Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9151
Peer-review started: April 19, 2021
First decision: June 24, 2021
Revised: July 3, 2021
Accepted: August 24, 2021
Article in press: August 24, 2021
Published online: October 26, 2021
Processing time: 184 Days and 17.8 Hours
Hepatocellular carcinoma is an aggressive tumor, and its latency and lack of clinical symptoms mean that most patients are already in the late stage when diagnosed. Large tumor volume and metastasis are the main reasons for not attempting surgery. Portal vein embolization and associated liver partition and portal vein ligation for staged hepatectomy are commonly used in clinical practice to increase the volume of remnant liver to allow surgical resection; however, research in this area is currently lacking.
A 48-year-old male patient with a history of viral hepatitis B for at least 30 years attended our center with a hepatic space-occupying lesion detected 3 d previously. Enhanced computed tomography scanning of the upper abdomen revealed a large mass in the right lobe of the liver, centered on the right posterior lobe, with the larger section measuring about 14 cm × 10 cm × 14 cm. He successfully underwent conversion therapy for a large right liver tumor after combined hepatic artery ligation and transcatheter arterial chemoembolization, and finally had an opportunity to undergo right hemi-hepatectomy and cholecystectomy. He remained asymptomatic with no obvious abnormalities on computed tomography scanning review at 2 mo after surgery.
This case highlights new ideas and provides a reference for conversion therapy of large liver tumors.
Core Tip: Portal vein embolization associated liver partition and portal vein ligation for staged hepatectomy, and transcatheter arterial chemoembolization (TACE) are commonly used in conversion therapy of advanced liver cancer. However, the therapeutic effects of portal vein embolization and TACE are limited, and associating liver partition and portal vein ligation for staged hepatectomy is associated with high morbidity and mortality. We therefore combined hepatic artery ligation with TACE, which has a longer treatment cycle and slower compensation of the contralateral liver compared with the above treatments but which may make R0 resection feasible with less surgical complications and with good postoperative recovery.
- Citation: Feng GY, Cheng Y, Xiong X, Shi ZR. Conversion therapy of hepatic artery ligation combined with transcatheter arterial chemoembolization for treating liver cancer: A case report. World J Clin Cases 2021; 9(30): 9151-9158
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9151.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9151
Hepatocellular carcinoma (HCC) is an aggressive tumor, and its latent nature, together with the lack of obvious clinical symptoms means that most patients are already in the late stage of the disease when they are diagnosed. In these cases, the tumor may already be large and/or be accompanied by metastasis and invasion, leading to a mean survival time as short as 10 mo[1]. In patients with large liver tumors, insu
A 48-year-old man attended our center with a hepatic space-occupying lesion detected 3 d previously.
The patient was found to have a hepatic space-occupying lesion detected by regular abdominal color Doppler ultrasound, with abdominal distension as the only manifestation.
The patient had a history of viral hepatitis B for at least 30 years and had never received antiviral therapy.
No specific personal and family history.
No obvious abnormal physical signs.
Initial laboratory examinations: The patient’s alfa-fetoprotein (AFP) level was 5789.0 ng/mL. Blood analysis, blood biochemical parameters, prothrombin and partial thromboplastin times, d-dimers, arterial blood gas, and electrocardiogram were all normal.
At 93 d after the initial operation: AFP had reduced to 3119.0 ng/mL, and the indocyanine green 15-minute clearance rate was 4.0%.
At 2 mo after the initial operation: AFP was 9.2 ng/mL.
