] and international cardiac failure guidelines[27-29], the diagnostic criteria for SIC used in the present study were as follows: (1) The admission and discharge diagnoses including sepsis, severe sepsis or septic shock[24-26]; (2) Existing left ventricular systolic dysfunction with a LVEF < 50% or patients with no LVEF value reported but were reported in the echocardiography data as having global left ventricular hypokinesis or global left ventricular systolic dysfunction, considered to be due to sepsis; and (3) No concomitant cardiac disease by screening the discharge diagnosis of patients in the MIMIC-III database by ICD-9 and ICD-10 codes.
The following demographic data and admission information were collected: Age, gender, weight, height, body surface area (BSA), body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate, respiratory rate, temperature, Simplified Acute Physiology Score-I (SAPS-I), SAPS-II, Sequential Organ Failure Assessment (SOFA), Elixhauser comorbidity index (ECI), admission type (emergency or elective), and sepsis type (sepsis, severe sepsis, or septic shock).
Additionally, data regarding the use of mechanical ventilation (MV) or renal replacement therapy (RRT) within the first day of ICU admission were collected.
Microbiology events were recorded and the following laboratory results were also collected: White blood cell (WBC) count, hemoglobin, blood platelet count (PLT), serum potassium, serum sodium, serum chloride, serum bicarbonate, pH, partial pressure of carbon dioxide, partial pressure of oxygen, and lactate value. The maximum levels of blood creatinine, blood urea nitrogen (BUN), cardiac troponin T, creatinine kinase (CK), and creatine kinase-MB (CK-MB) during hospital stay were also collected.
We recorded the chronic comorbidities of our study cohort. The MIMIC-III database contains over 15693 different diagnoses classified by ICD 9 and ICD 10 codes. For describing chronic diseases more concisely, we used Elixhauser’s comorbidity classification[30] according to an algorithm provided by the authors of the MIMIC-III database[31]. Chronic diseases can effectively be reflected by the Elixhauser comorbidity classification, and they have been validated for both ICD-9 and ICD-10 codes[32].
The primary outcomes were SIC incidence rate and hospital mortality rate, and the secondary outcomes included the number of patients who received MV or RRT during their hospital stay, the number of patients administered vasopressors (including norepinephrine, dopamine, epinephrine, and vasopressin), the length of ICU stay, and the length of hospital stay.
The details of the data screening strategies used are shown in the Supplementary File. Other source codes for our analyses, which were provided by the authors of the MIMIC-III database, can be found at GitHub[31,33]. Categorical variables including demographic data, admission information, and interventions are shown as frequencies, and continuous variables including vital signs and laboratory parameters are presented as the mean ± SD or median with interquartile range (25%, 75%). We used the analysis of variance or non-parametric tests to analyze continuous variables as appropriate. Categorical variables were analyzed using Pearson’s χ2 test or Fisher’s exact test.
We used binary multivariate logistic regression analysis (method: forward, LR) to analyze the risk factors for SIC. Variables with P values < 0.10 between groups, or if the variables could complicate the relationships of outcomes in biology, or if the variables were previously considered to be potential confounders were included in the logistic regression analysis model. The risk factors selected by multivariate logistic regression analysis are expressed as odds ratios (ORs) and 95% confidence intervals [CIs].
As some variables are missing a moderate amount of data, a complete case analysis in multivariable logistic regression analysis will exclude any patient with a single missing datapoint which will lead to a significant selection bias. Hence, multiple imputation strategies are used to overcome this deficit in the multivariate logistic regression analysis.
For the primary outcome of hospital mortality, in order to ensure the robustness of our results, we used the propensity score matching (PSM) method to adjust and balance the influence of confounding factors between groups. Variables that may be related to the incidence of SIC were included. Each SIC patient was matched with a non-SIC patient at a proportion of 1:1 with the closest propensity score. The matching tolerance was 0.02.
We also performed subgroup analyses to further investigate whether the primary outcome with regards to hospital mortality differed among the subgroups. The subgroups included age (< 60 years; ≥ 60 years), BMI (≥ 28 kg/m2; < 28 kg/m2), gender, SOFA score (≥ 2 points; < 2 points), SBP (≥ 90 mmHg; < 90 mmHg), MAP (< 65 mmHg; ≥ 65 mmHg), and use of MV or RRT in the first day of ICU admission.
We used PostgreSQL 10.0 software (University of California, Berkeley, California, USA) and Navicat premium 12.0 software (premiumSoft Cybertech Ltd, Kowloon, Hong Kong, China) for database management and data retrieval and screening; SPSS 23.0 software (IBM Corp., Armonk, NY, United States) was used for statistical analyses. P < 0.05 was considered to indicate statistical significance.
Initially, 38605 patients in the MIMIC-III database were screened for eligibility, and 6011 records were included. After removing duplicate records or readmissions to the ICU, 3622 sepsis patients were left. We further screened the echocardiography reports, and 92 patients were removed due to too much missing data, being difficult to assess left ventricle systolic function, severe valvular disease, severe cardiac arrhythmia, or pulmonary hypertension. Ultimately, 3530 patients were included in the present study (Figure 1). According to the presence or absence of SIC, the patients were divided into an SIC group or a non-SIC group. In total, there were 997 patients in the SIC group and 2533 patients in the non-SIC group. The SIC incidence rate was 28.20% (95%CI: 26.80%-29.70%). Of the included patients, 3044 were reported with the explicit LVEF value, 484 in the SIC group with no explicit LVEF value were reported with global left ventricular hypokinesis, or global left ventricular systolic dysfunction, and 2 in the non-SIC group were reported with normal cardiac index.
The clinical characteristics and laboratory results of the included patients are shown in Table 1. There were more male patients in the SIC group compared with those in the non-SIC group (634/997 patients, 63.6% vs 1383/2533 patients, 54.6%, P < 0.001). Compared to patients in the non-SIC group, patients in the SIC group had a significant older age (68.42 ± 15.21 years vs 64.46 ± 15.39 years, P < 0.001), higher SAPS-I score (21.40 ± 5.36 vs 20.90 ± 5.47, P = 0.013), SAPS-II score (46.57 ± 14.86 vs 44.09 ± 15.43, P < 0.001), and ECI score (15.30 ± 8.73 vs 13.76 ± 8.97, P < 0.001), and lower SBP (108.20 ± 14.05 mmHg vs 111.42 ± 14.89 mmHg, P < 0.001). Patients in both groups had similar BSA (1.86 ± 0.45 m2 vs 1.88 ± 0.45 m2, P = 0.224) and BMI (28.85 ± 13.46 kg/m2 vs 29.24 ± 8.77 kg/m2, P = 0.345), temperature (36.84 ± 0.79℃ vs 36.88 ± 0.77℃, P = 0.267), MAP (72.61 ± 10.15 mmHg vs 73.20 ± 10.22 mmHg, P = 0.130), and SOFA score (7.02 ± 3.71 vs 6.89 ± 3.88, P = 0.342) (Table 1).
