Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.677
Peer-review started: September 11, 2020
First decision: November 14, 2020
Revised: November 24, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: January 26, 2021
Processing time: 131 Days and 3.2 Hours
Takotsubo cardiomyopathy (TCM) is characterized by reversible left ventricular dysfunction triggered by emotional or physical stress. Only 1%-2% of patients with acute coronary syndrome are diagnosed with TCM. Although obstructive coronary artery disease is frequently considered to be the cause of chest pain, TCM should be considered in some clinical settings. In this case, clinicians did not make a timely and accurate diagnosis for TCM due to a lack of knowledge until the third hospitalization with a left ventriculogram.
A 55-year-old postmenopausal woman had intermittent chest pain following emotionally stressful events three times in the past 3 years. Cardiac troponin levels increased after each instance of symptom onset. A transthoracic echocardiogram showed reversible left ventricular dysfunction. The patient underwent three coronary angiograms without evidence of coronary artery disease. A left ventriculogram was first performed at the third hospitalization and revealed apical akinesia with ballooning of the apical region and consistent hypercontractile basal segments. The diagnosis of TCM was confirmed. The patient was treated with an angiotensin-converting-enzyme inhibitor (perindopril) and a β-blocker (metoprolol). No complications occurred during the patient’s hospitalization. The patient was told to avoid stressful events. During the 9-mo follow-up visit, the patient was asymptomatic with an ejection fraction of 55%.
Clinicians should be conscious of the possibility of TCM, especially in postmenopausal women presenting with clinical manifestations similar to acute coronary syndrome without coronary occlusion.
Core Tip: Although obstructive coronary artery disease is frequently considered to be the cause of chest pain, Takotsubo cardiomyopathy (TCM) should be considered in some clinical settings. Recurrent TCM triggered by emotional stress is rare. Early appropriate supportive treatments can avert adverse outcomes and ensure a good prognosis for patients with TCM. We hope that this case will increase our attention to the diagnosis of TCM.
- Citation: Wu HY, Cheng G, Liang L, Cao YW. Recurrent Takotsubo cardiomyopathy triggered by emotionally stressful events: A case report. World J Clin Cases 2021; 9(3): 677-684
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/677.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.677
Takotsubo cardiomyopathy (TCM) is characterized by reversible left ventricular apical ballooning without coronary occlusion. The underlying pathophysiological mechanisms may include catecholamine cardiotoxicity, coronary microvascular disorder and coronary artery spasm. Only 1%-2% of patients with acute coronary syndrome are diagnosed with TCM, and most patients are older women with the presence of triggers such as emotional or physical stressors[1].
We report a case of a postmenopausal woman with onset symptoms similar to acute coronary syndrome following emotionally stressful events that occurred three times from 2016 to 2019. Due to a lack of knowledge, clinicians failed to diagnose TCM until the third hospitalization. We hope that this case can increase attention to the diagnosis of TCM.
A 55-year-old postmenopausal woman had intermittent chest pain for 3 years.
Three years ago (on January 20, 2016), the patient experienced chest pain following an emotionally stressful event (quarrel with her husband) without any signs of infection, such as cough or diarrhea. Twelve-lead electrocardiograms (ECGs) indicated ST-segment and T-wave dynamic changes in the inferior and anterior leads. The peak troponin I level was 0.81 ng/mL (normal range < 0.03). A transthoracic echocardiogram (TTE) showed hypokinesis of the apical and mid-distal segments of the left ventricle with a reduced ejection fraction of 48%. A coronary angiogram showed no evidence of coronary artery disease. The patient was discharged home on diltiazem and an angiotensin-converting-enzyme inhibitor (perindopril) with a suspected diagnosis of coronary artery spasm. The TTE demonstrated completely normal cardiac structure and function with an ejection fraction of 68% on July 27, 2018. On August 9, 2018, the patient presented with chest pain again following the same emotionally stressful event (quarrel with her husband). The peak troponin I level was 0.338 ng/mL. The 12-lead ECG indicated ST-segment depression in the inferior leads and T-wave inversion in the inferior and anterior leads. The TTE showed hypokinesis of the apical and mid-distal segments of the left ventricle (ejection fraction of 52%). A coronary angiogram was performed again without evidence of coronary artery disease. A left ventriculogram was not performed again. The patient was discharged with trimetazidine and an angiotensin-converting-enzyme inhibitor (perindopril). On February 9, 2019, the patient presented with similar chest pain following another stressful event (business failure) without any signs of infection.