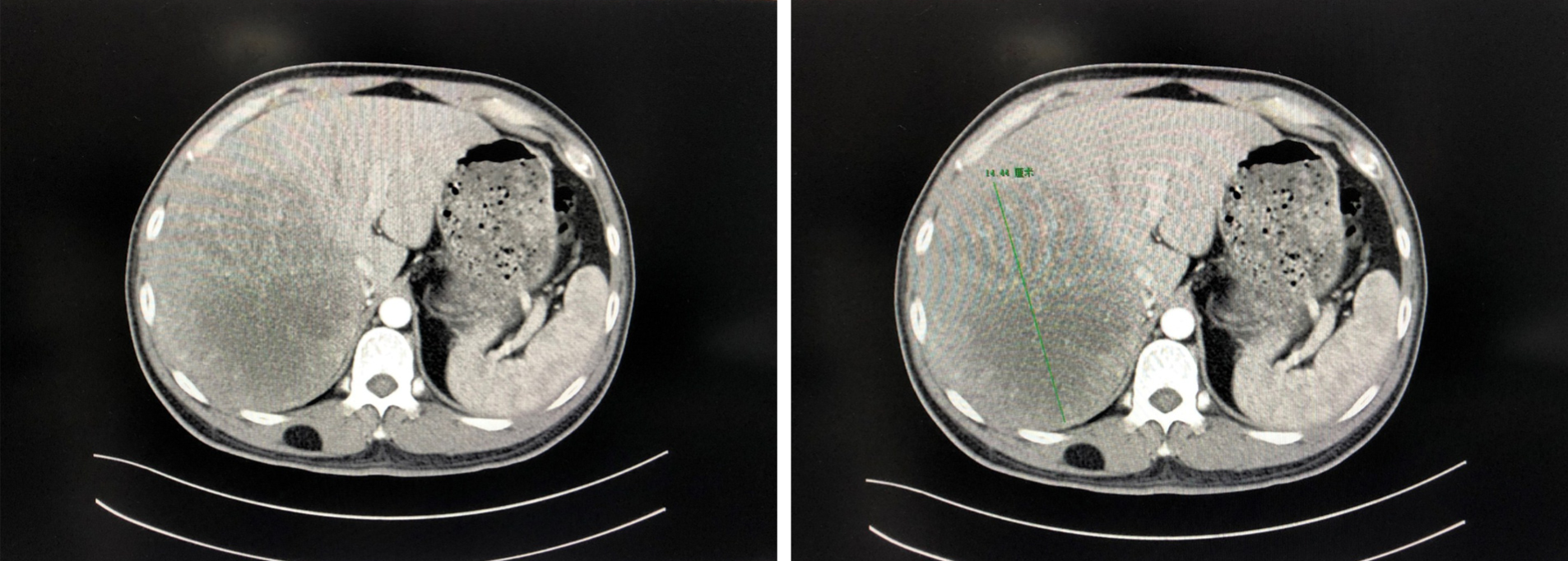
Pre-operative examination: Enhanced computed tomography (CT) scanning of the upper abdomen revealed a large mass in the right lobe of the liver, centered on the right posterior lobe, with the larger section measuring about 14 cm × 10 cm × 14 cm. The right hepatic vein mostly ran along the edge of the lesion, and the right hepatic vein and right portal vein were affected by pressure (Figure 1). The total liver volume was 3103.959 cm³, and the left hemi-liver volume was 662.376 cm³.
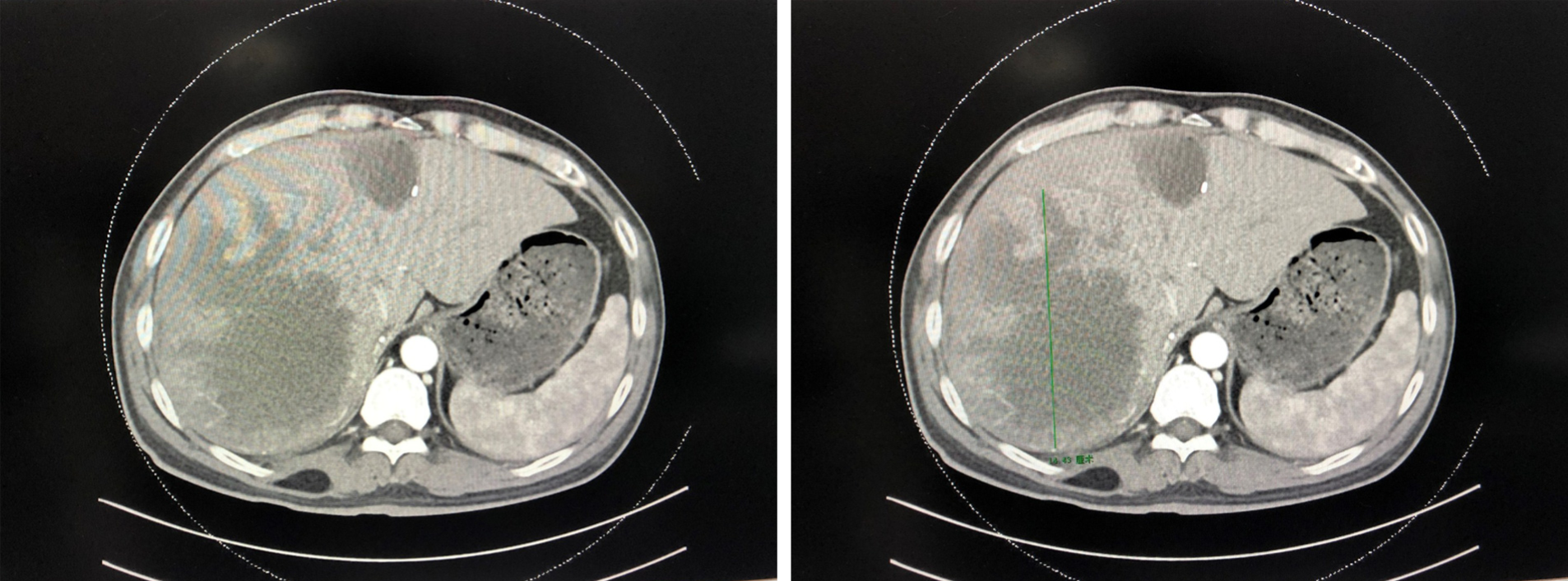
At 53 d after the initial operation: Repeat enhanced CT scanning of the upper abdomen showed no significant changes in the patient’s left or right liver (Figure 2), with a total liver volume of 3002.174 cm³ and a left liver volume of 846.648 cm³. CT angiography indicated that the tumor was mainly supplied with blood via the middle hepatic artery, with a smaller contribution from the left hepatic artery.
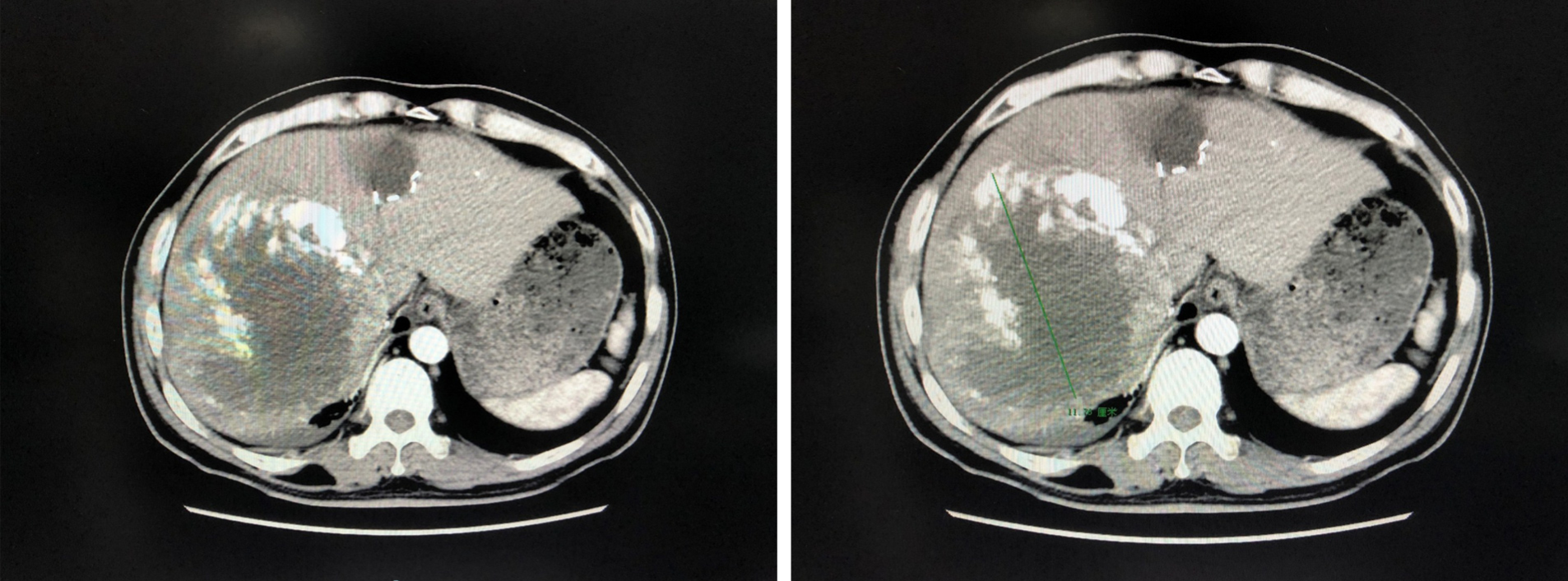
At 93 d after the initial operation: Repeat enhanced CT scan of the upper abdomen revealed a large soft tissue mass in the right lobe of the liver, with dot-like or sheet-like high-density shadows, slightly smaller than previously. Other imaging findings were similar to previously (Figure 3). The total liver volume was 2526.944 cm³ and the left liver volume was 780.415 cm³.