| Covariate | Original patients (before matching) | PSM adjusted patients (after matching) | Missing data (%) | ||||
| Non-SIC (n = 2533) | SIC (n = 997) | P value | Non-SIC (n = 809) | SIC (n = 809) | P value | ||
| Age (yr) | 64.46 ± 15.39 | 68.42 ± 15.21 | 0.000 | 68.06 ± 14.34 | 67.86 ± 15.37 | 0.791 | 0.0 |
| Gender (Male), n (%) | 1383 (54.6) | 634 (63.6) | 0.000 | 513 (63.4) | 510 (63.0) | 0.877 | 0.0 |
| Height (cm) | 169.22 ± 10.52 | 169.74 ± 12.16 | 0.236 | 169.34 ± 10.74 | 169.74 ± 11.28 | 0.466 | 12.1 |
| Weight (kg) | 84.19 ± 27.24 | 81.91 ± 25.49 | 0.027 | 80.74 ± 23.35 | 82.21 ± 26.16 | 0.233 | 6.2 |
| BSA (m2) | 1.88 ± 0.45 | 1.86 ± 0.45 | 0.224 | 1.91 ± 0.33 | 1.93 ± 0.36 | 0.218 | 12.6 |
| BMI (kg/m2) | 29.24 ± 8.77 | 28.85 ± 13.46 | 0.345 | 28.14 ± 7.69 | 28.59 ± 9.58 | 0.295 | 12.6 |
| Temperature (℃) | 36.88 ± 0.77 | 36.84 ± 0.79 | 0.267 | 37.02 ± 0.79 | 36.85 ± 0.76 | 0.000 | 2.1 |
| SBP (mmHg) | 111.42 ± 14.89 | 108.20 ± 14.05 | 0.000 | 110.55 ± 15.17 | 108.69 ± 14.21 | 0.011 | 1.6 |
| DBP (mmHg) | 57.60 ± 10.08 | 57.69 ± 10.09 | 0.821 | 59.18 ± 10.22 | 57.90 ± 9.99 | 0.011 | 1.6 |
| MAP (mmHg) | 73.20 ± 10.22 | 72.61 ± 10.15 | 0.130 | 75.09 ± 10.52 | 72.68 ± 9.96 | 0.000 | 1.5 |
| HR (beats min-1) | 91.97 ± 17.38 | 92.32 ± 17.10 | 0.594 | 91.50 ± 17.23 | 92.41 ± 17.27 | 0.289 | 1.5 |
| RR (min-1) | 21.20 ± 4.73 | 21.15 ± 4.45 | 0.789 | 21.32 ± 4.64 | 21.20 ± 4.46 | 0.572 | 1.5 |
| SpO2 (%) | 96.84 ± 2.70 | 96.91 ± 2.92 | 0.458 | 96.75 ± 3.02 | 96.91 ± 2.75 | 0.269 | 1.6 |
| SOFA score | 6.89 ± 3.88 | 7.02 ± 3.71 | 0.342 | 6.86 ± 3.72 | 6.99 ± 3.72 | 0.496 | 0.0 |
| SAPS-I score | 20.90 ± 5.47 | 21.40 ± 5.36 | 0.013 | 22.03 ± 5.32 | 21.29 ± 5.27 | 0.005 | 0.0 |
| SAPS-II score | 44.09 ± 15.43 | 46.57 ± 14.86 | 0.000 | 46.15 ± 15.18 | 46.32 ± 15.00 | 0.825 | 0.0 |
| ECI | 13.76 ± 8.97 | 15.30 ± 8.73 | 0.000 | 14.56 ± 8.63 | 15.20 ± 8.83 | 0.142 | 0.0 |
| Microbiology, n (%) | |||||||
| Positive | 1387 (54.8) | 560 (56.2) | 0.448 | 463 (57.2) | 453 (56.0) | 0.616 | 0.0 |
| Negative | 1146 (45.2) | 437 (43.8) | 346 (42.8) | 356 (44.0) | |||
| Interventions, n (%) | |||||||
| Renal replacement use (1st d) | 249 (9.8) | 120 (12.0) | 0.054 | 81 (10.0) | 98 (12.1) | 0.178 | 0.0 |
| Mechanical ventilation use (1st d) | 1232 (48.6) | 516 (51.8) | 0.095 | 341 (42.2) | 414 (51.2) | 0.000 | 0.0 |
| Comorbidities, n (%) | |||||||
| Renal failure | 614 (24.2) | 324 (32.5) | 0.000 | 282 (34.9) | 249 (30.8) | 0.081 | 0.0 |
| Liver failure | 418 (16.5) | 74 (7.4) | 0.000 | 17 (2.1) | 71 (8.8) | 0.000 | 0.0 |
| Diabetes | 809 (31.9) | 424 (42.5) | 0.000 | 337 (41.7) | 333 (41.2) | 0.840 | 0.0 |
| COPD | 552 (21.8) | 195 (19.6) | 0.144 | 186 (23.0) | 162 (20.0) | 0.146 | 0.0 |
| Coagulopathy | 869 (34.3) | 317 (31.8) | 0.155 | 283 (35.0) | 255 (31.5) | 0.140 | 0.0 |
| Admission type, n (%) | |||||||
| Emergency | 2396 (94.6) | 960 (96.3) | 0.036 | 780 (96.4) | 785 (97.0) | 0.485 | 0.0 |
| Elective | 137 (5.4) | 37 (3.7) | 29 (3.6) | 24 (3.0) | 0.0 | ||
| Sepsis type, n (%) | |||||||
| Sepsis | 548 (21.6) | 168 (16.9) | 0.003 | 174 (21.5) | 143 (17.7) | 0.064 | 0.0 |
| Severe sepsis | 1315 (51.9) | 567 (56.9) | 411 (50.8) | 454 (56.1) | 0.0 | ||
| Septic shock | 670 (26.5) | 262 (26.3) | 224 (27.7) | 212 (26.2) | 0.0 | ||
| Laboratory tests | |||||||
| WBC (109/L) | 16.50 ± 12.92 | 15.93 ± 9.65 | 0.206 | 17.15 ± 12.94 | 15.61 ± 9.17 | 0.006 | 0.3 |
| Hemoglobin (g/dL) | 9.37 ± 1.88 | 9.57 ± 1.81 | 0.004 | 9.42 ± 1.91 | 9.57 ± 1.79 | 0.099 | 0.3 |
| Platelet (109/L) | 195.01 ± 137.10 | 199.63 ± 124.20 | 0.356 | 202.55 ± 139.20 | 200.23 ± 125.24 | 0.725 | 0.2 |
| Sodium (mmol/L) | 139.96 ± 5.62 | 140.04 ± 5.17 | 0.705 | 139.92 ± 5.59 | 140.02 ± 5.20 | 0.695 | 0.1 |