The patient had a history of pacemaker implantation.
The patient had a free personal history. The patient had no family history of premature coronary artery disease.
Vital signs were stable. There was no obvious abnormality during pulmonary or cardiac examination. There was no jugular vein engorgement or peripheral edema.
The peak troponin I level was 2.228 ng/mL (normal range < 0.03), and the peak B-type natriuretic peptide level was 166 pg/mL (normal range < 76).
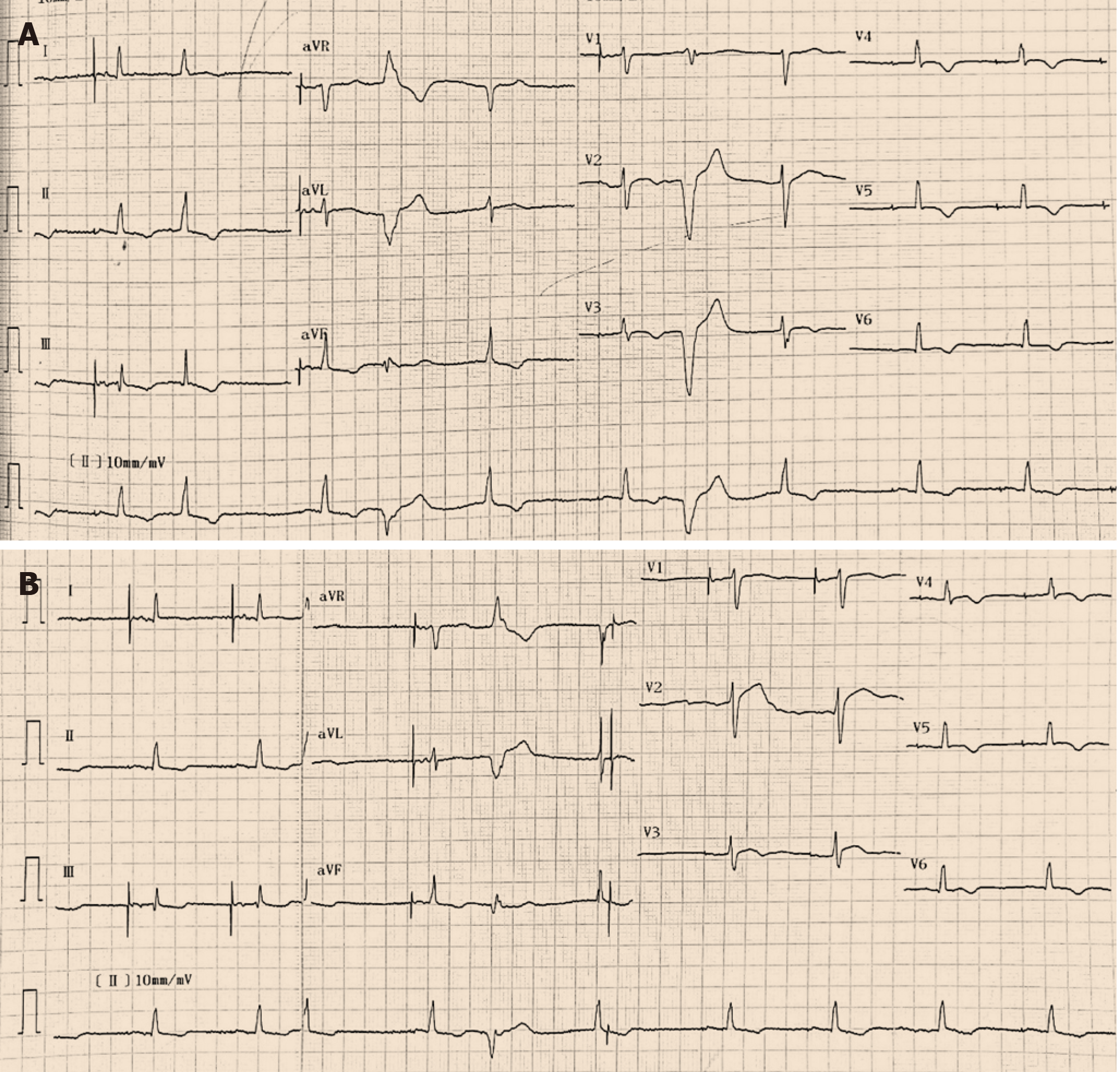
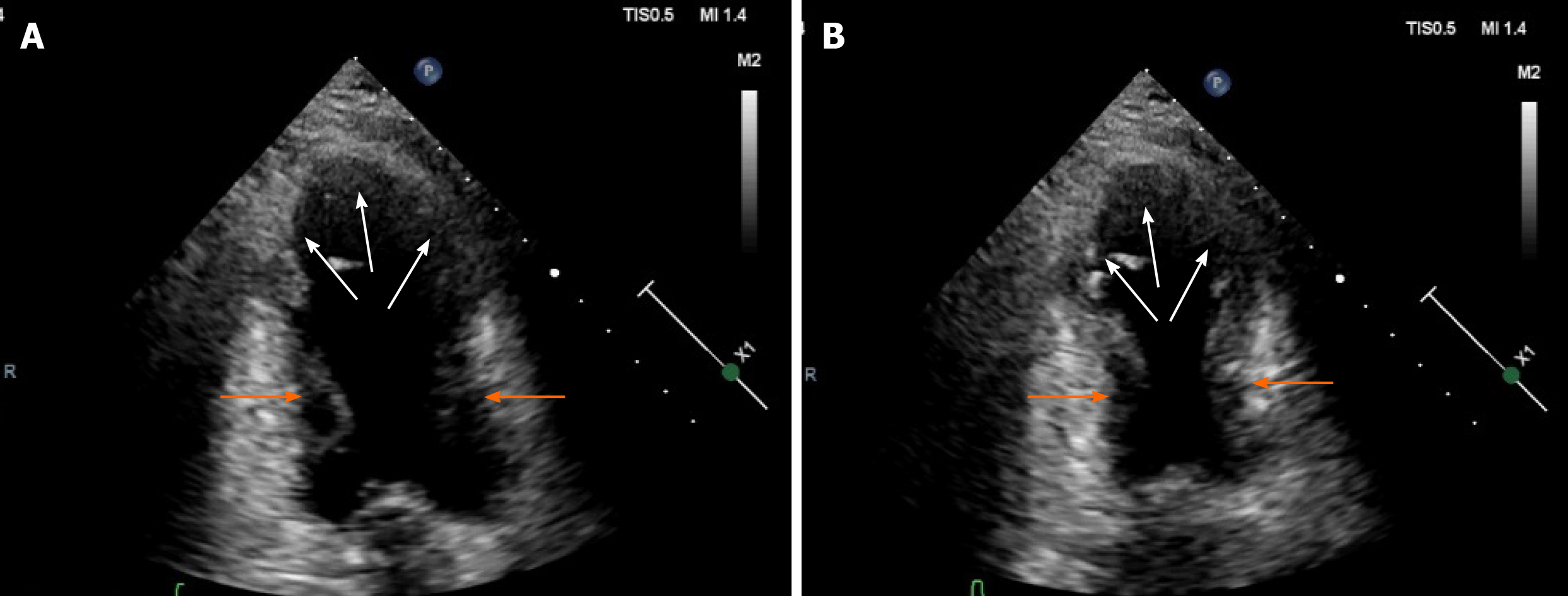
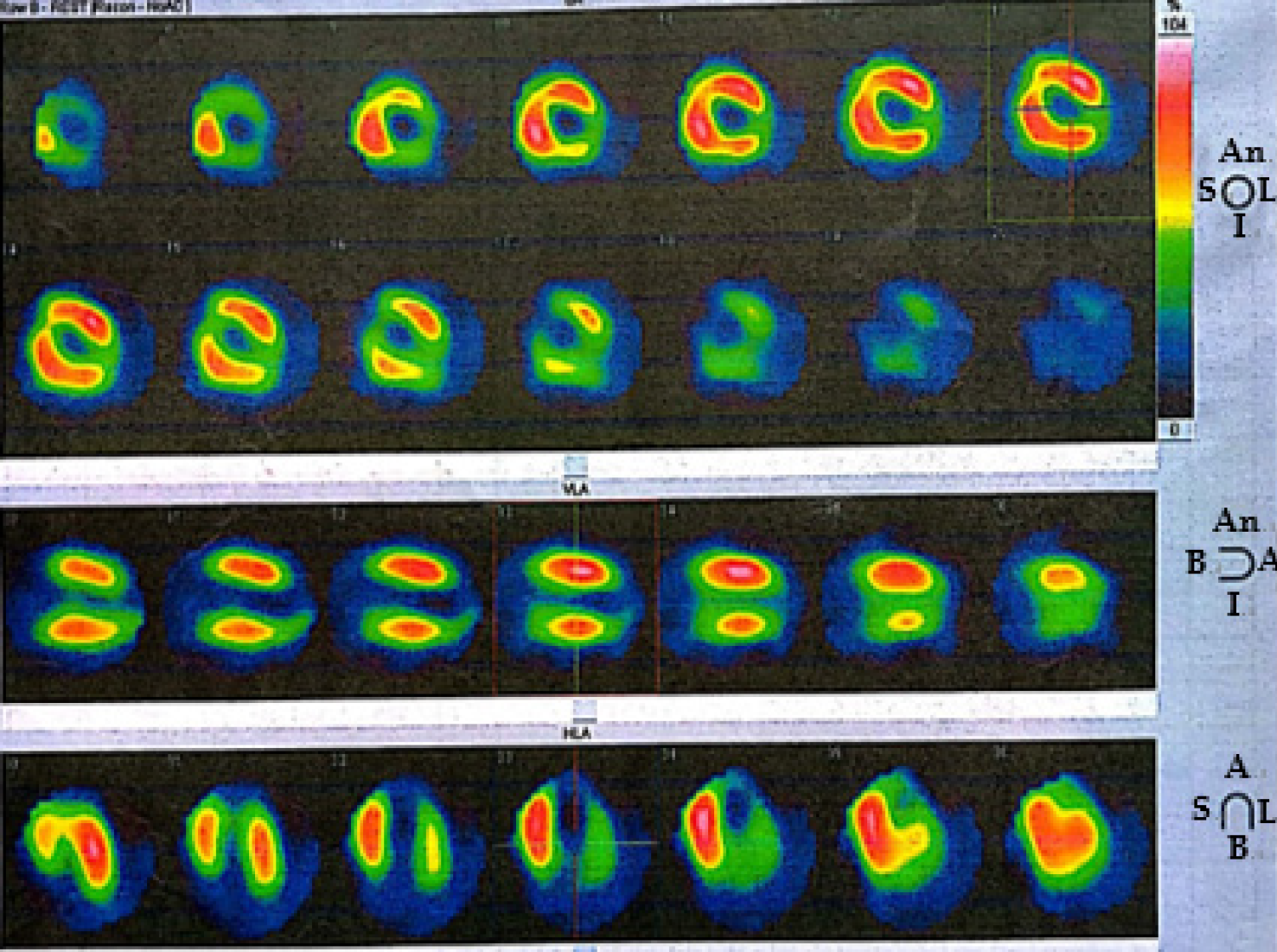
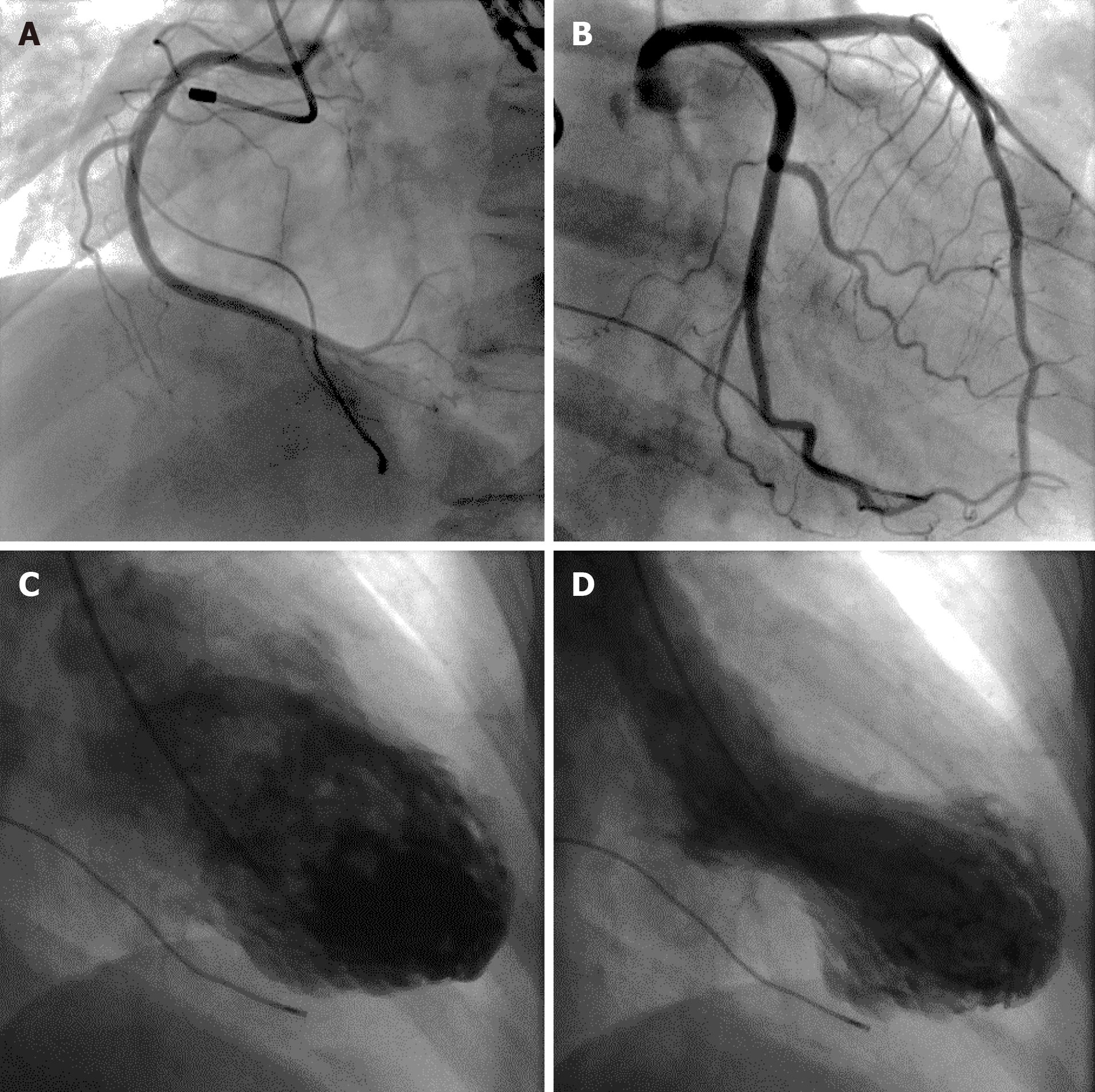
Twelve-lead ECGs indicated ST-segment and T-wave changes in the inferior and anterior leads (Figure 1). The TTE showed hypokinesis of the apical and mid-distal segments and a hyperdynamic basal segment of the left ventricle with a depressed ejection fraction of 47% (Figure 2). Myocardial perfusion single photon emission computed tomography imaging (resting state) demonstrated decreased uptake in the left ventricular apical, anterior, inferior and lateral walls of the myocardium (Figure 3). Adrenal computed tomography and hormone results excluded pheochromocytoma. She had no evidence of coronary artery disease detected by the coronary angiogram; however, the left ventriculogram revealed apical akinesia with ballooning of the apical region and hypercontractile basal segments consistent with the typical diagnosis of TCM (Figure 4).
