Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8923
Peer-review started: June 17, 2021
First decision: July 5, 2021
Revised: July 6, 2021
Accepted: August 4, 2021
Article in press: August 4, 2021
Published online: October 16, 2021
Processing time: 120 Days and 3.7 Hours
The duration of surveillance after curative resection of colorectal cancer (CRC) is generally 5 years. The overall incidence of recurrence more than 5 years after surgery for CRC in Japan has been reported to be 0.6%. Moreover, it is rare for CRC to have metachronous liver metastasis more than 10 years after surgery. Here, we present a case of liver metastasis detected 11 years after the curative resection of rectal cancer.
A 72-year-old man was referred to our hospital after a liver tumor was detected by abdominal ultrasonography at another hospital. He had undergone surgery for rectal cancer 11 years previously. Contrast-enhanced computed tomography (CT) showed a tumor with a diameter of approximately 8 cm in the posterior segment, which was weakly and gradually enhanced. 18F-fluorodeoxyglucose-positron emission tomography/CT showed an abnormally high uptake on the tumorous lesion, which showed that the tumor appeared to spread convexly along the intrahepatic bile ducts. Intrahepatic cholangiocarcinoma was therefore diagnosed, and he had an extended right posterior sectionectomy and regional lymph node dissection. Histopathological examination showed that the tumor was a mode
It is possible that a liver tumor is metastatic in a patient with a previous history of CRC, even if it was more than 10 years earlier.
Core Tip: This case report presents a case with an isolated liver metastasis 11 years after surgery for rectal cancer. In addition, it is difficult to differentiate a late liver metastasis of colorectal cancer (CRC) from intrahepatic cholangiocarcinoma (ICC) preoperatively. We should note the possibility that a liver tumor in a patient with a history of curatively resected CRC might not be ICC nor hepatocellular carcinoma but metastasis from CRC, even if CRC was diagnosed and resected more than 10 years earlier.
- Citation: Yonenaga Y, Yokoyama S. Isolated liver metastasis detected 11 years after the curative resection of rectal cancer: A case report. World J Clin Cases 2021; 9(29): 8923-8931
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8923.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8923
Globally, colorectal cancer (CRC) is the third-most commonly diagnosed cancer in males and the second-most in females, with 1931590 new cases and 935173 deaths in 2020 according to the Global Cancer Observatory database[1]. In Japan, the number of deaths from CRC continues to increase, exceeding 50000 in 2016. In principle, the duration of surveillance globally is 5 years after curative resection of CRC[2,3]. The results of the Japanese Society for Cancer of the Colon and Rectum (JSCCR) colorectal cancer registry in 2007 showed that 96.5% of recurrences were detected within 5 years of surgery, and that the overall incidence of recurrence more than 5 years after surgery was 0.6%[2]. They also showed that the incidence of recurrence in the liver more than 5 years after surgery was only 0.24%. It is also rare for CRC to have metachronous liver metastasis more than 10 years after surgery. Only a few studies in the English literature have reviewed patients who were followed for more than 10 years after the initial operation for CRC[4]. Moreover, as for now, there have been few data regarding the characteristics of the tumors with very late recurrence (≥ 10 years)[5,6]. Here, we present a case of liver metastasis detected 11 years after the curative resection of rectal cancer and report the characteristics and course of the tumor for 14 years and 9 mo after the first surgery. We also found that it was difficult to diagnose a liver metastasis preoperatively.
A 72-year-old male presented with no chief complaints.
In July 2017, the patient attended a health check-up at another hospital where he underwent an abdominal ultrasonography. A hepatic mass was detected by the ultrasonography, and intrahepatic cholangiocarcinoma (ICC) was suspected from the findings of a computed tomography (CT) scan. He was therefore referred to our department for further investigation.
In May 2006, the patient underwent a low anterior reresection for rectal cancer. The preoperative serum level of carcinoembryonic antigen (CEA) was 28.4 ng/mL (normal range, < 5.0 ng/dL). Histopathological examination showed that the tumor was a 70 mm × 50 mm type-3, moderately differentiated adenocarcinoma. According to the staging system described in the Union for International Cancer Control 8th edition[7], the pathological stage of the tumor was pT3N0M0 Stage IIA. As adjuvant chemo
The patient had no significant personal or family history.
The vitals on admission in August 2017 were within normal reference range and the patient’s physical examination was unremarkable.
Routine blood analysis on admission showed that complete blood counts, liver function, renal function, and electrolytes were within normal limits except for slightly decreased levels of hemoglobin (13.5 g/dL; normal range, 14.0-18.0 g/dL) and hematocrit (39.5%; normal range, 40.0%-52.0%) and slightly elevated levels of prothrombin time (PT) (123%; normal range, 85%-120%) (Table 1). The serum levels of CEA and CA19-9 were elevated with 1120 ng/mL and 305.9 U/mL (normal range, < 37.0 U/mL), respectively (Table 1). However, the serum levels of AFP and PIVKA-II were within normal range. This profile of tumor marker positivity was consistent with ICC.
| Patient result | |
| WBC | 6200 /μL |
| RBC | 443 × 104 /μL |
| Hb | 13.5 g/dL |
| Ht | 39.5% |
| PLT | 17.8 × 104 /μL |
| PT | 123% |
| APTT | 29.6 s |
| CRP | 0.23 mg/dL |
| AST | 26 IU/L |
| ALT | 16 IU/L |
| TP | 7.4 g/dL |
| ALB | 4.42 g/dL |
| T-Bil | 0.6 mg/dL |
| D-Bil | 0.0 mg/dL |
| CRE | 0.74 mg/dL |
| BUN | 10 mg/dL |
| Na | 136 mEq/L |
| K | 4.1 mEq/L |
| Cl | 102 mEq/L |
| CEA | 1120.0 ng/mL |
| CA19-9 | 305.9 U/mL |
| AFP | 1.9 ng/mL |
| PIVKA-II | 28 mAU/mL |
| ICG-R15 | 8% |
| Child-Pugh score | class A (5 points) |
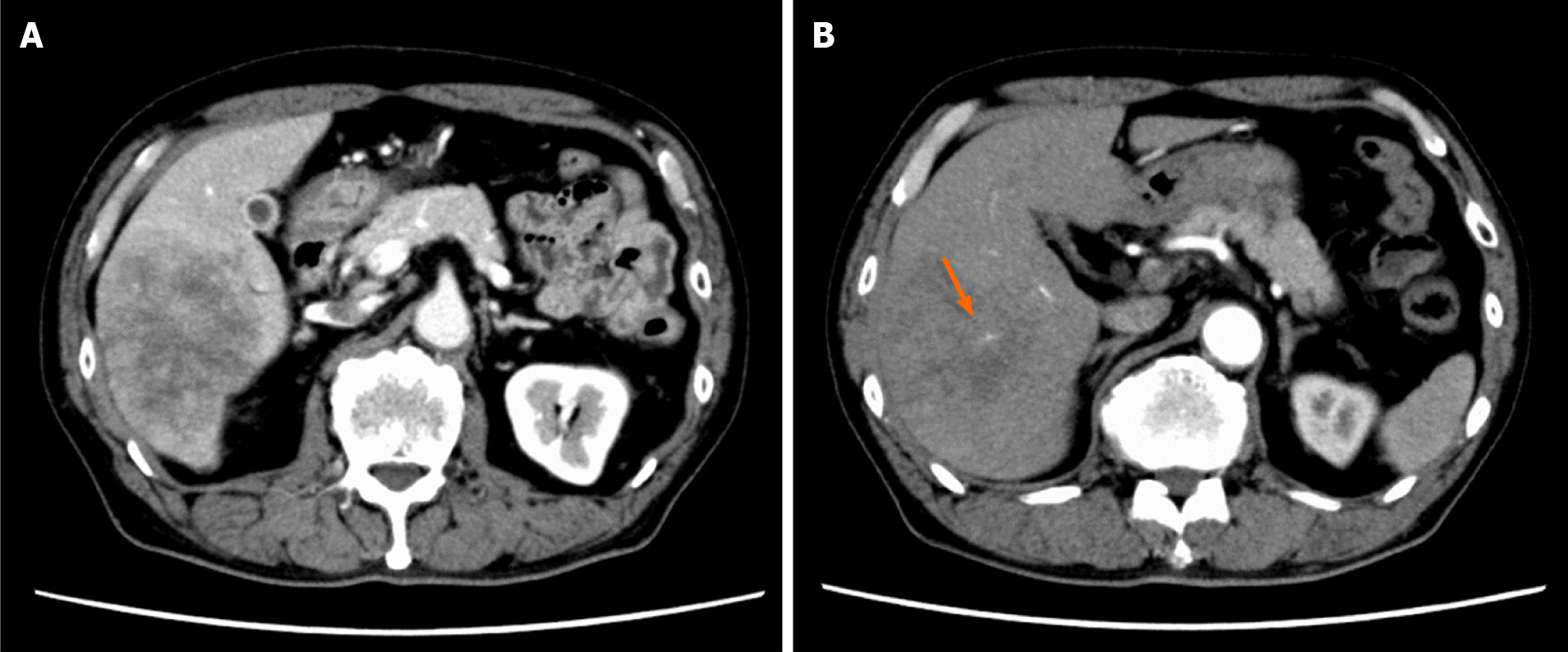
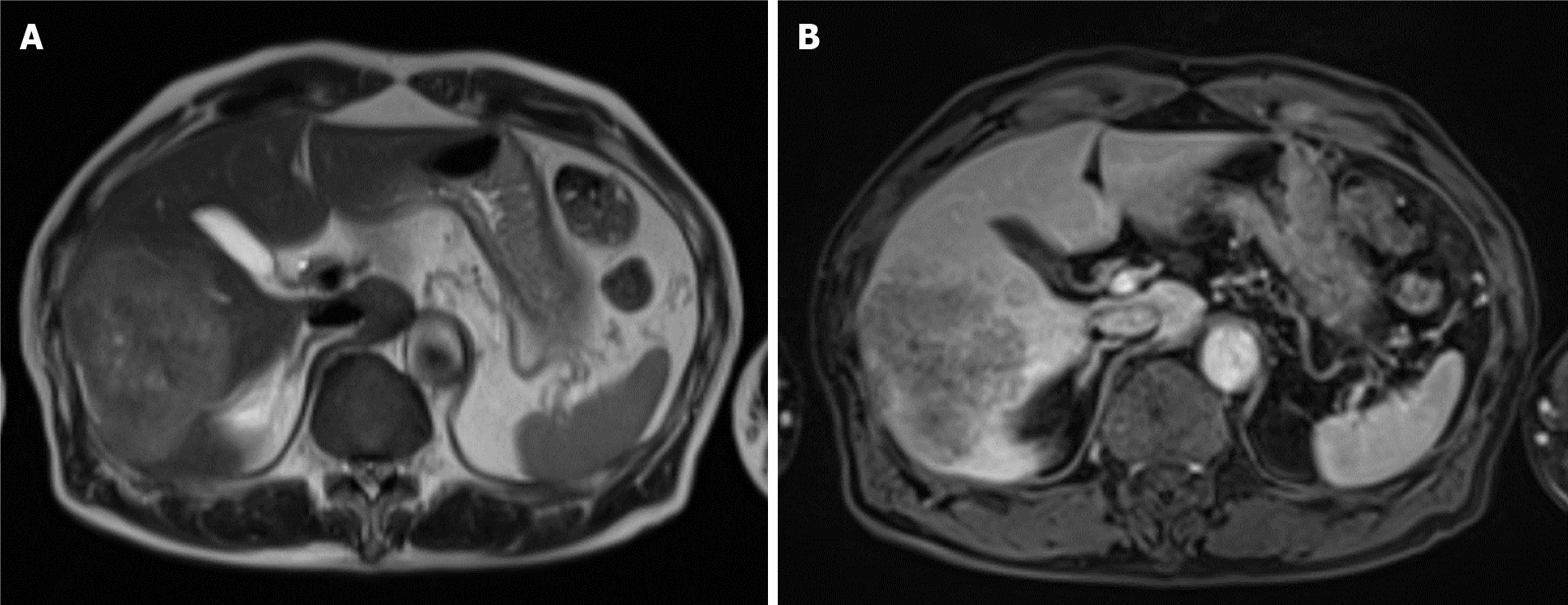
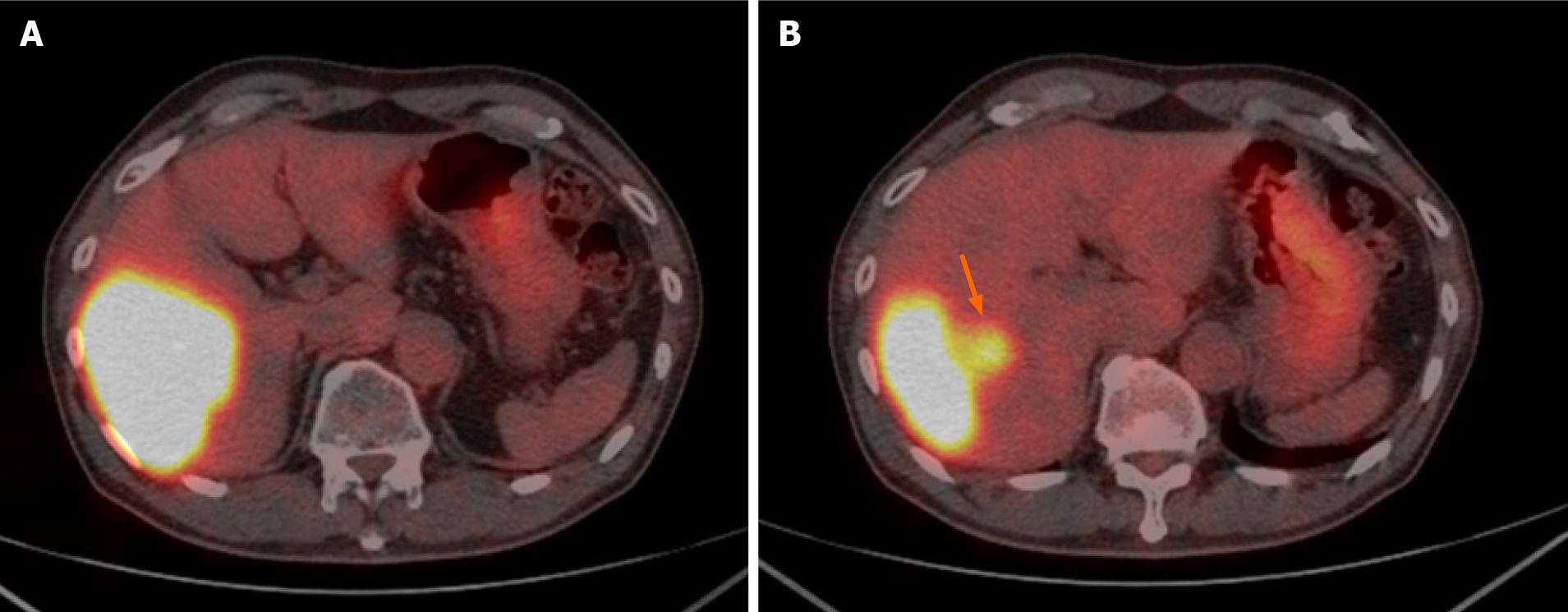
Contrast-enhanced dynamic-CT showed a tumor with a diameter of approximately 8 cm in the posterior segment of the liver which was weakly and gradually enhanced (Figure 1A). It also showed an intratumoral artery in the arterial phase (Figure 1B), suggesting that this tumor had an infiltrating replacement growth pattern, and that this finding was compatible with ICC[8]. In the magnetic resonance imaging (MRI) study, the tumor showed high signal intensity on T2-weighted images (Figure 2A) and weak enhancement compared to surrounding liver parenchyma in the portal venous phase after administration of gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) (Figure 2B). These findings were also consistent with ICC. 18F-fluorodeoxyglucose-positron emission tomography (PET)/CT showed an abnormally high uptake on the tumorous lesion in the posterior segment, with a maximum standardized uptake value (SUVmax) of 20.0 (Figure 3A). In addition, the abnormally high uptake showed that the tumor appeared to spread convexly along the intra
ICC was diagnosed based on the findings of imaging examinations and the profile of tumor marker positivity.