| Potassium (mmol/L) | 3.78 ± 0.62 | 3.82 ± 0.60 | 0.064 | 3.76 ± 0.63 | 3.81 ± 0.61 | 0.094 | 0.1 |
| Chloride (mmol/L) | 102.19 ± 7.57 | 102.09 ± 6.80 | 0.716 | 101.71 ± 7.25 | 102.25 ± 6.78 | 0.117 | 0.1 |
| BUN (mg/dL) | 39.02 ± 27.97 | 43.79 ± 27.74 | 0.000 | 40.22 ± 27.95 | 42.90 ± 27.39 | 0.052 | 0.2 |
| Creatine (mg/dL) | 2.21 ± 2.08 | 2.38 ± 2.02 | 0.023 | 2.43 ± 2.26 | 2.33 ± 2.04 | 0.389 | 0.1 |
| Lactate (mmol/L) | 3.30 ± 2.77 | 3.59 ± 3.16 | 0.012 | 3.20 ± 2.55 | 3.46 ± 3.01 | 0.074 | 14.5 |
| Albumin (g/dL) | 2.68 ± 0.67 | 2.78 ± 0.60 | 0.001 | 2.71 ± 0.66 | 2.76 ± 0.60 | 0.196 | 39.3 |
| Bilirubin (mg/dL) | 3.69 ± 7.35 | 1.82 ± 3.58 | 0.000 | 2.70 ± 5.50 | 1.78 ± 3.57 | 0.001 | 25.6 |
| PH | 7.34 ± 0.11 | 7.34 ± 0.11 | 0.358 | 7.34 ± 0.10 | 7.34 ± 0.11 | 0.592 | 27.3 |
| PO2 (mmHg) | 132.70 ± 86.45 | 140.77 ± 92.91 | 0.037 | 128.11 ± 81.34 | 136.85 ± 90.36 | 0.081 | 27.2 |
| PCO2 (mmHg) | 41.20 ± 14.08 | 39.92 ± 11.76 | 0.030 | 40.70 ± 14.25 | 39.81 ± 11.62 | 0.239 | 27.3 |
| Bicarbonate (mmol/L) | 20.74 ± 5.61 | 20.32 ± 5.34 | 0.042 | 20.35 ± 5.49 | 20.32 ± 5.18 | 0.916 | 0.1 |
| Glucose (mg/dl) | 140.88 ± 46.90 | 146.09 ± 51.54 | 0.004 | 145.48 ± 48.58 | 144.89 ± 48.96 | 0.808 | 1.6 |
| CK-MB (%) | 6.83 ± 6.87 | 5.82 ± 6.00 | 0.063 | 6.87 ± 7.11 | 5.40 ± 5.64 | 0.037 | 80.7 |
| CK (U/L) | 68.00 (31.00-194.00) | 74.50 (32.00-229.00) | 0.374 | 66.50 (30.00-212.25) | 76.00 (32.00-269.00) | 0.382 | 24.9 |
| Troponin T (ng/mL) | 0.33 ± 1.06 | 0.38 ± 1.24 | 0.319 | 0.41 ± 1.41 | 0.39 ± 1.25 | 0.814 | 34.0 |
There were no significant differences between two groups with regard to RRT use (249/2533 patients vs 120/997 patients, P = 0.054) and MV use (1232/2533 patients vs 516/997 patients, P = 0.095) in the first day of ICU admission. The culture positive rates of microbiology samples were similar between the two groups (1387/2533 patients vs 560/997 patients, P = 0.448) (Table 1).
Compared with the non-SIC group, there were more severe sepsis patients (567/997 patients vs 1315/2533 patients, P = 0.003), and more emergency admission to ICU patients in the SIC group (960/997 patients vs 2396/2533 patients, P = 0.036) (Table 1). In terms of comorbidities, patients in the SIC group had more renal failure (324/997 patients vs 614/2533 patients, P < 0.001), diabetes (424/997 patients vs 809/2533 patients, P < 0.001), and less liver failure (960/997 patients vs 2396/2533 patients, P < 0.001) compared with non-SIC patients.
Patients in the SIC group had similar WBC count (15.93 ± 9.65 × 109/L vs 16.50 ± 12.92 × 109/L, P = 0.206) and PLT count (199.63 ± 124.20 × 109/L vs 195.01 ± 137.10 × 109/L, P = 0.356), but a higher hemoglobin level (9.57 ± 1.81 g/dL vs 9.37 ± 1.88 g/dL, P = 0.004) compared with those in the non-SIC group. There were no significant differences with regard to the serum sodium level, potassium level, and chloride level (Table 1).
Compared with patients in the non-SIC group, patients in the SIC group had a higher creatine level (2.38 ± 2.02 mg/dL vs 2.21 ± 2.08 mg/dL, P = 0.023), BUN level (43.79 ± 27.74 mg/dL vs 39.02 ± 27.97 mg/dL, P < 0.001), lactate level (3.59 ± 3.16 mmol/L vs 3.30 ± 2.77 mmol/L, P < 0.001), and blood glucose level (146.09 ± 51.54 mg/dL vs 140.88 ± 46.90 mg/dL, P = 0.023) (Table 1).
There were no significant differences with regard to CK, CK-MB, and troponin T levels between the two groups. However, there were a large number of missing values for CK, CK-MB, and troponin T (Table 1). The results should be interpreted with great caution.
For the primary outcome of hospital mortality, 367 (36.8%) of 997 patients in the SIC group and 818 (32.3%) of 2533 patients in the non-SIC group died in the hospital, which resulted in a significant between-group difference (OR = 1.22, 95%CI: 1.05-1.42; P = 0.011) (Table 2).