The diagnosis of TCM was confirmed.
The patient was treated with an angiotensin-converting-enzyme inhibitor (perindopril, 4 mg/d) and a β-blocker (metoprolol, 47.5 mg/d).
No complications occurred during the patient’s hospitalization. The patient was told to avoid stressful events. The follow-up TTE showed that cardiac function had remarkably improved with an ejection fraction of 55% on June 28, 2019 (Table 1). At the 1-, 3- 6- and 9-mo follow-up visits, the patient was asymptomatic.
| Date | Left ventricular diameter in systole/diastole, mm | Left ventricular ejection fraction | ||
| Anteroposterior axis | Transverse axis | Major axis | ||
| September 17, 2014 | 32/48 | 32/48 | 55/65 | 62% |
| January 22, 2016 | 48/59 | 32/43 | 62/72 | 48% |
| July 27, 2018 | 30/49 | 30/49 | 59/69 | 68% |
| August 10, 2018 | 41/54 | 41/54 | 63/75 | 52% |
| February 14, 2019 | ?/49 | ?/51 | ?/82 | 47% |
| June 28, 2019 | 31/50 | 30/46 | ?/75 | 55% |
The patient we reported was a postmenopausal woman with recurrent chest pain, ECG changes and increased cardiac troponin following emotionally stressful events from 2016 to 2019. The coronary angiogram revealed no evidence of coronary artery disease, and the left ventriculogram revealed left ventricular apical ballooning. The medical history and related examination results excluded pheochromocytoma and myocarditis. The patient fulfilled all of the Mayo Clinic’s diagnostic criteria for TCM[2].
TCM is also called stress-induced cardiomyopathy. More than 60% of patients have emotional or physical stress. However, up to 20% of TCM patients have no identifiable stress[3]. Women account for 90% of TCM cases, with an average age of 58-75 years and with only 3% of patients less than 50-years-old. Postmenopausal women are more likely to be attacked due to estrogen deficiency and altered catecholamine hormonal receptor sensitivity[4,5]. The age of the postmenopausal patient at onset in our case ranged from 52 to 55, and the patient had emotionally stressful events before every symptom attack.
TCM is also considered a myocardial infarction-like syndrome with reversible left ventricular systolic dysfunction of the septum, apical, anterior, posterior and sometimes lateral walls. Transient myocardial dysfunction is usually not within the scope of a single coronary artery but tends to be limited to horizontal areas along the left ventricular longitudinal axis[6,7]. The most plausible mechanism is that intense emotional or physical stressors lead to a surge in catecholamine that can adversely affect myocardial contractility, resulting in transient myocardial dysfunction. There are no evidence-based guidelines specifically for the treatment of TCM, but β-blockers and angiotensin-converting enzyme inhibitors have proven effective according to some case reports[8].
Usually, the prognosis of TCM is favorable. Myocardial dysfunction in most patients can be almost completely resolved within a few weeks[9]. The TTE findings in our patient showed hypokinesis of the apical and mid-distal segments of the left ventricle with reduced ejection fraction. The left ventriculogram revealed apical akinesia with ballooning of the apical region and hypercontractile basal segments. An angiotensin-converting-enzyme inhibitor and a β-blocker were used to reverse cardiac remodeling. The follow-up TTE results after presentation showed complete resolution of left ventricular systolic dysfunction, which makes myocarditis unlikely to be the cause of left ventricular dysfunction in this case.