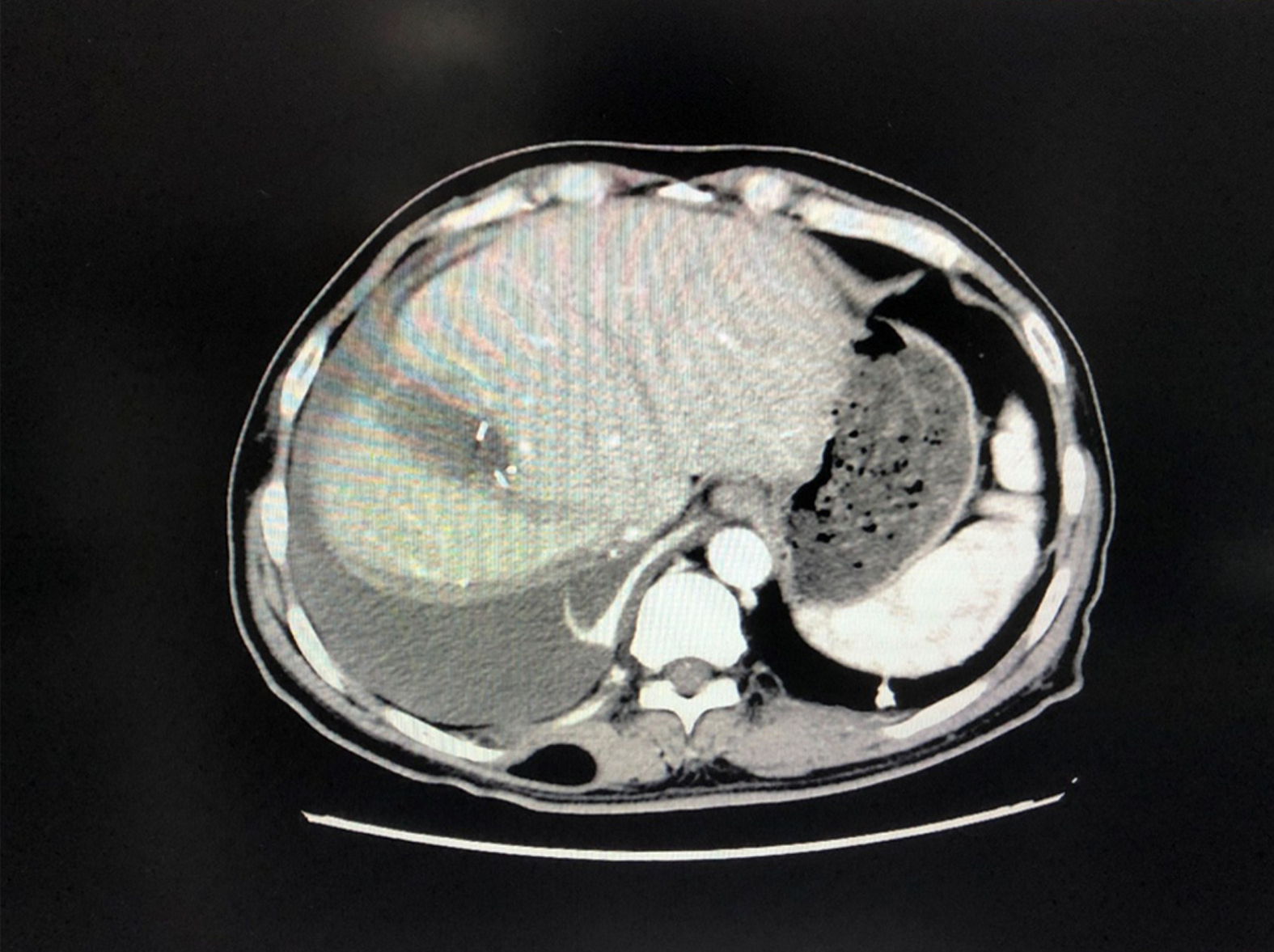
At 2 mo after the initial operation: Enhanced CT of the upper abdomen revealed a small amount of fluid in the operation area and no obvious abnormalities (Figure 4).
At 98 d after the first stage of surgery: Postoperative pathological examination revealed a moderately differentiated HCC with extensive necrosis, microvascular invasion grade M0, and the surrounding liver tissue with chronic hepatitis, G2S3.
The final diagnosis of the presented case was moderately differentiated HCC.
The patient was Child-Pugh grade A. Considering that the left hepatic volume was only 21.34% of the total liver volume, we planned liver partition and portal vein ligation to prepare for hepatectomy. However, it was difficult to expose the portal vein during surgery, resulting in bleeding, and intraoperative ultrasound detected suspicious lesions in the left liver, resulting in a switch to liver partition and right hepatic artery ligation. The patient developed Dindo-Clavien grade I complications after surgery and was discharged from hospital awaiting review.
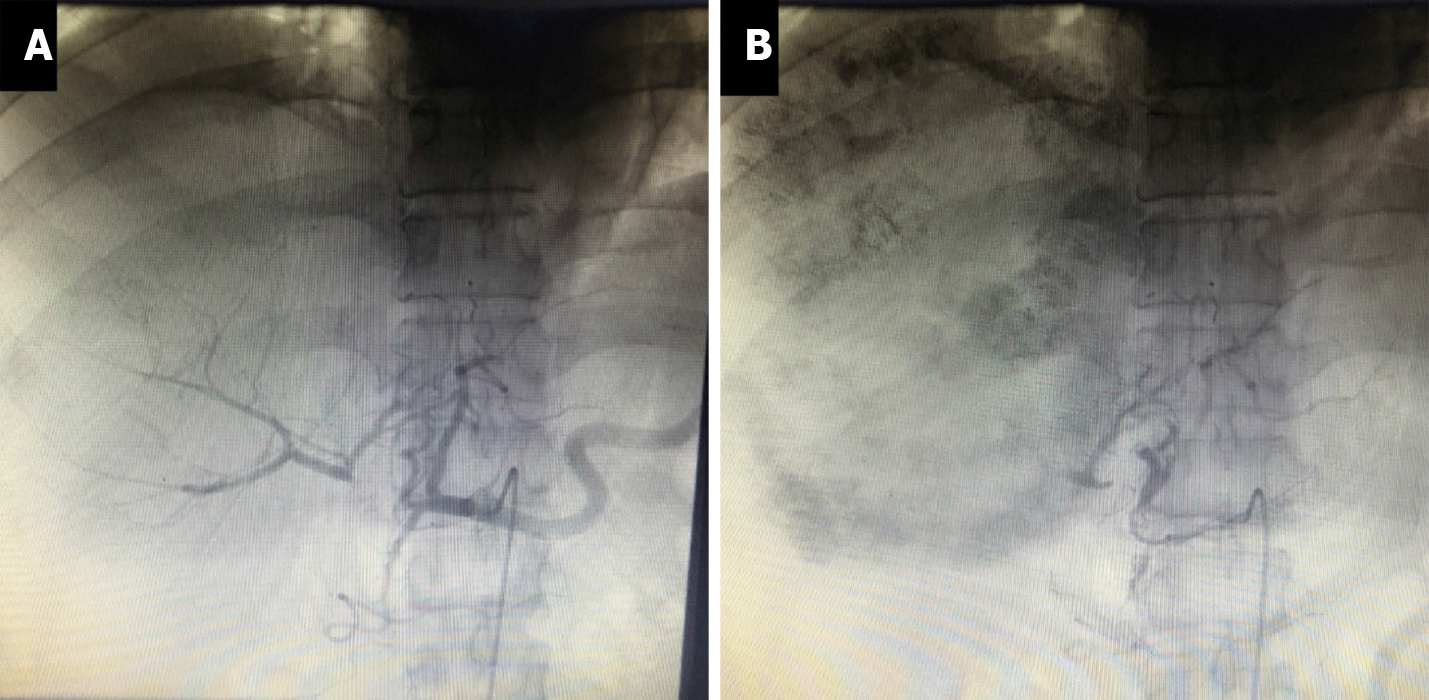
The patient returned to our center 53 d after his initial operation for reexamination. The volume of the left liver did not increase as expected. We therefore performed transcatheter arterial chemoembolization (TACE) 56 d after the first operation to limit the progression of the tumor. Intraoperative angiography showed that the right hepatic artery was ligated, and the right liver tumor was mainly supplied by the middle hepatic artery and to a lesser extent by the left hepatic artery via the collateral circulation (Figure 5A). He was treated with fluorouracil 750 mg + oxaliplatin 150 mg + pirarubicin 30 mg administered as a slow bolus, followed by iodized poppy seed oil and microspheres to embolize the branches of the middle and left hepatic arteries, respectively (Figure 5B). The patient recovered well and was discharged to await further review.