| Outcome | Original patients (before matching) | PSM adjusted patients (after matching) | ||||||
| Non-SIC (n = 2533) | SIC (n = 997) | OR (95%CI) | P value | Non-SIC (n = 809) | SIC (n = 809) | OR (95%CI) | P value | |
| Hospital mortality | 818 (32.3) | 367 (36.8) | 1.22 (1.05-1.42) | 0.011 | 243 (30.0) | 286 (35.4) | 1.27 (1.03-1.57) | 0.023 |
| Mechanical ventilation use | 1478 (58.3) | 619 (62.1) | 1.17 (1.01-1.36) | 0.042 | 431 (53.3) | 500 (61.8) | 1.42 (1.16-1.73) | 0.001 |
| RRT use | 290 (11.4) | 116 (11.6) | 1.02 (0.81-1.28) | 0.876 | 91 (11.2) | 94 (11.6) | 1.04 (0.76-1.41) | 0.815 |
| Norepinephrine use | 1276 (50.4) | 589 (59.1) | 1.42 (1.23-1.65) | 0.000 | 433 (53.5) | 474 (58.6) | 1.23 (1.01-1.50) | 0.040 |
| Epinephrine use | 63 (2.5) | 62 (6.2) | 2.59 (1.81-3.70) | 0.000 | 25 (3.1) | 46 (5.7) | 1.88 (1.14-3.09) | 0.012 |
| Dopamine use | 279 (11.0) | 188 (18.9) | 1.88 (1.54-2.30) | 0.000 | 105 (13.0) | 141 (17.4) | 2.08 (1.08-1.86) | 0.013 |
| Vasopressin use | 508 (20.1) | 258 (25.9) | 1.39 (1.17-1.65) | 0.000 | 178 (22.0) | 206 (25.5) | 1.21 (0.96-1.52) | 0.102 |
| Length of ICU stay (d) | ||||||||
| Overall | 9.25 ± 11.66 | 9.11 ± 11.33 | NA | 0.749 | 9.35 ± 12.00 | 8.69 ± 9.74 | NA | 0.222 |
| Survivors | 8.82 ± 12.00 | 8.70 ± 11.77 | 0.827 | 8.71 ± 11.85 | 8.25 ± 9.55 | 0.479 | ||
| Non-Survivors | 10.15 ± 10.85 | 9.81 ± 10.51 | 0.616 | 10.82 ± 12.24 | 9.49 ± 10.03 | 0.167 | ||
| Length of hospital stay (d) | ||||||||
| Overall | 24.83 ± 26.05 | 21.45 ± 20.84 | NA | 0.000 | 23.38 ± 23.01 | 21.22 ± 19.46 | NA | 0.042 |
| Survivors | 23.71 ± 23.69 | 21.92 ± 20.28 | 0.093 | 21.62 ± 19.17 | 21.40 ± 17.13 | 0.844 | ||
| Non-Survivors | 27.19 ± 30.29 | 20.61 ± 21.75 | 0.000 | 27.44 ± 29.69 | 20.84 ± 23.13 | 0.004 | ||
In order to test the robustness of the primary outcomes, PSM analysis was performed. The variables included in PSM are as follows: Age, gender, height, weight, BSA, BMI, SBP, MAP, SOFA score, SAPS-I score, SAPS-II score, ECI, sepsis type, admission type, diabetes, renal failure, liver failure, hemoglobin, BUN, creatine, and lactate. When the baseline demographic data and clinical characteristics were adjusted, results were consistent with the overall findings. The propensity score matched hospital mortality rates for the SIC group and non-SIC group were 35.4% (286/809 patients) vs 30.0% (243/809 patients). The adjusted OR was 1.27 (95%CI: 1.03-1.57, P = 0.023) (Table 2).
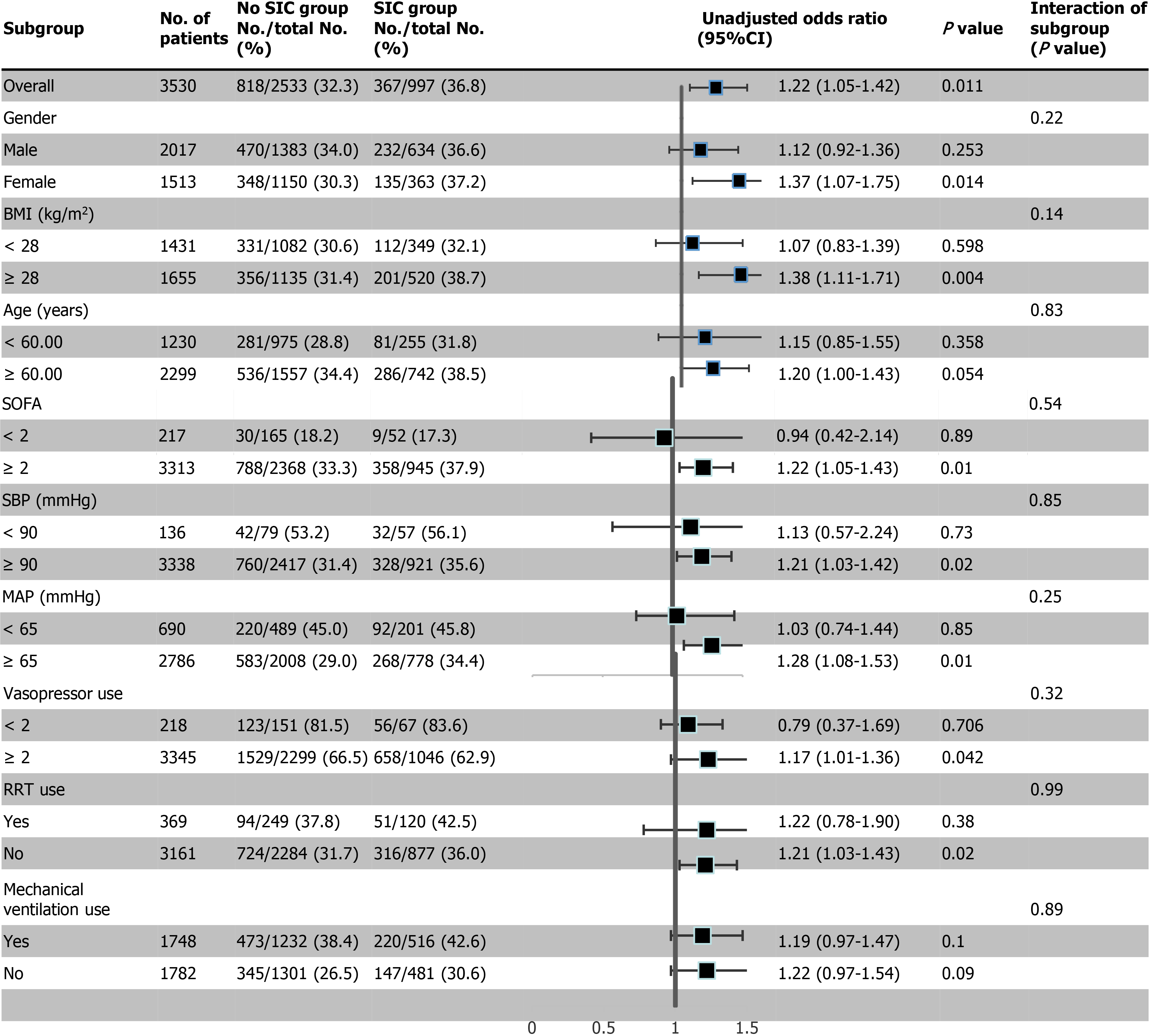
Subgroup analyses with regard to hospital mortality according to gender, age (≥ 60 years, < 60 years), BMI (≥ 28 kg/m2, < 28 kg/m2), SOFA score (≥ 2 points, < 2 points), SBP (≥ 90 mmHg, < 90 mmHg), MAP (≥ 65 mmHg, < 65 mmHg), and MV and RRT use in the first day of ICU admission revealed that the patients in the SIC group with a BMI ≥ 28 kg/m2, or SOFA score ≥ 2 points, SBP ≥ 90 mmHg, MAP ≥ 65 mmHg, no RRT use in the first day of ICU admission, or female gender had a higher risk of hospital death. However, there are no significant interactions between subgroups, and further studies are needed with respect to these aspects (Figure 2).