Patients with TCM present with different ECG abnormalities, including ST elevation, ST depression, QT prolongation, T wave inversion, left bundle branch block, abnormal Q waves and nonspecific abnormalities[10]. The characteristics of ECG are affected by the region of left ventricular ballooning, time from symptom onset, degree of myocardial edema and recovery rate of cardiomyocytes. T waves may be inverted when the sinus rhythm is restored in patients with pacemakers[11-13]. Therefore, cardiac troponin elevation is helpful for the diagnosis of TCM.
The clinical and laboratory manifestations of TCM are similar to those of acute coronary syndrome. Almost all patients with TCM show evidence of myocardial necrosis[14-17]. Most patients have elevated cardiac troponin levels as a predictor for in-hospital prognosis, which may be due to the increase of troponin in membrane leakage caused by acute myocardial necrosis, and troponin is elevated in proportion to the hypodynamic region[18-20]. B-type natriuretic peptide is a recognized marker of left ventricular dysfunction. The level of B-type natriuretic peptide is associated with ventricular distention with or without cardiac myocyte necrosis. Because TCM is a disease mainly causing ventricular distention and characterized by reversible myocardial dysfunction, the level of B-type natriuretic peptide is significantly higher in TCM patients than in patients with acute coronary syndrome related to the degree of left ventricular systolic dysfunction[21-23]. However, up to 5%-18% of TCM patients have no B-type natriuretic peptide elevation[24-26].
Our patient had three instances of chest pain, and ECG changes and cardiac troponin elevation were similar to acute coronary syndrome following emotionally stressful events. Urgent coronary angiograms were performed to exclude coronary artery disease. The patient underwent three coronary angiograms without evidence of coronary artery disease. The TTE showed reversible left ventricular dysfunction. Clinicians did not make a timely and accurate diagnosis for TCM due to a lack of knowledge until the third hospitalization with a left ventriculogram. The typical wall motion abnormalities of TCM may restore within hours, and delayed imaging may lead to a missed diagnosis. Urgent left ventriculogram is frequently used in the diagnosis of TCM. Nearly 6000 coronary angiograms were performed in our hospital for chest pain in 2019. However, fewer than 15 patients underwent left ventriculogram, which may be related to insufficient awareness of TCM. We believe that there may be some missed diagnosis of TCM in our hospital. Although obstructive coronary artery disease is frequently considered to be the cause of chest pain, TCM should be considered in some clinical settings.
TCM should receive much more attention, and clinicians should maintain a high suspicion for TCM, especially for postmenopausal women presenting with clinical symptoms similar to acute coronary syndrome. Early appropriate supportive treatments can avert adverse outcomes and ensure a good prognosis for patients with TCM.
| 1. | Chams S, El Sayegh S, Hamdon M, Kumar S, Kulairi Z. Zumba-induced Takotsubo cardiomyopathy: a case report. J Med Case Rep. 2018;12:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol. 2016;209:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Huynh K. Cardiomyopathies: Clinical features of patients with Takotsubo syndrome. Nat Rev Cardiol. 2015;12:684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Barbaryan A, Bailuc SL, Patel K, Raqeem MW, Thakur A, Mirrakhimov AE. An Emotional Stress as a Trigger for Reverse Takotsubo Cardiomyopathy: A Case Report and Literature Review. Am J Case Rep. 2016;17:137-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Dawson DK. Acute stress-induced (takotsubo) cardiomyopathy. Heart. 2018;104:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Kido K, Guglin M. Drug-Induced Takotsubo Cardiomyopathy. J Cardiovasc Pharmacol Ther. 2017;22:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Agarwal S, Sanghvi C, Odo N, Castresana MR. Perioperative takotsubo cardiomyopathy: Implications for anesthesiologist. Ann Card Anaesth. 2019;22:309-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Kato K, Lyon AR, Ghadri JR, Templin C. Takotsubo syndrome: aetiology, presentation and treatment. Heart. 2017;103:1461-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 9. | Y-Hassan S, Tornvall P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin Auton Res. 2018;28:53-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 10. | Oindi FM, Sequeira E, Sequeira HR, Mutiso SK. Takotsubo cardiomyopathy in pregnancy: a case report and literature review. BMC Pregnancy Childbirth. 2019;19:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Alvarez Retamales V, Lara Garcia OE, Ranjha S, Koester C, Labedi MR. Takotsubo Cardiomyopathy and QTc Prolongation with Subsequent Improvement of QTc Interval and Resolution of Apical Ballooning: A Case Report. Cureus. 2020;12:e9143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Yoshikawa T. Takotsubo cardiomyopathy, a new concept of cardiomyopathy: clinical features and pathophysiology. Int J Cardiol. 2015;182:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Angelini P. Recurrent Takotsubo Cardiomyopathy: An Opportunity to Clarify Causation and Prognosis. Tex Heart Inst J. 2018;45:252-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo Syndrome. Circulation. 2017;135:2426-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 492] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 15. | Goel S, Sharma A, Garg A, Chandra A, Shetty V. Chemotherapy induced Takotsubo cardiomyopathy. World J Clin Cases. 2014;2:565-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 16. | Roshanzamir S, Showkathali R. Takotsubo cardiomyopathy a short review. Curr Cardiol Rev. 2013;9:191-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Tofield A. Hikaru Sato and Takotsubo cardiomyopathy. Eur Heart J. 2016;37:2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Nguyen TH, Neil CJ, Sverdlov AL, Mahadavan G, Chirkov YY, Kucia AM, Stansborough J, Beltrame JF, Selvanayagam JB, Zeitz CJ, Struthers AD, Frenneaux MP, Horowitz JD. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am J Cardiol. 2011;108:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Jeong HS, Lee TH, Bang CH, Kim JH, Hong SJ. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: A comparative retrospective study. Medicine (Baltimore). 2018;97:e0263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Heyse A, Anne F, Lagae B, Van Haver H, Van Durme F. Serial strain imaging in takotsubo syndrome with concomitant coronary artery disease. Acta Clin Belg. 2019;74:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Jesel L, Berthon C, Messas N, Lim HS, Girardey M, Marzak H, Marchandot B, Trinh A, Ohlmann P, Morel O. Atrial arrhythmias in Takotsubo cardiomyopathy: incidence, predictive factors, and prognosis. Europace. 2019;21:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Ahmed KA, Madhavan M, Prasad A. Brain natriuretic peptide in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): comparison with acute myocardial infarction. Coron Artery Dis. 2012;23:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Finkel-Oron A, Olchowski J, Jotkowitz A, Barski L. Takotsubo cardiomyopathy triggered by wasabi consumption: can sushi break your heart? BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Stiermaier T, Santoro F, Graf T, Guastafierro F, Tarantino N, De Gennaro L, Caldarola P, Di Biase M, Thiele H, Brunetti ND, Möller C, Eitel I. Prognostic value of N-Terminal Pro-B-Type Natriuretic Peptide in Takotsubo syndrome. Clin Res Cardiol. 2018;107:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Lee M, Oh JH, Lee KB, Kang GH, Park YH, Jang WJ, Chun WJ, Lee SH, Lee IC. Clinical and Echocardiographic Characteristics of Acute Cardiac Dysfunction Associated With Acute Brain Hemorrhage - Difference From Takotsubo Cardiomyopathy. Circ J. 2016;80:2026-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Yang HS, Kim HJ, Shim HJ, Kim SJ, Hur M, Di Somma S; GREAT Network. Soluble ST2 and troponin I combination: Useful biomarker for predicting development of stress cardiomyopathy in patients admitted to the medical intensive care unit. Heart Lung. 2015;44:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Perrault LP S-Editor: Huang P L-Editor: Filipodia P-Editor: Li JH