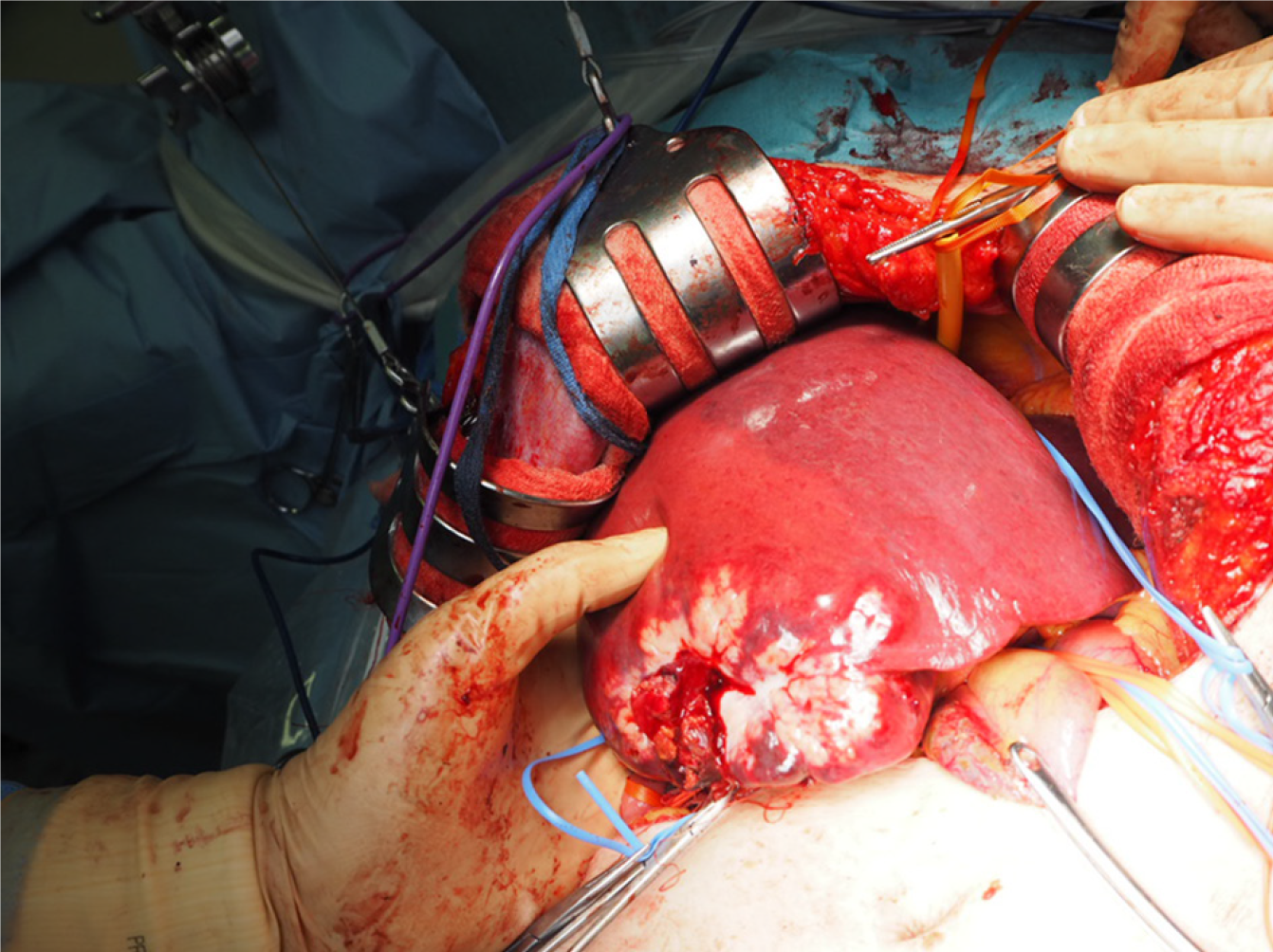
In August 2017, the patient had an extended right posterior sectionectomy, cholecystectomy, and en bloc regional lymph node dissection (Figure 4).
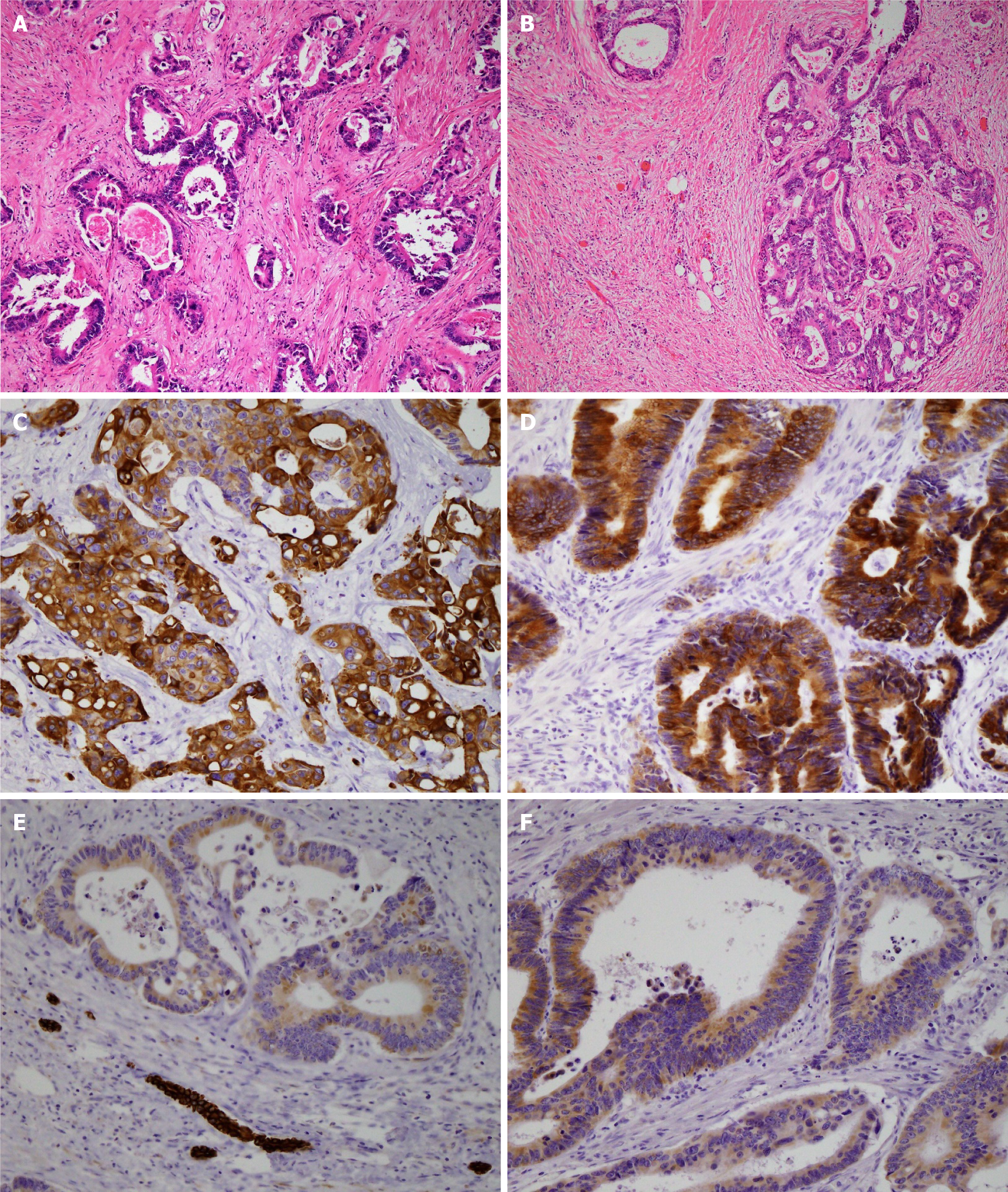
The resected specimen weighed 495 g, including a 9.0 cm × 6.0 cm × 5.4 cm tumor. Histopathological examination of the resected specimen showed that the tumor was a moderately differentiated adenocarcinoma with tubular and cribriform growth patterns, and it showed the same pathological characteristics as the rectal cancer resected 11 years earlier (Figure 5A and B). Immunohistological examination showed that the cancer cells of both the liver tumor and rectal cancer were positive for cyto
We identified two important clinical issues. CRC can develop distant metastases even 11 years after a curative operation for CRC. At diagnosis, it is difficult to differentiate a late liver metastasis of CRC from ICC preoperatively.
First, CRC can develop distant metastases even 11 years after a curative operation for CRC. Some studies have reported the characteristics of late recurrences after curative resection for CRC. Cho et al[9] reported on 18 of 1136 patients who had undergone curative colorectal resection and experienced late recurrence (> 5 years). The latest of these occurred at 8 years, and there were 3 patients with late liver metastasis. The characteristics of late recurrence of CRC could be summarized as male predominance, low level of preoperative CEA, location in the left colon or rectum, grossly polypoid type, small-sized tumor, and histopathologically well-differentiated adenocarcinoma. Lung metastasis was more frequently observed than liver metastasis. Sadahiro et al[4] reviewed 418 patients who had undergone curative resection for CRC, whose follow-up period