Before PSM, more patients in the SIC group received MV (619/997patients vs 1478/2533 patients; OR = 1.17, 95%CI: 1.01-1.36; P = 0.042) compared with patients in the non-SIC group. Furthermore, the proportion of each commonly used vasoactive medication in the SIC group was significantly higher than that in the non-SIC group, including norepinephrine (589/997 patients vs 1276/2533 patients, OR = 1.42, 95%CI: 1.23-1.65; P < 0.001), dopamine (188/997 patients vs 279/2533 patients, OR = 1.88, 95%CI: 1.54-2.30; P < 0.001), epinephrine (62/997 patients vs 63/2533 patients, OR = 2.59, 95%CI: 1.81-3.70; P < 0.001), and vasopressin (258/997 patients vs 508/2533 patients, OR = 1.39, 95%CI: 1.17-1.65; P < 0.001). No significant differences were observed between the two groups for the use of RRT and length of ICU stay (Table 2).
It is interesting that the length of hospital stay was shorter in SIC group patients compared with those in the non-SIC group (21.45 ± 20.84 d vs 24.83 ± 26.05 d, P < 0.001). This may be due to the fact that non-survivors died earlier in the SIC group compared to the non-SIC group (20.61 ± 21.75 d vs 27.19 ± 30.29 d, P < 0.001) (Table 2).
These results were consistent after PSM analysis except for vasopressin use.
We also analyzed the clinical characteristics between survivors and non-survivors in the SIC group. The results showed that both groups differed in many aspects (Table 3). Compared with the surviving group, the non-surviving group of SIC patients were older (70.42 ± 13.97 years vs 67.26 ± 15.80 years, P = 0.002), had a lower temperature (36.74 ± 0.88℃ vs 36.90 ± 0.73℃, P = 0.002), SpO2 (96.43 ± 3.60% vs 97.19 ± 2.40%, P < 0.001), SBP (105.59 ± 14.34 mmHg vs 109.72 ± 13.65 mmHg, P < 0.001), DBP (56.53 ± 10.70 mmHg vs 58.36 ± 9.67 mmHg, P = 0.006), and MAP (71.41 ± 10.91 mmHg vs 73.32 ± 9.62 mmHg, P = 0.005), had a higher SOFA score (8.29 ± 3.91 vs 6.28 ± 3.38, P < 0.001), SAPS-I score (23.19 ± 5.66 vs 20.37 ± 4.89, P < 0.001), SAPS-II score (52.87 ± 14.94 vs 42.90 ± 13.54, P < 0.001), and ECI score (16.92 ± 8.93 vs 14.36 ± 8.47, P < 0.001). Additionally, compared to survivors in the SIC group, a higher proportion of non-surviving SIC patients received MV in the first day of ICU admission (220/367 patients vs 296/630 patients, P < 0.001). And the non-surviving group of SIC patients had more comorbidities of renal failure (135/367 patients vs 189/630 patients, P < 0.001) and coagulopathy (145/367 patients vs 172/630 patients, P < 0.001), a lower platelet count (174.26 ± 113.66×109/L vs 214.36 ± 127.73×109/L, P = 0.000) and albumin level (2.69 ± 0.61 g/dL vs 2.83 ± 0.58 g/dL, P = 0.004), and a higher creatine level (2.63 ± 2.13 mg/dL vs 2.23 ± 1.94 mg/dL, P = 0.003), BUN level (51.24 ± 31.80 mg/dL vs 39.46 ± 24.07 mg/dL, P < 0.001), lactate level (4.62 ± 4.19 mmol/L vs 2.98 ± 2.12 mmol/L, P < 0.001), and bilirubin level (2.34 ± 4.10 mg/dL vs 1.49 ± 3.16 mg/dL, P = 0.002) (Table 3).
| Covariate | Survivors (n = 630) | Non-survivors (n = 367) | P value | Missing data (%) |
| Age (yr) | 67.26 ± 15.80 | 70.42 ± 13.97 | 0.002 | 0 |
| Gender (Male), n (%) | 402 (63.8) | 232 (63.2) | 0.851 | 0 |
| Height (cm) | 169.92 ± 12.52 | 169.44 ± 11.51 | 0.575 | 12.4 |
| Weight (kg) | 83.44 ± 26.22 | 79.15 ± 23.91 | 0.014 | 6.7 |
| BSA (m2) | 1.88 ± 0.46 | 1.83 ± 0.43 | 0.128 | 12.8 |
| BMI (kg/m2) | 29.38 ± 15.02 | 27.92 ± 10.06 | 0.124 | 12.8 |
| Temperature (℃) | 36.90 ± 0.73 | 36.74 ± 0.88 | 0.002 | 2.9 |
| SBP (mmHg) | 109.72 ± 13.65 | 105.59 ± 14.34 | 0 | 1.9 |
| DBP (mmHg) | 58.36 ± 9.67 | 56.53 ± 10.70 | 0.006 | 1.9 |
| MAP (mmHg) | 73.32 ± 9.62 | 71.41 ± 10.91 | 0.005 | 1.8 |
| HR (beats/min) | 91.36 ± 16.88 | 93.95 ± 17.38 | 0.022 | 1.8 |
| RR (/min) | 20.98 ± 4.33 | 21.44 ± 4.65 | 0.119 | 1.8 |
| SpO2 (%) | 97.19 ± 2.40 | 96.43 ± 3.60 | 0 | 2 |
| SOFA score | 6.28 ± 3.38 | 8.29 ± 3.91 | 0 | 0 |
| SAPS-I score | 20.37 ± 4.89 | 23.19 ± 5.66 | 0 | 0 |
| SAPS-II score | 42.90 ± 13.54 | 52.87 ± 14.94 | 0 | 0 |
| ECI | 14.36 ± 8.47 | 16.92 ± 8.93 | 0 | 0 |
| Microbiology, n (%) | ||||
| Positive | 354 (56.2) | 206 (56.1) | 0.985 | 0 |
| Negative | 276 (43.8) | 161 (43.9) | ||
| Interventions, n (%) | ||||
| Renal replacement use (1st 24 h) | 69 (11.0) | 51 (13.9) | 0.168 | 0 |
| Mechanical ventilation use | 296 (47.0) | 220 (59.9) | 0 | 0 |
| Comorbidities, n (%) | ||||
| Renal failure | 189 (30.0) | 135 (36.8) | 0.027 | 0 |
| Liver failure | 46 (7.3) | 28 (7.6) | 0.849 | 0 |
| Diabetes | 272 (43.2) | 152 (41.4) | 0.588 | 0 |
| COPD | 117 (18.6) | 78 (21.3) | 0.303 | 0 |
| Coagulopathy | 172 (27.3) | 145 (39.5) | 0 | 0 |
| Admission type, n (%) | ||||
| Emergency | 608 (96.5) | 352 (95.9) | 0.632 | 0 |
| Elective | 22 (3.5) | 15 (4.1) | 0 | |
| Sepsis type, n (%) | ||||
| Sepsis | 143 (22.7) | 25 (6.8) | 0 | 0 |
| Severe sepsis | 336 (53.3) | 231 (62.9) | 0 | |
| Septic shock | 151 (24.0) | 111 (30.2) | 0 | |
| Laboratory tests | ||||
| WBC (109/L) | 15.88 ± 9.23 | 16.03 ± 10.36 | 0.809 | 0.1 |
| Hemoglobin (g/dL) | 9.56 ± 1.84 | 9.59 ± 1.76 | 0.812 | 0.2 |
| Platelet (109/L) | 214.36 ± 127.73 | 174.26 ± 113.66 | 0 | 0.1 |
| Sodium (mmol/L) | 140.05 ± 4.93 | 140.03 ± 5.58 | 0.965 | 0 |
| Potassium (mmol/L) | 3.78 ± 0.61 | 3.88 ± 0.59 | 0.018 | 0 |
| Chloride (mmol/L) | 102.26 ± 6.58 | 101.78 ± 7.18 | 0.287 | 0.1 |
| BUN (mg/dL) | 39.46 ± 24.07 | 51.24 ± 31.80 | 0 | 0.2 |
| Creatine (mg/dL) | 2.23 ± 1.94 | 2.63 ± 2.13 | 0.003 | 0.2 |
| Lactate (mmol/L) | 2.98 ± 2.12 | 4.62 ± 4.19 | 0 | 14.1 |
| Albumin (g/dL) | 2.83 ± 0.58 | 2.69 ± 0.61 | 0.004 | 39.8 |
| Bilirubin (mg/dL) | 1.49 ± 3.16 | 2.34 ± 4.10 | 0.002 | 26.8 |
| PH | 7.35 ± 0.11 | 7.32 ± 0.12 | 0.001 | 26.3 |
| PO2 (mmHg) | 142.73 ± 93.83 | 137.98 ± 91.67 | 0.495 | 26.3 |
| PCO2 (mmHg) | 40.36 ± 10.79 | 39.30 ± 13.01 | 0.23 | 26.3 |
| Bicarbonate | 21.01 ± 5.00 | 19.13 ± 5.69 | 0 | 0.1 |
| (mmol/L) | ||||
| Glucose (mg/dL) | 144.45 ± 47.67 | 148.89 ± 57.53 | 0.193 | 1.8 |
| CK-MB (%) | 5.73 ± 5.64 | 6.00 ± 6.73 | 0.767 | 78.5 |
| CK (U/L) | 413.37 ± 1425.54 | 313.84 ± 842.98 | 0.284 | 22.4 |
| Troponin (ng/mL) | 0.42 ± 1.41 | 0.31 ± 0.87 | 0.264 | 33.4 |
| Outcomes, n (%) | ||||
| Mechanical ventilation use | 343 (54.4) | 276 (75.2) | 0 | 0 |
| RRT use | 44 (7.0) | 72 (19.6) | 0 | 0 |
| Norepinephrine use | 322 (51.1) | 267 (72.8) | 0 | 0 |
| Epinephrine use | 27 (4.3) | 35 (9.6) | 0.001 | 0 |
| Dopamine use | 94 (14.9) | 94 (25.6) | 0 | 0 |
| Vasopressin use | 95 (15.1) | 163 (44.4) | 0 | 0 |
| Length of ICU stay (d) | 8.71 ± 11.77 | 9.81 ± 10.51 | 0.138 | 0 |
| Length of hospital stay (d) | 21.94 ± 20.29 | 20.61 ± 21.75 | 0.332 | 0 |
With regard to outcomes, compared with the survivor group, a higher proportion of patients in the non-survivor group received MV (276/367 patients vs 343/630 patients, P < 0.001) and RRT (72/367 patients vs 44/630 patients, P < 0.001) during hospital stay. Furthermore, the proportion of each commonly used vasoactive medication in the non-survivor group was significantly higher than that in the survivor group, including norepinephrine (267/367 patients vs 322/630 patients, P < 0.001), dopamine (94/367 patients vs 94/630 patients, P < 0.001), epinephrine (35/367 patients vs 27/630 patients, P = 0.001), and vasopressin (163/367 patients vs 95/630 patients, P < 0.001). No significant differences were observed between the two groups for the length of ICU stay and hospital stay (Table 3).
The multivariate logistic regression analysis showed that age (OR = 1.012, 95%CI: 1.006-1.017; P < 0.001), male gender (OR = 1.498, 95%CI: 1.264-1.776; P < 0.001), ECI (OR = 1.036, 95%CI: 1.025-1.046; P < 0.001), hemoglobin (OR = 1.067, 95%CI: 1.020-1.116; P = 0.005), MV use in the first day of ICU admission (OR = 1.003, 95%CI: 1.000-1.006; P = 0.041), and diabetes (OR = 1.538, 95%CI: 1.298-1.823; P < 0.001) were risk factors for SIC (Table 4). SBP (OR = 0.983, 95%CI: 0.977-0.989; P < 0.001) and liver failure (OR = 0.340, 95%CI: 0.251-0.459; P < 0.001) were protective factors for SIC (Table 4).
| Covariate | OR | 95%CI | P value |
| Age | 1.012 | 1.006-1.017 | 0.000 |
| Male | 1.498 | 1.264-1.776 | 0.000 |
| ECI | 1.036 | 1.025-1.046 | 0.000 |
| Hemoglobin | 1.067 | 1.020-1.116 | 0.005 |
| MV use1 | 1.003 | 1.000-1.006 | 0.041 |
| Diabetes | 1.538 | 1.298-1.823 | 0.000 |
| Liver failure | 0.340 | 0.251-0.459 | 0.000 |
| SBP | 0.983 | 0.977-0.989 | 0.000 |
Furthermore, the multivariate logistic regression analysis showed that lactate level (OR = 1.107, 95%CI: 1.038-1.180; P = 0.002), SAPS-II score (OR = 1.035, 95%CI: 1.021-1.049; P < 0.001), sepsis type (OR = 1.386, 95%CI: 1.066-1.801; P = 0.015), and BUN (OR = 1.009, 95%CI: 1.003-1.015; P = 0.006) were risk factors for hospital death of SIC patients (Table 5). SBP (OR = 0.986, 95%CI: 0.973-1.000; P < 0.001) and platelet level (OR = 0.998, 95%CI: 0.997-1.000; P < 0.001) were protective factors for hospital death of SIC patients (Table 5).
| Covariate | OR | 95%CI | P value |
| Lactate | 1.107 | 1.038-1.180 | 0.002 |
| SAPS-II score | 1.035 | 1.021-1.049 | 0.000 |
| Sepsis types | 1.386 | 1.066-1.801 | 0.015 |
| BUN | 1.009 | 1.003-1.015 | 0.006 |
| SBP | 0.986 | 0.973-1.000 | 0.044 |
| Platelet level | 0.998 | 0.997-1.000 | 0.031 |
A total of 3530 patients with sepsis who met the inclusion criteria were included in the present study. Among them, 997 patients met the SIC diagnostic criteria. The incidence of SIC was 28.20% (95%CI: 26.80%-29.70%). We searched the PubMed, EMBASE, and Web of Science databases, and the current epidemiological studies of SIC are mainly small studies[13-20]; large-scale studies are still lacking. To the best of our knowledge, the current study is the largest scale research with regard to the clinical characteristics of SIC.
