Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9218
Peer-review started: May 17, 2021
First decision: June 24, 2021
Revised: July 1, 2021
Accepted: August 27, 2021
Article in press: August 27, 2021
Published online: October 26, 2021
Processing time: 156 Days and 17.5 Hours
Severe acute pancreatitis (SAP) is a common critical disease of the digestive sys
We report a 66-year-old Asian male with severe acute pancreatitis who presented with intermittent abdominal pain and an increasing abdominal mass. The abscess spread from the retroperitoneum to the anterior abdominal wall and the right groin. In the described case, drainage tubes were placed in the retroperitoneal and anterior abdominal wall by percutaneous puncture. After a series of symptomatic supportive therapies, the patient was discharged from the hospital with a retroperi
We believe that patients with SAP complicated with anterior abdominal abscess can be treated conservatively to avoid unnecessary exploration or operation.
Core Tip: Severe acute pancreatitis is a common critical disease of the digestive system. Its local complications are pancreatic pseudocyst, walled off necrosis and infected pancreatic necrosis. However, so far, there are few cases of severe acute pancreatitis with anterior abdominal abscess. We report a rare case of anterior abdominal abscess with severe acute pancreatitis. We think that for such cases, conservative treatment can be carried out to avoid unnecessary exploration or operation.
- Citation: Jia YC, Ding YX, Mei WT, Xue ZG, Zheng Z, Qu YX, Li J, Cao F, Li F. Anterior abdominal abscess - a rare manifestation of severe acute pancreatitis: A case report. World J Clin Cases 2021; 9(30): 9218-9227
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9218.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9218
Severe acute pancreatitis (SAP) is a common critical disease of the digestive system. It often has local complications such as acute peripancreatic fluid collection, acute necrotic collection, pancreatic pseudocyst, walled off necrosis and infectious pancreatic necrosis. Sometimes, SAP may have atypical complications due to the course of the disease, the degree of inflammation, the anatomical variation and other factors. Here, we present a rare complication of SAP, characterized by an abscess of the anterior abdominal wall.
A 66-year-old male patient was sent to our center for intermittent abdominal pain for 8 wk and an increasing abdominal mass for 5 wk.
Eight weeks ago, after the treatment of SAP, the patient presented with intermittent abdominal pain, fat diarrhea, diabetes, malnutrition and other chronic pancreatitis symptoms. Five weeks ago, the peri-umbilical and lower abdomen skin began to appear purple, and a lower abdominal mass gradually appeared. Computed tomo
Ten weeks prior, the patient was sent to our center for treatment due to SAP. After drinking a lot of alcohol, the patient had sudden, continuous and severe abdominal colic. Vital signs were as follows: heart rate 136 times/min, respiratory rate 26 times/min, blood pressure 92/52 mmHg, temperature 38.0 ºC, body mass index 18.1. Laboratory examination was as follows: serum amylase 804 IU/L, serum lipase 259.9 U/L, serum calcium 1.44 mmol/L, lactate 3.2 mmol/L, serum glucose 23.29 mmol/L, serum sodium 119.7 mmol/L, triglyceride 0.79 mmol/L, lactate dehydrogenase 579 IU/L, prealbumin 38 mg/L, white cell count 18.77 × 109/L, neutrophil count 16.03 × 109/L and plasma D-dimer 5.45 µg/mL. The modified Marshall score was 3, the APACHE II score was 23, the Ranson score was 6 and the modified computed tomography severity index was 10. A CT scan showed bilateral pleural effusion and atelectasis of the right lower lung lobe. The outline of the uncinate process of the head of the pancreas was unclear, and a large amount of exudate could be seen around the pancreas. Pancreatic parenchyma atrophy, multiple spotted calcifications, and pan
The patient had a history of chronic pancreatitis and had multiple acute pancreatitis episodes. The first diagnosis of acute pancreatitis in the patient was 14 years prior to presentation. Comorbidities of the patient included 12 years of diabetes, 30 years of smoking and 30 years of alcohol abuse.
At the time of admission, the size of the lower abdominal mass was approximately 22.5 cm × 14.2 cm, accompanied by obvious skin cyanosis and swelling, a significant increase in skin temperature and an obvious palpable tenderness. Heart rate 121 times/min, breathing 28/min, blood pressure 94/35 mmHg and body temperature 38.6 °C.
Laboratory values were as follows: 13.86 × 109/L white blood cell count, 10.23 × 109/L neutrophil count, 76 g/L hemoglobin, 405 × 109/L platelet count, 5.4 mmol/L lactate, 7.543 pH, 61 IU/L serum amylase, 34.9 U/L serum lipase, 25.10 g/L albumin, 19 mg/L prealbumin, 12.05 mmol/L glucose, 587 µmol/L uric acid, 124 mmol/L sodium, 3.990 ng/mL procalcitonin and 154.00 mg/L C-reactive protein.
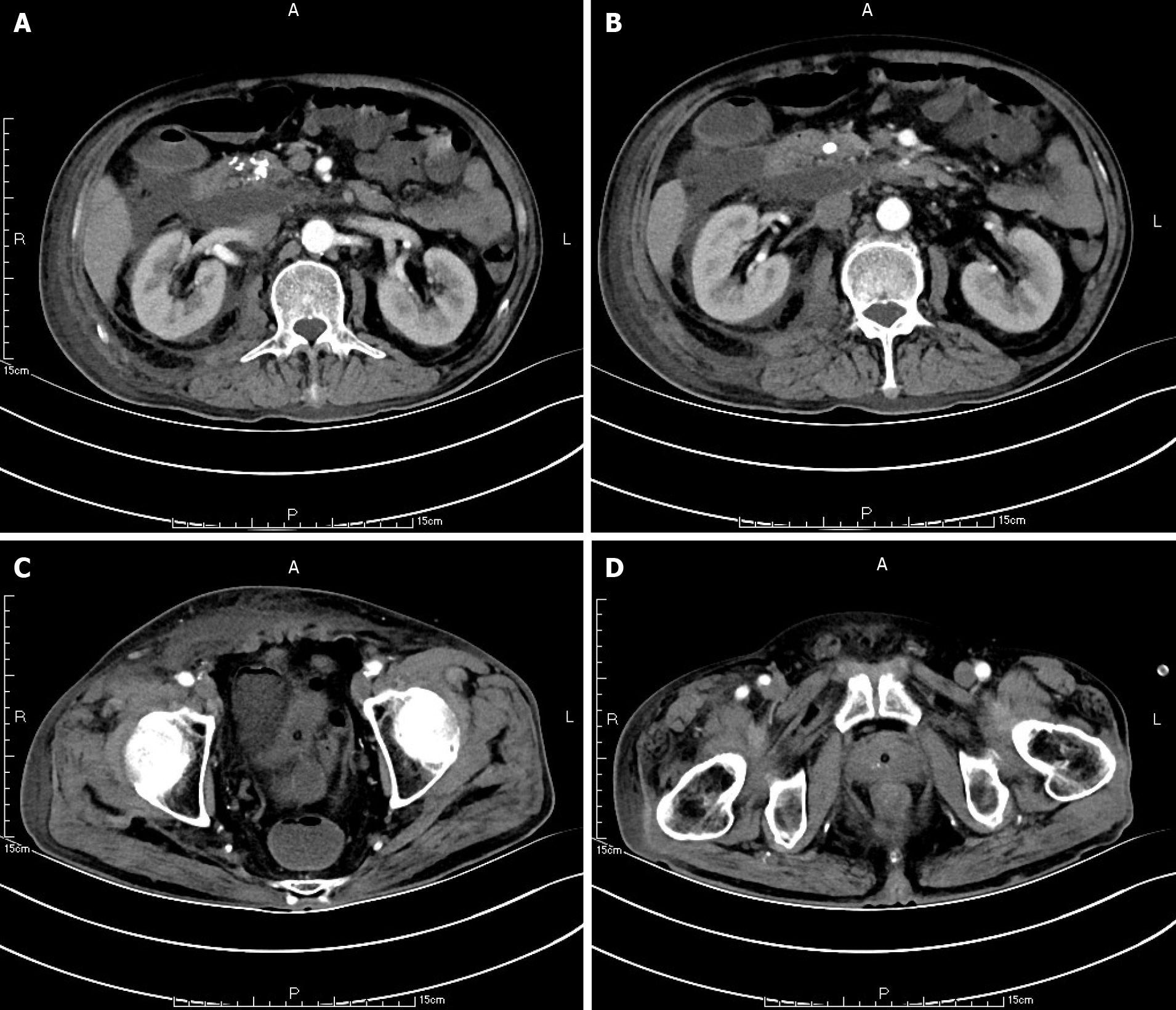
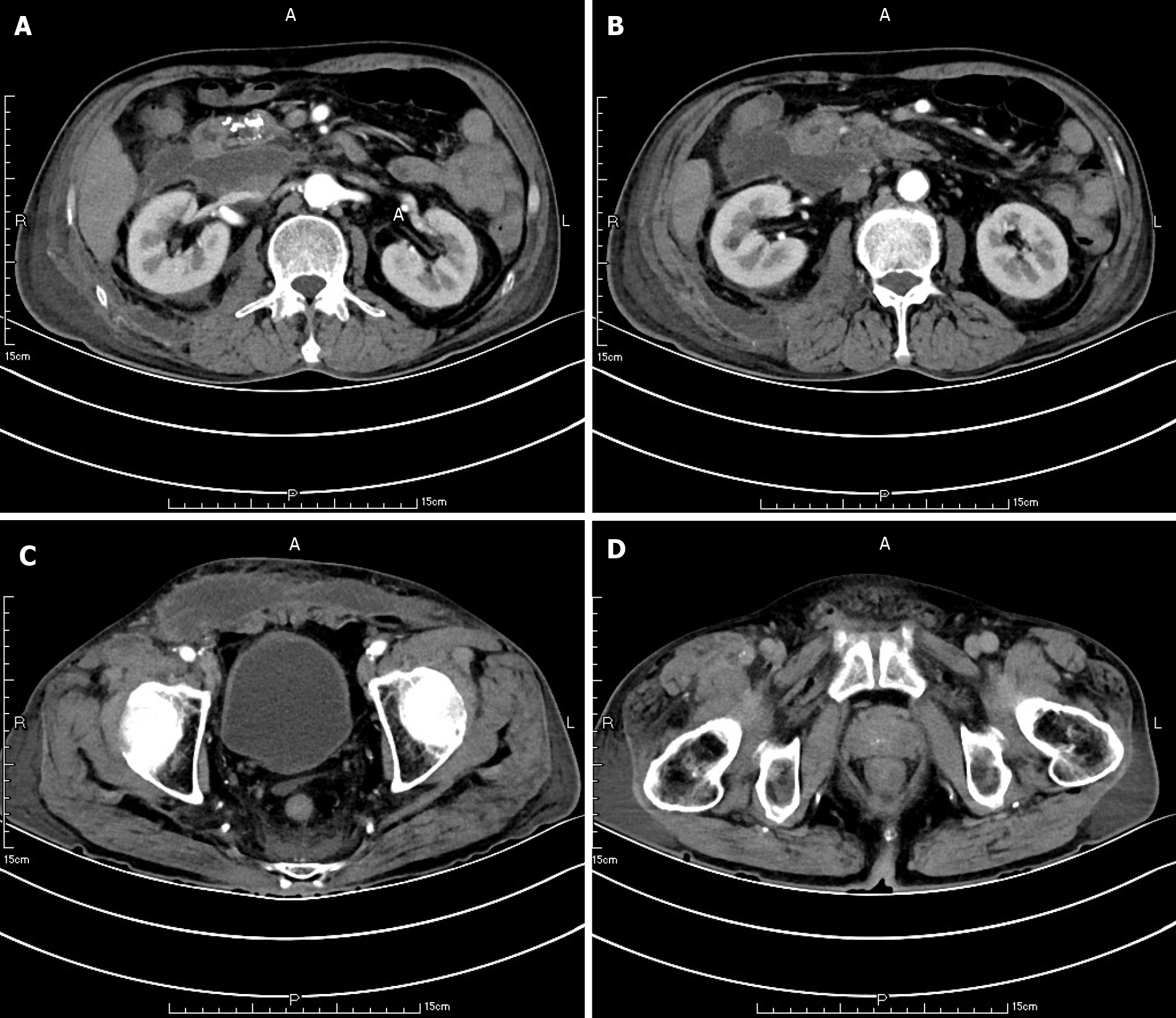
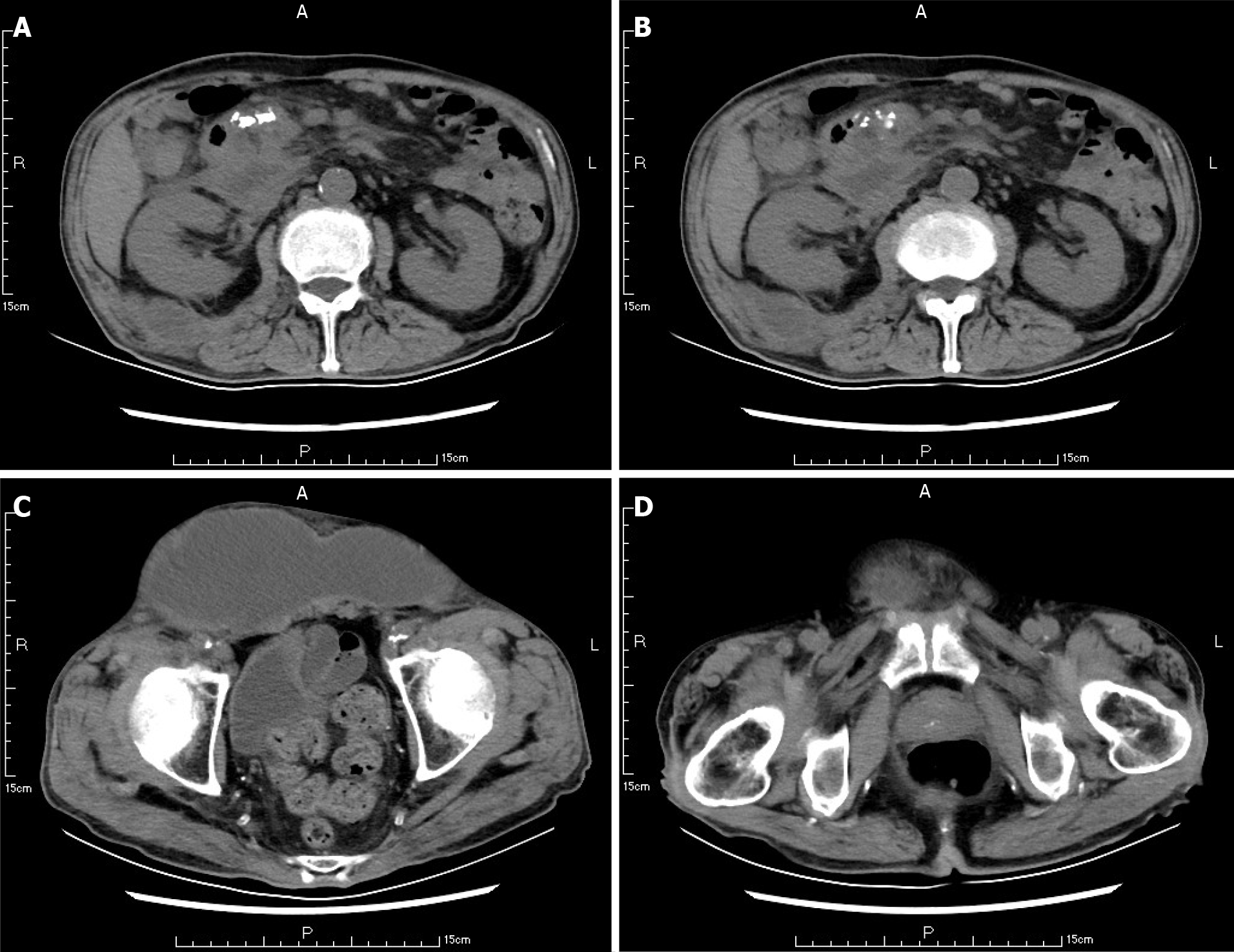
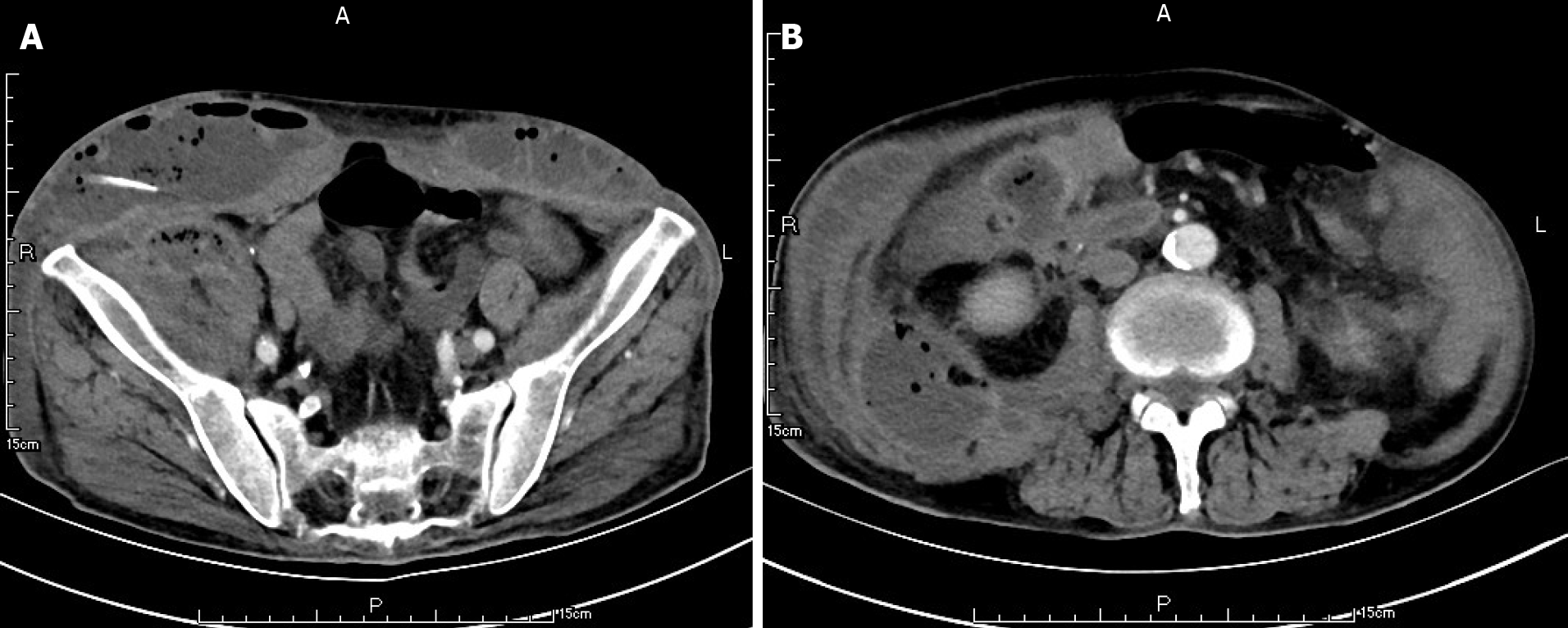
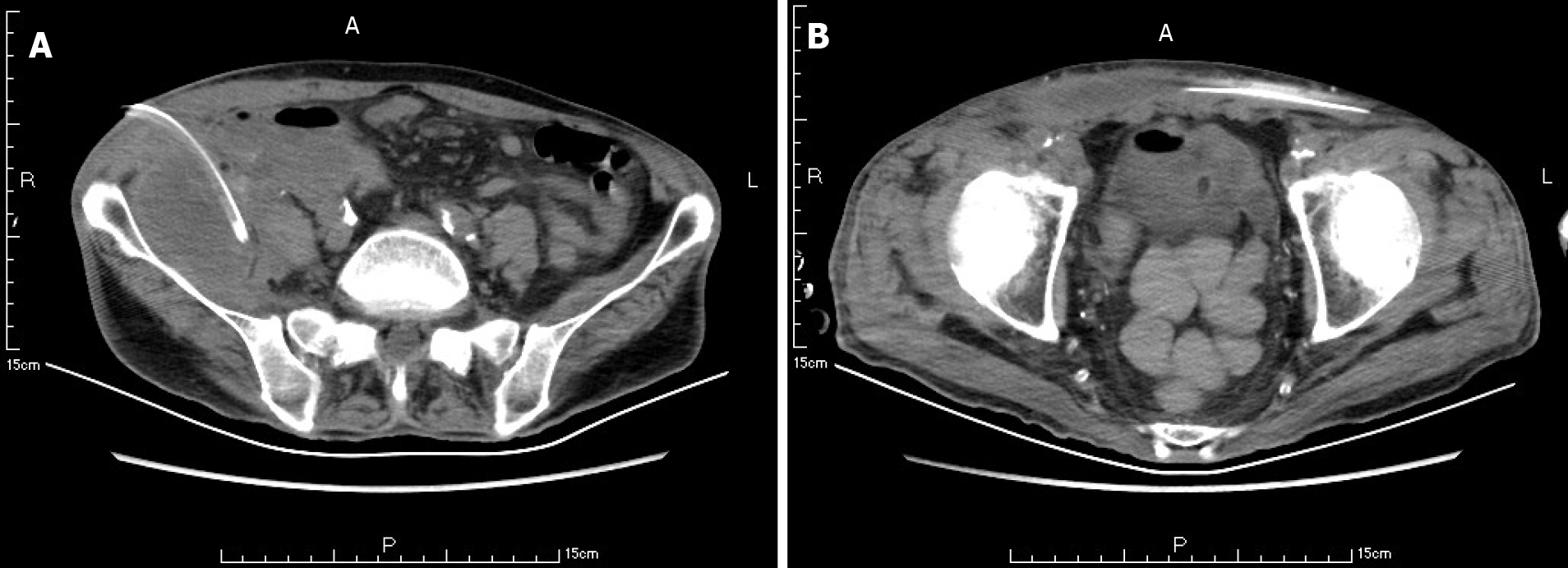
Ten weeks before this hospitalization, the patient was sent to our center for treatment due to SAP. A CT scan showed bilateral pleural effusion and atelectasis of the right lower lung lobe. The outline of the uncinate process of the head of the pancreas was unclear, and a large amount of exudate could be seen around the pancreas. Pancreatic parenchyma atrophy, multiple spotted calcifications and pancreatic duct widening were also noted. On the right side of the kidney, there was an encapsulated effusion in the anterior and posterior spaces, and a density shadow of fluid could be seen in the subcutaneous and intramuscular areas of the abdominal wall. On the right side of the kidney, the volume of fluid was approximately 6.9 cm × 1.6 cm (Figure 1). Eight weeks before this hospitalization, CT scan showed that the ranges of liquid density shadow of the right anterior renal space, and the right posterior renal space were slightly larger than that of the front, extending to the right iliac fossa. The density of the abdominal wall was increased in the subcutaneous and intermuscular areas, and the size of the right side was approximately 8.3 cm × 3.1 cm. The right peritoneum and perirenal fascia were thickened, and the liquid density shadow in the right renal fat capsule was decreased slightly. After intravenous contrast agent administration, the right anterior renal space, right posterior renal space, right iliac fossa and abdominal wall were subcutaneously wrapped with a liquid density shadow wall (Figure 2). A week before this hospitalization, CT scan showed that the fluid in the right renal space was less than before. The range of encapsulated liquid density shadows in the right retrorenal space was significantly larger than previously, and it extended to the right iliac fossa. The range of subcutaneous and intramuscular fluid density shadows in the right abdominal wall was significantly larger than measured before, measuring approximately 20.1 cm × 7.3 cm, and it went down to the right groin without obvious pelvic effusion (Figure 3).
Gram-positive cocci were found in the smear of drained fluid. Enterococcus faecalis and Escherichia coli were found in the drainage medium.
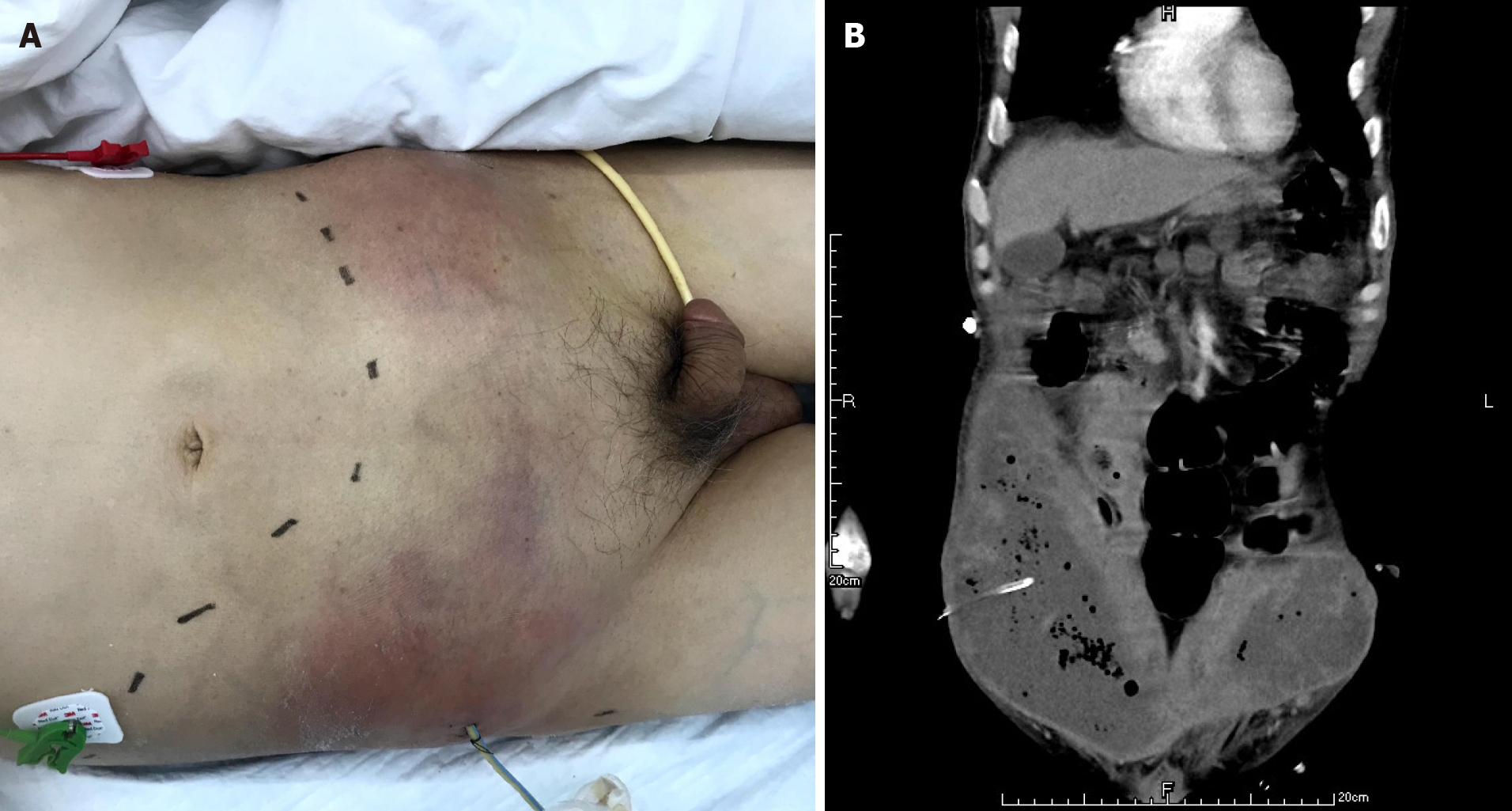
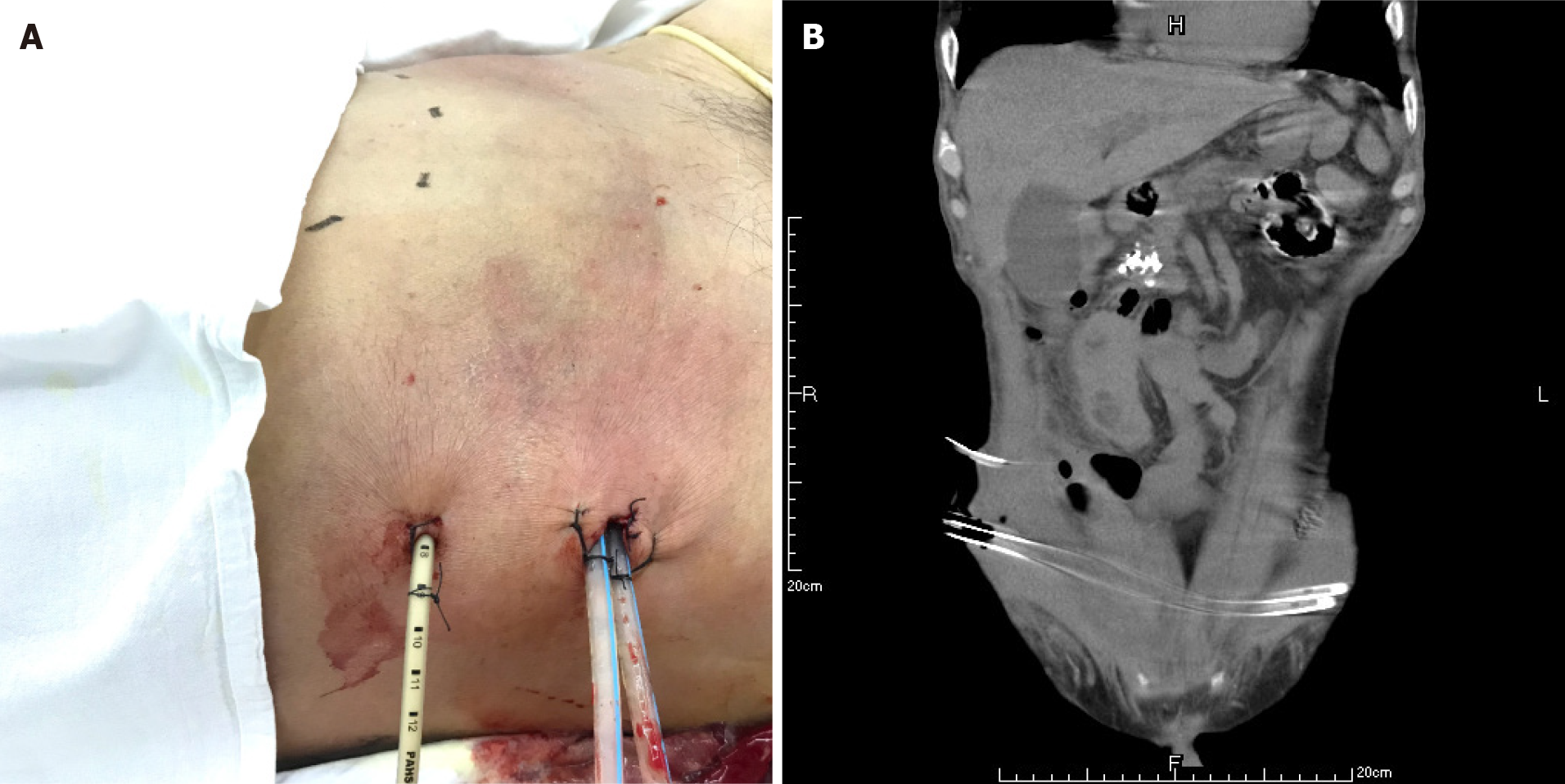
The patient had definite sepsis and septic shock, the Glasgow Coma Score was 12, and the Visual Analog Scale score was 6. After the initial fluid resuscitation, vasoactive drug infusion and broad-spectrum antibiotic administration, puncture and drainage of right lower abdominal wall abscess was performed under ultrasound guidance within 24 h at the most obvious wave motion of right lower abdominal wall abscess. A silicone rubber drainage tube of 8 Fr was placed (Figure 4). A total of 350 mL of gray-white purulent exudate was extracted, with an obvious odor. The amylase in the drained fluid was 5 IU/L, and gram-positive cocci were found in the smear of drained fluid. Enterococcus faecalis and Escherichia coli were found in the drainage medium. According to the drug sensitivity results, vancomycin combined with imipenem was used for treatment.
A CT scan showed new air bubbles in the right anterior renal space and in the right pararenal posterior space to the right of the iliac fossa. The amount of subcutaneous and intramuscular encapsulated effusion on the right side was significantly increased, and multiple air bubbles could be seen in it, which went down to the right groin. The lesion crossed the center into the left anterior inferior abdominal wall (Figure 5). Forty-eight hours after admission, ultrasound-guided drainage of right retroperitoneal abscess and right lower abdominal wall abscess was performed. First, the right retroperitoneal abscess was punctured to the abscess cavity under ultrasound gui
On the 12th day of admission, a liquid diet was given orally. Two more abdominal drainage tubes were removed on the 35th day after admission. The patient was dis
SAP is characterized by acute peripancreatic fluid collection, acute necrotic collection, pancreatic pseudocysts, walled off necrosis, infected pancreatic necrosis, circulatory failure, acute lung injury, water electrolyte and acid-base balance derangements and other complications[1-6]. However, the occurrence of anterior abdominal abscess in SAP is rare, and only two cases have been reported[7,8].
In 1918, Thomas Stephen Cullen[9] had initially described a female patient with a ruptured ectopic pregnancy and with blue discoloration of the periumbilical skin, namely, Cullen’s sign. In 1920, George Grey Turner[10] reported a case of acute pancreatitis with similar skin ecchymoses, but the location of the ecchymoses was located on the lateral side of the abdominal wall. Dickson et al[11] found that approximately 1%-3% of patients with acute pancreatitis may have body wall ecchymoses. Meyers et al[12] described the specific anatomical path of the two signs via CT scan
At the first admission for SAP caused by alcoholism, the patient had obvious fluid accumulations in the right anterior renal space, the right posterior renal space, the right anterior abdominal wall and the right groin (Figure 1). With the progression of the disease, the skin around the umbilicus and lower abdomen of the patient began to exhibit cyanosis, and a lower abdominal mass gradually appeared. According to the CT scan, we found that the abscess of the patient’s anterior abdominal wall gradually increased in size from the right side to the left through the midline of the abdominal wall, and the abscess eventually involved the right groin (Figures 2 and 3). This indicated that the appearance of the anterior abdominal wall abscess was continuous, which further excluded the possibility of anterior abdominal wall abscess caused by poor nutritional status, decreased immune function or diabetes mellitus.
According to the experience of our center, in the early stage of SAP (onset time < 4 wk), due to pancreatic duct disruption, the amylase content in the drainage fluid after percutaneous catheter drainage (PCD) is usually very high, but the amylase content in the drainage fluid is usually not high after infectious necrosis is formed about 4 wk after the onset. The patient was treated with PCD for the first time at the fifth week, and the amylase in the drainage fluid was 5 IU/L.
After reviewing the anatomy of the peritoneum and abdominal wall, we found that an adjacent potential connection between the retrorenal space and the abdomen may be the anatomical basis of the abdominal wall involvement before SAP. Four pairs of lumbar arteries originate from the posterior level of the abdominal aorta, enter the posterior part of the psoas major and quadratus psoas along the front and side of the 1st-4th lumbar body and then move forward between abdominal muscles. Its branches supply the muscles and skin of the lumbar and ventral walls. Inflammation spreads between the two layers of the prerenal fascia in the prerenal space, breaks through or dissolves the retrorenal fascia into the retrorenal space and may reach the lateral edge of the quadratus psoas through the gap between the lumbar artery and the peri
The abscess of the anterior abdominal wall of this patient was divided into two abscess cavities, and the right abscess cavity was significantly larger than the left abscess cavity. There was a certain pressure difference, and the production of the left abscess cavity may have been the result of the inflammation on the right side gradually eroding the abdominal white line to the left (Figure 3). Under the premise that the first puncture and drainage did not enter the retroperitoneum, the CT scan after the puncture showed extensive encapsulated hydrops and pneumatosis in the subcutaneous and intramuscular abdominal wall and in the right retrorenal space (Figures 4 and 5). This suggests that the anterior abdominal wall abscesses and the retroperitoneal abscesses may be interlinked.
In the case reported in this paper, the patient had obvious effusion in the right iliac fossa and in the right groin (Figure 2). In addition, the testicular artery originates below the starting point of the renal artery and originates from the anterior wall of the abdominal aorta, goes down along the surface of the psoas major muscle obliquely, crosses the front of the ureter at the height of the fourth lumbar spine, enters the scrotum through the inguinal canal and forms the spermatic cord, which is distributed in the testis and epididymis. Inflammation may also travel down the testicular artery into the inguinal area and even further involve the scrotum. In 2011, Kamble et al[8] reported a case of acute pancreatitis with the initial symptoms of an anterior abdominal wall abscess and epididymorchitis. The fluid then accumulated in the retroperitoneum until preperitoneal, and retroperitoneal abscesses formed in the lower abdomen and scrotum. The patient recovered after puncture and drainage of the abscesses in the anterior abdominal wall and supportive care.
Among the local complications caused by SAP, anterior abdominal wall abscesses are very rare. The patient described here developed an anterior abdominal wall abscess with severe hypoproteinemia after the diagnosis of SAP. We believe that severe hypoproteinemia is an important factor in the development of anterior abdo
In conclusion, drainage and other conservative treatment strategies can be preferred for anterior abdominal wall abscess, which is a rare complication of SAP. We should pay attention to and evaluate the nutritional status of patients in time and carry out PCD and nutritional support treatment as early as possible to avoid unnecessary exploration or operation.
| 1. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 685] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 2. | Garg PK, Singh VP. Organ Failure Due to Systemic Injury in Acute Pancreatitis. Gastroenterology. 2019;156:2008-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 436] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 3. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 980] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 4. | Nazar MA, D'Souza FR, Ray A, Memon MA. Unusual presentation of acute pancreatitis: an irreducible inguinoscrotal swelling mimicking a strangulated hernia. Abdom Imaging. 2007;32:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Athappan G, Ariyamuthu VK, Rajamani VK. Bilateral renal halo sign in acute pancreatitis. Med J Aust. 2008;189:228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Moorthy N, Raveesha A, Prabhakar K. Pancreaticopleural fistula and mediastinal pseudocyst: an unusual presentation of acute pancreatitis. Ann Thorac Med. 2007;2:122-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Manji N, Hulyalkar AR, Keroack MA, Vekshtein VI, Kirshenbaum JM, Sugarman DI, Chopra S. Cutaneous pseudo abscesses: an unusual presentation of severe pancreatitis. Am J Gastroenterol. 1988;83:177-179. [PubMed] |
| 8. | Kamble PM, Patil A, Jadhav S, Rao SA. Anterior abdominal wall abscess with epididymo-orchitis: an unusual presentation of acute pancreatitis. J Postgrad Med. 2011;57:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Bosmann M, Schreiner O, Galle PR. Coexistence of Cullen's and Grey Turner's signs in acute pancreatitis. Am J Med. 2009;122:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Bem J, Bradley EL 3rd. Subcutaneous manifestations of severe acute pancreatitis. Pancreas. 1998;16:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Dickson AP, Imrie CW. The incidence and prognosis of body wall ecchymosis in acute pancreatitis. Surg Gynecol Obstet. 1984;159:343-347. [PubMed] |
| 12. | Meyers MA, Feldberg MA, Oliphant M. Grey Turner's sign and Cullen's sign in acute pancreatitis. Gastrointest Radiol. 1989;14:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Marks SC Jr, Raptopoulos V, Kleinman P, Snyder M. The anatomical basis for retrorenal extensions of pancreatic effusions: the role of the renal fasciae. Surg Radiol Anat. 1986;8:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1075] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aseni P, Kikuyama M S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wu RR