Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9168
Peer-review started: April 20, 2021
First decision: July 15, 2021
Revised: July 16, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 26, 2021
Processing time: 184 Days and 1.5 Hours
Visceral disseminated varicella-zoster virus (VZV) infection is a rare but life-threatening disease. In transplant recipients with VZV infection, visceral dissemination may develop without skin eruptions, which leads to the failure of early diagnosis.
The patient was a 33-year-old male renal recipient who was referred to our hospital with severe upper abdominal pain of 3-d duration. On admission, the patient rapidly developed septic shock and multiple organ dysfunction syndrome with liver dysfunction and acute kidney injury. Next-generation sequencing of peripheral blood yielded 39224 sequence reads of VZV, and real-time polymerase chain reaction for VZV was positive, with 1.2 × 107 copies/mL. The final diagnosis was visceral disseminated VZV infection. Acyclovir and supportive therapy were started, but the patient died of severe visceral organ damage 16 h after admission.
Visceral disseminated VZV infection is possible in renal transplant recipients presenting abdominal pain and rapidly-evolving organ damage without skin involvement.
Core Tip: In transplant recipients, visceral disseminated varicella-zoster virus (VZV) infection may develop without skin eruptions, which leads to the failure of early diagnosis and fatal outcome. Early diagnosis and prompt antiviral therapy is the key to successful treatment. Next-generation sequencing is a promising tool for early de
- Citation: Wang D, Wang JQ, Tao XG. Fatal visceral disseminated varicella-zoster virus infection in a renal transplant recipient: A case report. World J Clin Cases 2021; 9(30): 9168-9173
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9168.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9168
Varicella-zoster virus (VZV), also known as human alphaherpesvirus 3, causes acute varicella (chickenpox) as a primary infection, in which VZV travels to sensory nerve ganglia where it becomes dormant as a potentially pathogenic virus[1]. Years later, VZV can reactivate and cause herpes zoster (shingles) when cell-mediated immunity to VZV wanes with aging or becomes disrupted in a compromised immune state. In solid organ transplant recipients, reactivation of VZV is likely to involve dissemination to multiple visceral organs, which induces hepatitis, pneumonia, encephalitis, and even pancreatitis. Viscerally disseminated VZV infection is life-threatening and early diagnosis is challenging because visceral complications often precede skin eruptions[2]. This case of viscerally disseminated VZV infection in a kidney recipient empha
A 33-year-old renal transplant recipient was referred to our hospital with severe upper abdominal pain of 3-d duration.
Six months previously, the patient underwent parent-to-child kidney transplantation for end-stage renal disease and was then started on immunosuppressive therapy with methylprednisolone, tacrolimus, and mycophenolate mofetil (MMF). Three days before hospital admission, he developed severe acute upper abdominal pain radiating towards his back and was admitted to a local hospital. As abdominal computed tomography (CT) and ultrasonography results were normal, only analgesic treatment was given. However, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) increased significantly, and he was referred to our hospital. On admission to the organ transplant center at our hospital, he complained of nausea and upper abdominal pain. Following a diagnosis of acute abdominal pain and liver damage, the patient was prescribed flurbiprofen tapes for pain relief, intravenous magnesium isoglycyrrhizinate against liver damage, and empirical antimicrobial therapy with intravenous cefoperazone sodium and sulbactam sodium. The patient rapidly developed septic shock and multiple organ dysfunction syndrome (commonly known as MODS) with liver dysfunction and acute kidney injury. Two days later, the patient was transferred to our intensive care unit (ICU).
He had a 3-year history of end-stage renal failure.
He had no other specific diseases or familial medical history.
On admission, the patient was lethargic, with a heart rate of 136 beats/min, respira
Laboratory test results revealed high levels of ALT (4814 U/L), AST (8574 U/L), alkaline phosphatase (251 U/L), and gamma-glutamyltransferase (165 U/L), high concentrations of C-reactive protein (53.1 mg/L), procalcitonin (0.33 ng/mL), and creatinine (203 μmol/L), prolonged activated partial thromboplastin time and throm
Abdominal ultrasonography revealed a normal pancreas, spleen, and gallbladder. The common bile duct was without stones and had a normal diameter. Ascites or abnormal fluid collection were not seen, and there were no focal liver lesions. Abdominal and pelvic CT revealed no notable abnormalities or evidence of intestinal obstruction, perforation, or mesenteric artery thrombosis. Chest X-ray and electrocardiogram were both normal.
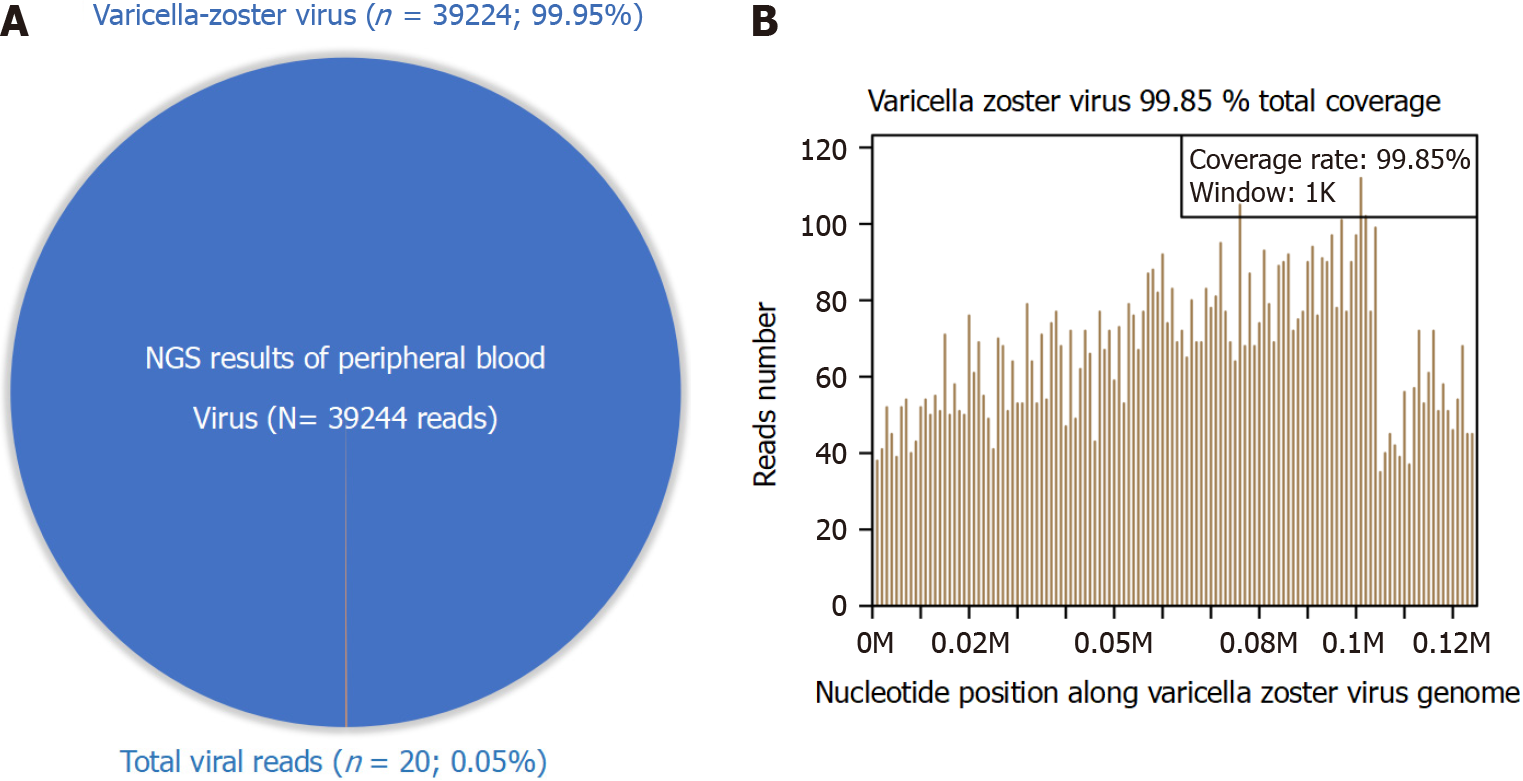
Separate samples of peripheral blood were collected for next-generation sequencing (NGS), real-time PCR, and IgG assays. The patient was VZV IgG-negative. NGS found 39224 reads mapped on the VZV genome sequence that covered 99.8% of the total VZV genome and accounted for 99.95% of the total viral reads (Figure 1). Real-time PCR for VZV was positive, with 1.2 × 107 copies/mL.
The patient’s primary complaint, symptoms, and physical examination, NGS, and real-time PCR results supported a final diagnosis of visceral disseminated VZV infection, septic shock, and MODS with liver dysfunction and acute kidney injury.
On admission, we considered the possibility of visceral disseminated VZV infection because of acute upper abdominal pain and rapidly evolving MODS after renal transplantation. We promptly initiated antiviral therapy with intravenous acyclovir (750 mg q8 h) combined with intravenous meropenem, linezolid, and caspofungin as empirical antibiotic treatment. Noradrenaline and terlipressin were given to maintain blood pressure, and intravenous sodium bicarbonate was given to correct metabolic acidosis. After the diagnosis of visceral disseminated VZV infection was confirmed, acyclovir treatment and supportive treatment were continued.