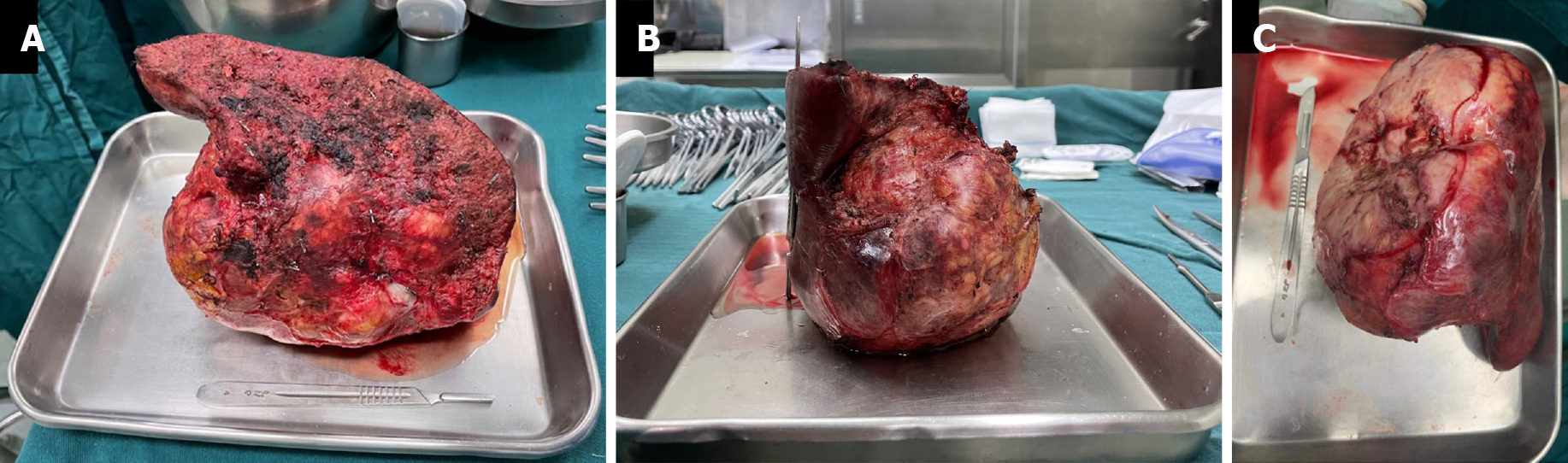
CT scans at 93 d after the first stage of surgery indicated that the left liver volume had increased to 30.88% of the total liver volume. The patient was still Child-Pugh grade A. He therefore underwent right hemi-hepatectomy and cholecystectomy 98 d after the first stage of surgery (Figure 6A-C).
The patient had a relatively uneventful postoperative clinical course. Dindo-Clavien grade I-II complications occurred after surgery, and he was discharged 113 d after the primary operation. The patient returned to the hospital for review 2 mo later. He was asymptomatic, and enhanced CT of the upper abdomen found no obvious abnormalities. The patient remained satisfied with the results and continued to be reviewed at our center as planned.
Conversion therapy of HCC is a recent area of interest. TACE involves injecting chemotherapeutic drugs and embolic agents into the tumor-supplying blood vessels via the hepatic artery to cause tumor necrosis. It is the first-choice standard non-surgical treatment for liver cancer and can effectively control tumor progression and increase the opportunities for liver resection and transplantation[4]. The problem of insufficient remnant liver volume can be addressed in several ways, including PVE, portal vein ligation (PVL), and ALPPS, which aim to limit the growth of the liver tumor on the affected side whilst enabling the healthy side of the liver to obtain sufficient portal vein blood flow and nutrients, reducing the volume of the affected-side liver and tumor, increasing the remnant liver volume after surgery, and improving the surgical resection rate and safety.
ALPPS was initially used in patients with liver metastases from colon cancer and has since been increasingly used for treating liver cancer. It provides a reasonable chance of complete resection for patients with initially unresectable primary liver tumors[5]. As a surgical technique, ALPPS can cause liver hypertrophy on the contralateral side in a shorter period of time compared with PVE or PVL. During this period, the patient’s condition progresses less, and more than 90% of patients can undergo secondary radical hepatectomy[6-8]. However, ALPPS is associated with high morbidity and mortality[9]. The procedure has thus not been widely used for the treatment of HCC, and further studies are needed to assess its safety relative to PVE[8].
PVE and PVL involve blocking the branches of the affected hepatic portal vein through surgery and intervention, respectively. Previous studies[10] found no significant difference in safety and future liver remnant hypertrophy rate between PVE and PVL, while its minimally invasive nature suggests that PVE should be considered the preferred strategy. Because PVL is often implemented in ALPPS, the current literature mostly compares the advantages and disadvantages of PVE and ALPPS. In a comparison between ALPPS and PVE, the ALPPS group had a greater increase in future liver remnant and a higher completion rate of second-stage surgery, but PVE had lower morbidity and mortality rates after the second stage[11].
TACE utilizes the characteristics of liver cancer, including nourishment mainly by the hepatic artery. The injection of embolic agents such as iodized oil, microspheres, and chemotherapeutics, such as cisplatin and fluorouracil, via the hepatic artery leads to necrosis of liver cancer tissues, thereby allowing some tumors to be downgraded sufficiently to allow radical resection. The possibility of surgery has greatly improved the prognosis of patients with advanced liver cancer, and it has been widely used for the treatment of these patients. However, issues such as the choice of indications and number of preoperative TACE procedures require more evidence.
As one of the main blood vessels of the liver, the hepatic artery accounts for 50% of the liver’s oxygen supply, with an average of 25% of the hepatic blood flow. Unlike many other organs, the blood supply to normal liver cells is mainly via venous blood distributed by the branches of the portal vein, with only 10%-35% of the blood supply of normal liver cells provided by the hepatic artery. However, malignant cells in the liver are mainly supplied by the branches of the hepatic artery, and the number of arteries around liver tumor tissues is three to four times higher than the number of veins[12]. We therefore suggest that even blocking only the hepatic artery supplying blood to the affected side of the liver tumor during surgery can achieve good results in terms of restricting tumor growth and, to a certain extent, restricting the growth of the affected side of the liver. Compared with ALPPS and PVE conversion therapies, the current treatment strategy can produce satisfactory results with relatively minor surgery. The degree of one-stage surgery required is much less than that with ALPPS, by only dissecting and ligating the branch of the hepatic artery that supplies blood to the tumor, without breaking the liver. Experienced surgeons can even complete the operation laparoscopically, potentially reducing the likelihood of postoperative liver failure[13] and improving the chances of a smooth recovery after the first-stage operation. Even though hepatic artery ligation combined with TACE may have less effect on the tumor than ALPPS, its effect is better than that of PVE alone. The effect of this strategy in terms of increasing residual liver volume is not obvious, compared with ALPPS and PVE. However, despite the current lack of evidence, the strategy warrants further research in terms of surgical operability and safety, especially when combined with TACE to re-embolize the tumor blood vessel branch.
Notably, although the current minimally invasive surgical technique has been applied to patients with Child-Pugh grade B liver cancer with good results[13,14], we do not recommend its implementation in patients with liver cancer of Child-Pugh grade B or below. The risk of postoperative liver failure remains high in patients with advanced giant liver tumors and liver decompensation, even if second-stage TACE is not considered immediately and only minimally invasive surgery is used to ligate the hepatic artery. In addition, the benefits of TACE in patients with Child-Pugh grade B remain controversial[15]. As for ALPPS, the current conversion treatment plan is thus currently only reproducible in patients with huge liver tumors, whose liver function is sufficient to compensate.
After first-stage liver partition and right hepatic artery ligation combined with second-stage TACE, the proportion of the current patient’s left liver volume to total liver volume increased from 21.34% to 30.88%. Liver tumor R0 resection was then completed in the third stage, with no surgical complications and good postoperative recovery. The treatment was therefore considered to be relatively successful. However, the long treatment cycle and slow compensation of the contralateral liver still represent shortcomings compared with ALPPS and PVE. In addition, the unplanned nature of the first-stage operation means that this treatment still lacks good repeatability and evaluability. Nevertheless, the successful treatment of this patient demonstrated the effective, safe, and operable characteristics and suggests that this strategy might help provide a solution to the problem of insufficient residual liver volume after surgery and a reference for future treatment strategies.
| 1. | Intaraprasong P, Siramolpiwat S, Vilaichone RK. Advances in Management of Hepatocellular Carcinoma. Asian Pac J Cancer Prev. 2016;17:3697-3703. [PubMed] |
| 2. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 3. | Shindoh J, Vauthey JN, Zimmitti G, Curley SA, Huang SY, Mahvash A, Gupta S, Wallace MJ, Aloia TA. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg. 2013;217:126-33; discussion 133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (2)] |
| 4. | Kulik L, Heimbach JK, Zaiem F, Almasri J, Prokop LJ, Wang Z, Murad MH, Mohammed K. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology. 2018;67:381-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 5. | Baili E, Tsilimigras DI, Filippou D, Ioannidis A, Bakopoulos A, Machairas N, Papalampros A, Petrou A, Schizas D, Moris D. Associating liver partition and portal vein ligation for staged hepatectomy in patients with primary liver malignancies: A systematic review of the literature. J BUON. 2019;24:1371-1381. [PubMed] |
| 6. | Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, Man N, Poon R, Lo CM. ALPPS Versus Portal Vein Embolization for Hepatitis-related Hepatocellular Carcinoma: A Changing Paradigm in Modulation of Future Liver Remnant Before Major Hepatectomy. Ann Surg. 2021;273:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, Baumgart J, Croome K, Hernandez-Alejandro R, Lang H, de Santibaňes E, Clavien PA. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | Li J, Ewald F, Gulati A, Nashan B. Associating liver partition and portal vein ligation for staged hepatectomy: From technical evolution to oncological benefit. World J Gastrointest Surg. 2016;8:124-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Lau WY, Lai EC, Lau SH. Associating liver partition and portal vein ligation for staged hepatectomy: the current role and development. Hepatobiliary Pancreat Dis Int. 2017;16:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Isfordink CJ, Samim M, Braat MNGJA, Almalki AM, Hagendoorn J, Borel Rinkes IHM, Molenaar IQ. Portal vein ligation versus portal vein embolization for induction of hypertrophy of the future liver remnant: A systematic review and meta-analysis. Surg Oncol. 2017;26:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Eshmuminov D, Raptis DA, Linecker M, Wirsching A, Lesurtel M, Clavien PA. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg. 2016;103:1768-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Bozkurt MF, Salanci BV, Uğur Ö. Intra-Arterial Radionuclide Therapies for Liver Tumors. Semin Nucl Med. 2016;46:324-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Vega EA, Kutlu OC, Joechle K, De La Cruz N, Ko D, Conrad C. Preoperative Prognosticators of Safe Laparoscopic Hepatocellular Carcinoma Resection in Advanced Cirrhosis: a Propensity Score Matching Population-Based Analysis of 1799 Western Patients. J Gastrointest Surg. 2019;23:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Romano F, Chiarelli M, Garancini M, Scotti M, Zago M, Cioffi G, De Simone M, Cioffi U. Rethinking the Barcelona clinic liver cancer guidelines: Intermediate stage and Child-Pugh B patients are suitable for surgery? World J Gastroenterol. 2021;27:2784-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 15. | Zhao Y, Duran R, Chapiro J, Sohn JH, Sahu S, Fleckenstein F, Smolka S, Pawlik TM, Schernthaner R, Zhao L, Lee H, He S, Lin M, Geschwind JF. Transarterial Chemoembolization for the Treatment of Advanced-Stage Hepatocellular Carcinoma. J Gastrointest Surg. 2016;20:2002-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fakhradiyev I, Kapritsou M S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Zhang YL