ranged from 10 to 23 years, and the latest recurrence occurred at 7 years. They concluded that rectal cancer patients should be followed for a longer time than colon cancer patients and that adjuvant chemotherapy may prolong the interval until recurrence both in colon and rectal cancers. Frontali et al[10] showed that tumor recurrences after 5 years were noted in 15 of 218 CRC patients (6.9%), and the latest recurrence occurred at 9 years. They also showed that in rectal cancer, 69% of the late tumor recurrences were staged pN0. Seo et al[6] reported that the rate of late recurrence (> 5 years) after curative resection for CRC was 0.9% and that the latest recurrence occurred at 11 years and the location of the latest recurrence was the lung. They showed that the late recurrence group had lower preoperative CEA levels, tumors with expanding growth, tumors with a well-differentiated histological type, and lack of lymph node metastasis. They also reported that in the late recurrence group, the lung was the most common recurrence site. The characteristics of our case were male, high level of preoperative CEA, ulceroinfiltrative growth (type 3), large-sized tumor, moderately differentiated adenocarcinoma, rectum, lack of lymph node metastasis, adjuvant chemotherapy, and liver metastasis. Thus, while some factors, such as gender, tumor location, lack of lymph node metastasis, and adjuvant chemotherapy, were consistent with the previously described reports, the other factors were not consistent. Further research to accumulate data and analyze the characteristics of very late recurrence cases is needed.
Second, at diagnosis, it is difficult to differentiate a late liver metastasis of CRC from ICC preoperatively. Because the follow-up programs usually end 5 years after the curative resection, if a late metastasis is found, it will often be found as a large tumor. Thus, it can show various imaging features. In this case, the contrast-enhanced dynamic-CT showed an intratumoral artery in the arterial phase. Tsunematsu et al[8] reported that this finding suggests a tumor with an infiltrating replacement growth pattern and that the presence of an intratumoral artery in the artery phase is a predictable finding for ICC. Kozaka et al[11] showed that the growth pattern of liver tumors was classified as: (1) Fibrous encapsulation; (2) Compression growth; and (3) Infiltrating replacement. They reported that 19 of 24 peripheral ICCs showed infiltrating replacement, whereas 23 of 27 hepatocellular carcinomas (HCCs) showed fibrous encapsulation, and 17 of 24 metastatic colorectal adenocarcinoma showed compression growth. Thus, the tumor in this case was suspected to be ICC. In the MRI study of this case, the tumor showed high signal intensity on T2-weighted images and weak enhancement compared to surrounding liver parenchyma in the portal venous phase of Gd-EOB-DTPA-enhanced MRI. These findings were also consistent with ICC[12]. In the PET/CT of this case, the abnormally high uptake showed that the tumor appeared to spread convexly along the intrahepatic bile ducts. Taken together, we suspected ICC rather than hepatic metastasis. Therefore, it was very difficult to differentiate a very late liver metastasis of CRC from ICC preoperatively.
To date, although there have been some reports about follow-up more than 5 years after surgery for CRC, there have been very few reports about the history of patients with late distant metastases[4]. This case report also showed the postoperative follow-up after the resection of the liver metastasis as well as the preoperative period. The patient had no recurrence nor metastasis 3 years and 6 mo after the hepatic resection. Thus, the patient had only an isolated liver metastasis for 14 years and 9 mo in total after the curative resection for rectal cancer. This report is of importance in describing episodes during the very long-term postoperative period. The tumor in this case suggested low tumor aggressiveness. As a tumor with late recurrence generally appears to have low tumor aggressiveness, if the recurrence is operable, a curative operation should be considered. Many factors, such as the biological characteristics of the tumor, and the condition of the host, are involved in the recurrence patterns and the time to recurrence of CRC. For example, Eisenberg et al[13] reported that the majority of patients who died of cancers 5 years or more after surgery had suffered from cancer of the left colon or rectum, and therefore, cancer of the left colon or rectum was likely to be slowly progressive compared with cancers of other parts of the colon. It is important to accumulate and analyze individual cancer data of characteristics, progression pattern, and natural history. These analyses would help us to elucidate which patients with CRC should be monitored over a longer period to detect very late recurrence. Further research on oncological behavior might give new insights into identifying the high-risk group of very late recurrence.
This patient course demonstrated that CRC can develop distant metastases even 11 years after surgery and that it is difficult to differentiate a late liver metastasis of CRC from ICC preoperatively. We should note that it is possible that a liver tumor in a patient with a history of curatively resected CRC is not ICC or HCC but metastasis from CRC, even if CRC was diagnosed and resected more than 10 years earlier. Because the disease-free survival time is improving due to advances in adjuvant chemotherapy, the incidence of distant metastases at a very late stage is likely to increase.
| 1. | The Global Cancer Observatory. Available from: https://gco.iarc.fr/today/home. |
| 2. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1431] [Article Influence: 238.5] [Reference Citation Analysis (3)] |
| 3. | Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, Rafferty JF; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. Practice Guideline for the Surveillance of Patients After Curative Treatment of Colon and Rectal Cancer. Dis Colon Rectum. 2015;58:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, Mukoyama S, Yasuda S, Tajima T, Makuuchi H, Murayama C. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology. 2003;50:1362-1366. [PubMed] |
| 5. | Daniels AM, Vogelaar JFJ. Late onset pulmonary metastasis more than 10 years after primary sigmoid carcinoma. World J Gastrointest Pathophysiol. 2017;8:96-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Seo SI, Lim SB, Yoon YS, Kim CW, Yu CS, Kim TW, