Published online Jan 26, 2021. doi: 10.12998/wjcc.v9.i3.639
Peer-review started: September 17, 2020
First decision: November 26, 2020
Revised: December 6, 2020
Accepted: December 10, 2020
Article in press: December 10, 2020
Published online: January 26, 2021
Processing time: 124 Days and 23.9 Hours
As an established, simple, inexpensive, and surprisingly effective diagnostic tool, right-heart contrast echocardiography (RHCE) might help in solving a vexing diagnostic problem. If performed appropriately and interpreted logically, RHCE allows for differentiation of various usual and unusual right-to-left shunts based on the site of injection and the sequence of microbubble appearance in the heart.
A 31-year-old woman was readmitted to hospital with a 2-mo history of worsening palpitation and chest distress. Two years prior, she had been diagnosed with postpartum pulmonary embolism by conventional echocardiography and computed tomography angiography. While the latter showed no sign of pulmonary artery embolism, the former showed pulmonary artery hypertension, moderate insufficiency, and mild stenosis of the aortic valve. RHCE showed microbubbles appearing in the left ventricle, slightly delayed after right-heart filling with microbubbles; no microbubbles appeared in the left atrium and microbubbles’ appearance in the descending aorta occurred nearly simultaneous to right pulmonary artery filling with microbubbles. Conventional echocardiography was re-performed, and an arterial horizontal bidirectional shunt was found according to Doppler enhancement effects caused by microbubbles. The original computed tomography angiography findings were reviewed and found to show a patent ductus arteriosus.
RHCE shows a special imaging sequence for unexplained pulmonary artery hypertension with aortic valve insufficiency and simultaneous patent ductus arteriosus.
Core Tip: A 31-year-old woman diagnosed with postpartum pulmonary embolism was readmitted to hospital, when conventional echocardiography found pulmonary artery hypertension. Right-heart contrast echocardiography showed microbubbles appearing in the left ventricle, with a slight delay after right-heart filling with microbubbles but no microbubbles in the left atrium; microbubbles also appeared in the descending aorta almost simultaneously to the right pulmonary artery. Computed tomography angiography showed no sign of pulmonary artery embolism but did show a patent ductus arteriosus. Ultimately, right-heart contrast echocardiography showed a special imaging sequence for unexplained pulmonary artery hypertension with aortic valve insufficiency and simultaneous patent ductus arteriosus.
- Citation: Chen JL, Mei DE, Yu CG, Zhao ZY. Right-heart contrast echocardiography reveals missed patent ductus arteriosus in a postpartum woman with pulmonary embolism: A case report. World J Clin Cases 2021; 9(3): 639-643
- URL: https://www.wjgnet.com/2307-8960/full/v9/i3/639.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i3.639
Right-heart contrast echocardiography (RHCE) using agitated saline or 50% glucose is an established, simple, inexpensive, and surprisingly effective diagnostic tool which may help in clinical practice to overcome an ongoing vexing diagnostic problem[1]. Although RHCE was introduced over 50 years ago, in 1968[2], and the list of clinical entities that can be diagnosed by RHCE continues to grow, it remains largely underutilized. In this article, we highlight the special imaging sequence of microbubbles in RHCE, which disclosed a missed diagnosis of patent ductus arteriosus (PDA) in a postpartum woman with pulmonary embolism.
A 31-year-old woman was admitted to hospital with a 2-mo history of worsening palpitation and chest distress.
The patient had experienced the symptoms of palpitation and chest distress 20 years prior but reported their recurrence, with greater intensity, over the past 2 mo.
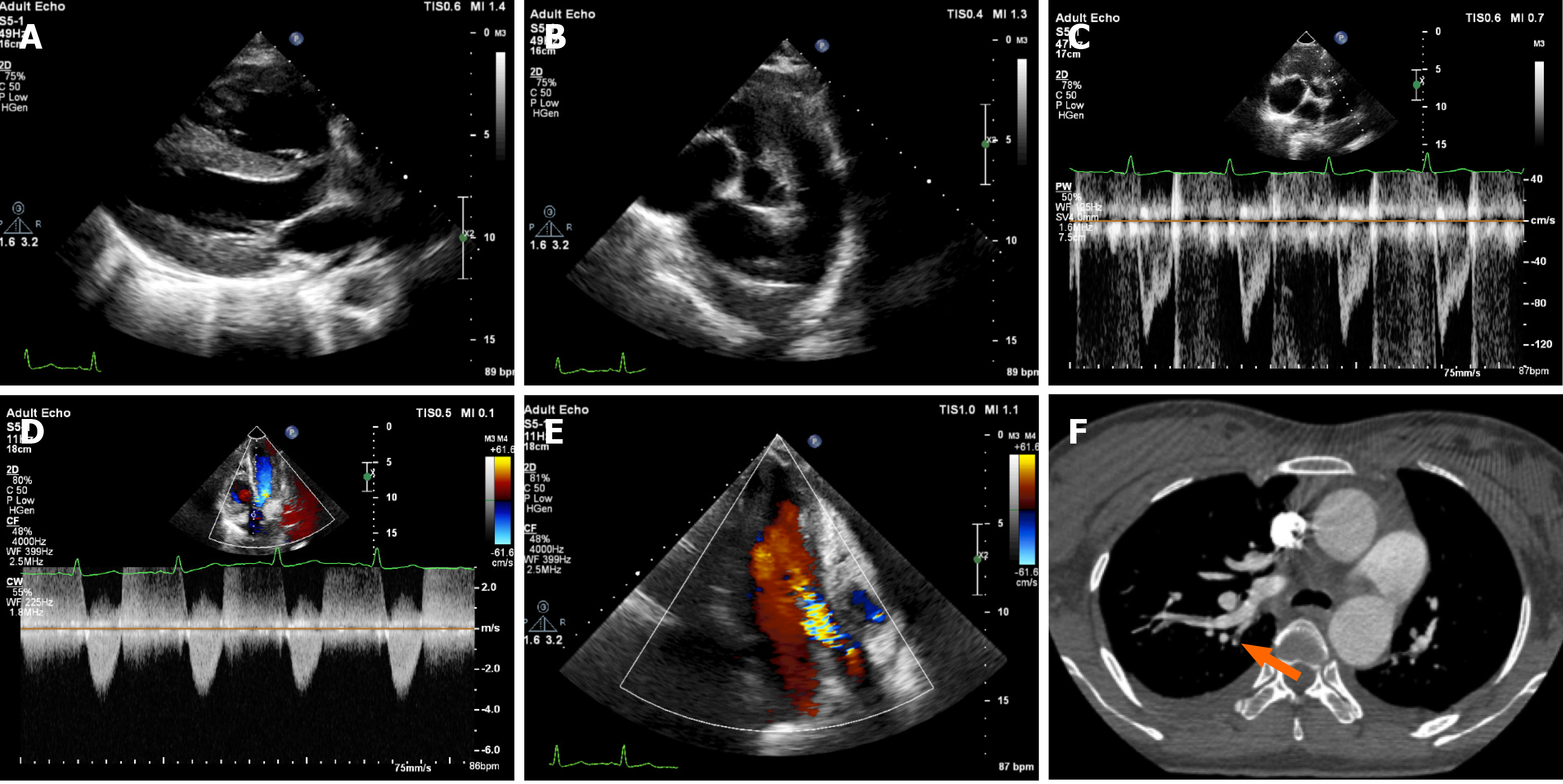
Two years prior, the patient had been hospitalized due to massive postpartum hemorrhage and dyspnea, lasting for 1 d. Physical examination revealed grade 3/6 diastolic murmur in the aortic valve auscultation area, and laboratory examination showed an increased blood D-dimer level (3.39 mg/L; normal range: 0-0.256 mg/L). Conventional echocardiography, at that time, revealed enlargement of the right heart, thickening of the right ventricular wall, obvious broadening of the pulmonary artery, shortening of the pulmonary artery systolic blood flow acceleration time, pulmonary hypertension (pulmonary artery systolic pressure, pulmonary artery diastolic pressure, and mean pulmonary artery pressure were 81 mmHg, 24 mmHg, and 43 mmHg, respectively), and moderate insufficiency and mild stenosis of the aortic valve (Figure 1A-E). Meanwhile, computed tomography angiography (CTA) revealed embolism in a branch of the pulmonary artery, evidenced by a filling defect in the superior lobe of the right lung and the lingual lobe of the left lung (Figure 1F). The patient was discharged after successful thrombolytic treatment.
The patient had no previous or family history of similar illnesses.
Physical examination at admission for the current complaints found grade 3/6 diastolic murmur in the aortic valve auscultation area.
Blood analysis showed a normal blood D-dimer level (0.2 mg/L).
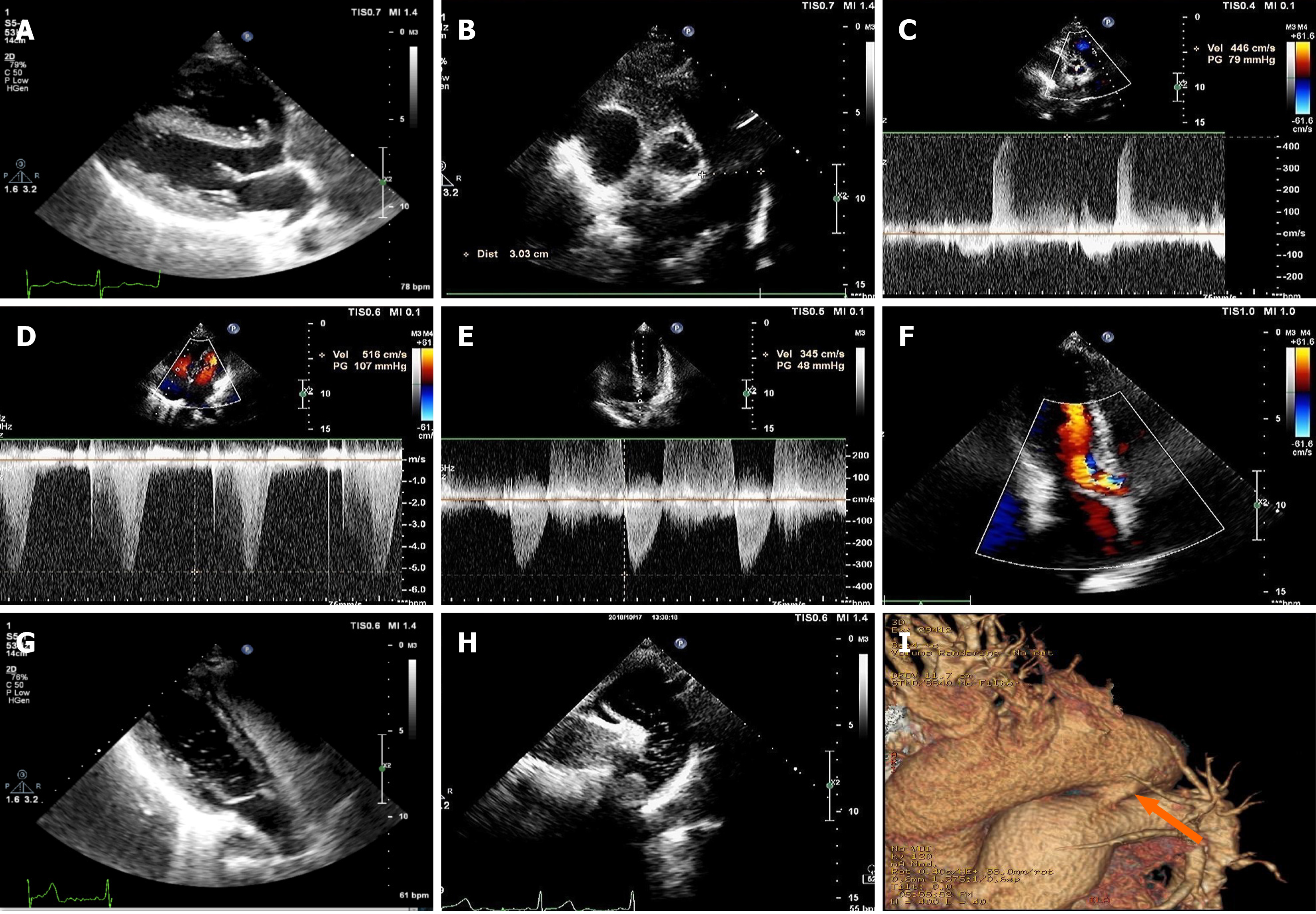
Conventional echocardiography upon admission gave results similar to those from the 2-year previous admission, but the pulmonary hypertension was more severe (pulmonary artery systolic pressure, pulmonary artery diastolic pressure, and mean pulmonary artery pressure were 115 mmHg, 73 mmHg, and 87 mmHg, respectively) (Figure 2A-F). CTA showed no sign of pulmonary artery embolism. To extend our search for the cause of the patient’s increased pulmonary artery hypertension, RHCE was performed. After agitated 50% glucose was injected into the left antecubital vein, the right atrium and right ventricle were found to immediately fill with microbubbles, as observed on the parasternal four-chamber view. The microbubbles appeared in the left ventricle with a slight delay (about three cardiac cycles) after the right heart was filled with microbubbles; no microbubbles appeared in the left atrium and no intracardiac shunt was found. We chose to re-perform the RHCE to look for any new clues on other echocardiography views. At the suprasternal aortic arch long axis view, many microbubbles were found in the descending aorta, appearing at almost the same time as those in the right pulmonary artery (Figure 2G and H).
Conventional echocardiography was re-performed and the findings from the CTA were re-reviewed. On the echocardiography, we found an arterial horizontal bidirectional shunt, evidenced by Doppler enhancement effects caused by microbubbles. On the CTA, we found a PDA (width: 4 mm; length: 8 mm) and no obvious pulmonary embolism (Figure 2I).
Pulmonary artery hypertension combined with aortic valve insufficiency and PDA.
The patient refused surgery and was treated with oral targeted drug therapy, consisting of sildenafil (phosphodiesterase-5 inhibitor; 50 mg, TID) and bosentan (endothelin receptor antagonist; within 4 wk, 62.5 mg bid; after 4 wk, 125mg bid).
The patient's symptoms were lessened after drug treatment but not resolved completely. The arterial horizontal bidirectional shunt was still observable by imaging analyses. At the follow-up visit 1 year after this hospitalization, the patient’s pulmonary artery pressure showed a slight decrease.
Since Gramiak et al[3] reported the utility of contrast echocardiography in 1969, it has been the most useful method for detecting right-to-left shunts. RHCE may be administered via a peripheral vein, to serve as a screening test that will also assess the degree of right to left shunting in patients who have hypoxemia out of proportion to their degree of lung parenchymal and/or pulmonary vascular disease; this may also be helpful for improvement of the spectral Doppler trace, providing more accurate measurement of tricuspid and pulmonary valve velocities[4]. If performed appropriately and interpreted logically, RHCE allows for differentiation of various usual and unusual right-to-left shunts based on the site of injection and the sequence of microbubble appearance in the heart[1].
In general, conventional echocardiography has high diagnostic value for PDA with a left to right shunt. However, in the case of PDA with pulmonary hypertension, a bidirectional or right-to-left shunt will often occur at the level of the aorta. Conventional echocardiography is prone to missed diagnosis due to the lack of typical continuous murmurs, the inherent difficulty in detecting blood flow signal, or the overall spectrum of PDA.
For the patient presented herein, RHCE showed a special sequence of microbubble appearance, which helped to reveal the previously missed diagnosis of PDA. The appearance of microbubbles in the left ventricle, coupled with the absence of bubbles in the left atrium and the slight delay of appearance after the right heart was filled with microbubbles may have been due to the PDA being combined with aortic insufficiency. The microbubbles in the pulmonary artery were observed to enter the descending aorta through the PDA, and then to enter the left ventricle through the insufficient aortic valve during diastole. The presence of aortic insufficiency in this patient was the key factor that led to the special imaging sequence during RHCE, which facilitated the detection of bilateral or right-to-left shunts at the level of the large artery.
Moreover, pulmonary hypertension caused by various reasons usually increases the possibility of missed diagnosis of congenital heart disease, such as PDA, in conventional echocardiography. In our case, pulmonary embolism could be reasonably used to explain the occurrence of pulmonary hypertension at the first admission, while other causes of pulmonary hypertension or shunt were more likely to be ignored. The diagnostic value of RHCE should be high in such cases. Performance of RHCE could confirm indistinctive right-to-left shunts when causes are not detected by routine clinical examinations. In addition, when tracing the etiology of pulmonary hypertension, full application of RHCE, reasonable and detailed analysis of the developing sequence of microbubbles, and selection of suitable image sections, especially for the long axis of the suprasternal aortic arch, can effectively help to improve the detection of PDA.
We highlight the special imaging sequence detected by RHCE in a patient with unexplained pulmonary artery hypertension combined with aortic valve insufficiency and simultaneous PDA. We recommend RHCE be performed in order to confirm indistinctive right-to-left shunts when causes are not detected by routine clinical examinations.
| 1. | Gupta SK, Shetkar SS, Ramakrishnan S, Kothari SS. Saline Contrast Echocardiography in the Era of Multimodality Imaging--Importance of "Bubbling It Right". Echocardiography. 2015;32:1707-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol. 1968;3:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 548] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Gramiak R, Shah PM, Kramer DH. Ultrasound cardiography: contrast studies in anatomy and function. Radiology. 1969;92:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 268] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Cordina RL, Playford D, Lang I, Celermajer DS. State-of-the-Art Review: Echocardiography in Pulmonary Hypertension. Heart Lung Circ. 2019;28:1351-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordonaro M S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Xing YX