Prior to this study, reports on the incidence of SIC varied greatly. Jardin and colleagues studied 90 patients with sepsis (aged 55 ± 18 years), 60% of whom had Gram-positive bacteremia, and monitored cardiac systolic and diastolic function by transthoracic echocardiography (TTE). Their results showed that 51% of patients had cardiac function depression[37]. Narváez et al[13] screened 57 patients with sepsis or septic shock who were admitted to the ICU from May 2014 to October 2015; of these, 13 patients met the diagnostic criteria for SIC, and the incidence of SIC was 22.8%. Sato et al[14] screened 210 patients with sepsis or septic shock who were admitted to the ICU in Japan; a total of 29 of those patients had SIC, with an incidence of SIC of 13.8%. Vieillard-Baron et al[17] screened 67 sepsis patients who had no previous cardiac disease and survived more than 48 h after being admitted to the ICU, and the results showed that the incidence of SIC was 60%. Other studies have reported an SIC incidence between 24%-40%[15,16,18,19]. Our study showed that the incidence of SIC was 28.20% (95%CI: 26.80%-29.70%), which was in the middle of the range reported in previous studies. We considered that the reasons for the varied SIC incidences reported among the different studies were related to the following factors: First, there is still no gold standard or unified consensus for the diagnosis of SIC. The diagnostic criteria for SIC in various studies were mainly based on the exclusion of previous cardiac diseases combined with cardiac ultrasound indicators, especially LVEF. However, the LVEF cut-off value for diagnosing SIC varied among studies. Some studies used LVEF < 45% as the diagnostic criterion, and some other studies used LVEF < 50%[13-16,18-20]. Vieillard-Baron et al[17] used LVEF < 40% and cardiac index < 3 L/min/m2 as diagnostic criteria. The above different diagnostic criteria might affect the epidemiological results of SIC. The criteria used in our research were based on previous studies and international cardiac failure guidelines[27-29]; the cut-off value of LVEF for diagnosing SIC in our study was < 50%. Second, since left ventricular diastolic dysfunction and right ventricular dysfunction (isolated or concurrent) are common in elderly and critically ill patients[38,39], it is difficult to directly attribute the above ventricular dysfunctions to sepsis. Therefore, the definition of SIC in our study was restricted to systolic dysfunction of the LV, which may explain why the incidence of SIC in our study is lower than that in some previous studies. Third, our study was a large sample study based on analysis of the MIMIC-III database, which included information on all patients who entered Beth Israel Deaconess Medical Center between 2002 and 2011, and we rigorously excluded patients with concomitant cardiac disease. As a result of the more rigorous inclusion and exclusion criteria, the research results were more reliable in our study.
In terms of secondary outcomes, our study showed that more patients in the SIC group than in the non-SIC group received MV and vasopressor therapy. The results were consistent with those after PSM. Sato et al[14] also reported that more patients in the SIC group received norepinephrine and vasopressin. In the study by Pulido et al[18], more patients in the SIC group received norepinephrine. Our results were consistent with those of previous studies.
We further studied the risk factors for SIC and the multivariate logistic regression analysis revealed that age, male, ECI, hemoglobin level, diabetes and MV use in the first day of ICU admission were risk factors for SIC (Table 4). Sato et al[14] reported that gender and age were risk factors for SIC, which was consistent with our findings. However, due to the limitation of the sample size, Sato et al[14] obtained few risk factors through logistic regression analysis. Based on the large sample size compared with previous studies, the risk factors for SIC in our study were more comprehensive.
Some previous studies showed that the mechanism of SIC was related to chemical mediators, such as endotoxins and cytokines[1,43]. Interestingly, we found that liver failure might be a protective factor against SIC. Estrogen has an inhibitory effect on cytokines[44], and the level of estrogen is usually high in liver failure. Further studies are warranted to determine the role of estrogen and liver failure in the pathogenesis of SIC.
There were some limitations in our study that should also be noted. First, this study was based on analysis of the MIMIC-III database, making this be a single-center retrospective study. Due to the nature of the research, there was unavoidable risk of bias. To decrease the influences of bias, our study adjusted for the baseline characteristics between the SIC and non-SIC groups using PSM method, and we further studied the primary outcomes through multiple subgroup analyses. Ultimately, the results were still consistent, which demonstrated that our results were reliable. Second, due to the lack of widely accepted diagnostic criteria for SIC, the cut-off values of LVEF used among studies varied widely[13-20]. Therefore, inconsistent SIC diagnostic criteria may influence the comparability of results between different studies. Third, the diagnosis of SIC in our study was mainly dependent on TTE. However, TTE is subjective and dependent on the operator’s technique and level of experience, and the interpretation of the results may vary among operators, which might partly influence our results. Recently, researchers reported that using two-dimensional speckle tracking echocardiography to evaluate patients’ cardiac function is more sensitive[45]. Therefore, future studies using more sensitive ultrasound techniques may increase the ability to evaluate cardiac function and improve the sensitivity of SIC diagnosis.
Our study showed that the incidence of SIC in patients with sepsis is 28.20% (95%CI: 26.80%-29.70%). Hospital mortality is higher in the SIC patients compared with the non-SIC patients.
Sepsis-induced cardiomyopathy (SIC) is a complication of sepsis and septic shock. The current epidemiological studies of SIC are mainly small ones.
At present, large-scale studies on the clinical characteristics of SIC, such as the incidence, prognosis, and risk factors, are lacking. The present study was intended to investigate these characteristics.
This study aimed to evaluate the SIC incidence rate and hospital mortality rate, as well as mechanical ventilation or renal replacement therapy use during hospital stay, the use of vasopressors (including norepinephrine, dopamine, epinephrine, and vasopressin), the length of intensive care unit (ICU) stay, and the length of hospital stay.
Based on the analysis of the MIMIC-III public database, we performed a large-scale retrospective study involving sepsis patients who were admitted to the ICU and had no concomitant cardiac disease. We used propensity score matching analysis and multivariate logistic regression to ensure the robustness of the results.
In the present study, we included 3530 sepsis patients. The incidence of SIC was 28.20% (95% confidence interval: 26.80%-29.70%). Compared to patients in the non-SIC group, patients in the SIC group had a significantly older age and higher SAPS-I score, SAPS-II score, and Elixhauser comorbidity index (ECI). Hospital mortality was higher in the SIC group than in the non-SIC group. For the secondary outcomes, more patients in the SIC group received mechanical ventilation and vasopressors. Multivariate logistic regression analysis showed that age, male sex, ECI, hemoglobin level, diabetes, and mechanical ventilation use on the first day of ICU admission were risk factors for SIC.
Our study showed that the incidence of SIC in patients with sepsis is 28.20%. Hospital mortality is higher in the SIC patients than in the non-SIC patients.
The current study is the largest-scale study with regard to the clinical characteristics of SIC. The incidence of SIC is high. The hospital mortality is higher in the SIC group than in the non-SIC group. Clinicians should pay more attention to these patients. Further multicenter large scale studies with regard to SIC are needed.
We acknowledge all staff who helped us in performing this study and we particularly acknowledge Professor Shao-Heng Zhang who provided advice in data analysis.
| 1. | Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care. 2015;3:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 2. | Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 941] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 3. | Huang SJ, Nalos M, McLean AS. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? Crit Care. 2013;17:R96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Flynn A, Chokkalingam Mani B, Mather PJ. Sepsis-induced cardiomyopathy: a review of pathophysiologic mechanisms. Heart Fail Rev. 2010;15:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 512] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 6. | Stanzani G, Duchen MR, Singer M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochim Biophys Acta Mol Basis Dis. 2019;1865:759-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 7. | Nabzdyk CS, Couture EJ, Shelton K, Cudemus G, Bittner EA. Sepsis induced cardiomyopathy: Pathophysiology and use of mechanical circulatory support for refractory shock. J Crit Care. 2019;54:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Wilson RL, Selvaraju V, Lakshmanan R, Thirunavukkarasu M, Campbell J, McFadden DW, Maulik N. Thioredoxin-1 attenuates sepsis-induced cardiomyopathy after cecal ligation and puncture in mice. J Surg Res. 2017;220:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Hobai IA, Edgecomb J, LaBarge K, Colucci WS. Dysregulation of intracellular calcium transporters in animal models of sepsis-induced cardiomyopathy. Shock. 2015;43:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Tsolaki V, Makris D, Mantzarlis K, Zakynthinos E. Sepsis-Induced Cardiomyopathy: Oxidative Implications in the Initiation and Resolution of the Damage. Oxid Med Cell Longev. 2017;2017:7393525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Waqar F, Jamali HK, Gerson MC. Role of I-123 MIBG in sepsis-induced cardiomyopathy. J Nucl Cardiol. 2018;25:492-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Liu YC, Yu MM, Shou ST, Chai YF. Sepsis-Induced Cardiomyopathy: Mechanisms and Treatments. Front Immunol. 2017;8:1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Narváez I, Canabal A, Martín C, Sánchez M, Moron A, Alcalá J, Giacoman S, Magro M. Incidence and evolution of sepsis-induced cardiomyopathy in a cohort of patients with sepsis and septic shock. Med Intensiva. 2018;42:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Sato R, Kuriyama A, Takada T, Nasu M, Luthe SK. Prevalence and risk factors of sepsis-induced cardiomyopathy: A retrospective cohort study. Medicine (Baltimore). 2016;95:e5031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Morelli A, De Castro S, Teboul JL, Singer M, Rocco M, Conti G, De Luca L, Di Angelantonio E, Orecchioni A, Pandian NG, Pietropaoli P. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. 2005;31:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Charpentier J, Luyt CE, Fulla Y, Vinsonneau C, Cariou A, Grabar S, Dhainaut JF, Mira JP, Chiche JD. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 316] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 17. | Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36:1701-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 402] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevich V, Brown DR, Oh JK. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc. 2012;87:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 19. | Arméstar F, Mesalles E, López-Ayerbe J, Roca J. [Moderate-severe myocardial depression during septic shock: a pilot study]. Med Intensiva. 2012;36:445-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | L'Heureux M, Sternberg M, Brath L, Turlington J, Kashiouris MG. Sepsis-Induced Cardiomyopathy: a Comprehensive Review. Curr Cardiol Rep. 2020;22:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 21. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2924] [Cited by in RCA: 3827] [Article Influence: 201.4] [Reference Citation Analysis (8)] |
| 22. | Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, Moody B, Szolovits P, Celi LA, Mark RG. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2345] [Cited by in RCA: 3296] [Article Influence: 329.6] [Reference Citation Analysis (0)] |
| 23. | Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215-E220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7935] [Cited by in RCA: 5482] [Article Influence: 210.8] [Reference Citation Analysis (1)] |
| 24. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 4035] [Article Influence: 310.4] [Reference Citation Analysis (0)] |
| 25. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1784] [Cited by in RCA: 2028] [Article Influence: 225.3] [Reference Citation Analysis (1)] |
| 26. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 18754] [Article Influence: 1875.4] [Reference Citation Analysis (4)] |
| 27. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10348] [Cited by in RCA: 9571] [Article Influence: 957.1] [Reference Citation Analysis (3)] |
| 28. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 4714] [Article Influence: 362.6] [Reference Citation Analysis (1)] |
| 29. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1413] [Article Influence: 157.0] [Reference Citation Analysis (0)] |
| 30. | van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1779] [Article Influence: 104.6] [Reference Citation Analysis (0)] |
| 31. | GitHub. MIT-LCP/mimic-code. [cited 29 October 2019]. Available from: https://github.com/MIT-LCP/mimic-code. |
| 32. | Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6122] [Cited by in RCA: 8733] [Article Influence: 415.9] [Reference Citation Analysis (0)] |
| 33. | Johnson AE, Stone DJ, Celi LA, Pollard TJ. The MIMIC Code Repository: enabling reproducibility in critical care research. J Am Med Inform Assoc. 2018;25:32-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 34. | Fathi M, Markazi-Moghaddam N, Ramezankhani A. A systematic review on risk factors associated with sepsis in patients admitted to intensive care units. Aust Crit Care. 2019;32:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Ahiawodzi PD, Kelly K, Massengill A, Thompson DK. Risk factors for sepsis morbidity in a rural hospital population: A case-control study. Am J Infect Control. 2018;46:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Martin-Loeches I, Guia MC, Vallecoccia MS, Suarez D, Ibarz M, Irazabal M, Ferrer R, Artigas A. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: a prospective, observational, multicenter cohort study. Ann Intensive Care. 2019;9:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 37. | Jardin F, Fourme T, Page B, Loubières Y, Vieillard-Baron A, Beauchet A, Bourdarias JP. Persistent preload defect in severe sepsis despite fluid loading: A longitudinal echocardiographic study in patients with septic shock. Chest. 1999;116:1354-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 39. | Suárez JC, López P, Mancebo J, Zapata L. Diastolic dysfunction in the critically ill patient. Med Intensiva. 2016;40:499-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 825] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 41. | Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 42. | Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4208] [Cited by in RCA: 4338] [Article Influence: 188.6] [Reference Citation Analysis (0)] |
| 43. | Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949-958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 579] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 44. | Anderson HV. Estrogen therapy, atherosclerosis, and clinical cardiovascular events. Circulation. 1996;94:1809-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Ng PY, Sin WC, Ng AK, Chan WM. Speckle tracking echocardiography in patients with septic shock: a case control study (SPECKSS). Crit Care. 2016;20:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kutti Sridharan G S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Li JH