Unfortunately, the patient’s condition deteriorated quickly, with acute liver failure, disseminated intravascular coagulation, acute respiratory failure, and acute renal injury. He died 16 h after ICU admission. An autopsy was not performed.
VZV is the second most common viral pathogen after cytomegalovirus in renal transplant recipients during the first year after transplantation[3]. Intensive immu
Several cases of visceral disseminated VZV infection in renal transplant recipients have been reported. A recent case occurred in a 66-year-old patient in Australia who initially presented with chest and abdominal pain and then simultaneously developed hepatitis and pancreatitis. He was diagnosed with visceral disseminated VZV and received intravenous acyclovir (10 mg/kg twice daily) only after developing a widespread vesicular rash 11 d following the onset of chest and back pain. Despite supportive care and antiviral therapy, the patient died after 6 d in the ICU[6]. Another case involved a renal transplant recipient in India who presented after 7 d of severe epigastric pain and 4 d of multiple vesiculopapular rashes over the entire body. The typical varicella rash plus increased serum lipase and liver enzyme levels led to a diagnosis of visceral disseminated VZV infection complicated by hepatitis and pancreatitis. Intravenous acyclovir was started on hospital admission. After 48 h, the patient’s pain was relieved and the liver enzymes returned to normal levels[7]. A report published in 2002 included 4 renal transplant recipients with visceral disseminated VZV infection[8]. One patient presented with acute epigastric pain, nausea, vomiting, and generalized pustulosis on admission. He was diagnosed by identification of VZV DNA in the pustule content, and the case was complicated with hepatitis, pneumo
The clinical characteristics of six previously reported cases and our case of renal transplant recipients with visceral disseminated VZV infection included initial symp
Early diagnosis is the key to prompt treatment and a good prognosis of visceral disseminated VZV infection, especially for cases with delayed or absent skin lesions. Real-time PCR and direct immunofluorescence can detect VZV DNA and VZV an
The case described herein indicates that visceral disseminated VZV infection should be considered if a renal transplant recipient initially presents with acute abdominal pain and rapidly develops visceral organ damage, even without skin rashes. Prompt acyclovir treatment is the key to achieving a good prognosis. NGS facilitates early, accurate diagnosis.
| 1. | Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PG, Oxman MN, Seward JF, Yamanishi K. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 558] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 2. | Pergam SA, Limaye AP; AST Infectious Diseases Community of Practice. Varicella zoster virus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Eidgahi ES, Lotfi Z, Tayefi M, Bahrami A, Shams SF, Shakeri S, Sheikhi M. Incidence and risk factors of common viral infections among renal transplant recipients during the first year post-transplant in North-eastern Iran. Saudi J Kidney Dis Transpl. 2019;30:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Lauzurica R, Bayés B, Frías C, Fontseré N, Hernandez A, Matas L, Jimenez A, Bonet J, Romero R. Disseminated varicella infection in adult renal allograft recipients: role of mycophenolate mofetil. Transplant Proc. 2003;35:1758-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Rommelaere M, Maréchal C, Yombi JC, Goffin E, Kanaan N. Disseminated varicella zoster virus infection in adult renal transplant recipients: outcome and risk factors. Transplant Proc. 2012;44:2814-2817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Loftus MJ, Yong MK, Wilson S, Peleg AY. Fatal disseminated visceral varicella zoster virus infection in a renal transplant recipient. Transpl Infect Dis. 2019;21:e13062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Chhabra P, Ranjan P, Bhasin DK. Simultaneous Occurrence of Varicella Zoster Virus-Induced Pancreatitis and Hepatitis in a Renal Transplant Recipient: A Case Report and Review of Literature. Perm J. 2017;21:16-083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Fehr T, Bossart W, Wahl C, Binswanger U. Disseminated varicella infection in adult renal allograft recipients: four cases and a review of the literature. Transplantation. 2002;73:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Tsuji H, Yoshifuji H, Fujii T, Matsuo T, Nakashima R, Imura Y, Yukawa N, Ohmura K, Sumiyoshi S, Mimori T. Visceral disseminated varicella zoster virus infection after rituximab treatment for granulomatosis with polyangiitis. Mod Rheumatol. 2017;27:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Datta S, Budhauliya R, Das B, Chatterjee S, Vanlalhmuaka, Veer V. Next-generation sequencing in clinical virology: Discovery of new viruses. World J Virol. 2015;4:265-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Kustin T, Ling G, Sharabi S, Ram D, Friedman N, Zuckerman N, Bucris ED, Glatman-Freedman A, Stern A, Mandelboim M. A method to identify respiratory virus infections in clinical samples using next-generation sequencing. Sci Rep. 2019;9:2606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salvadori M S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY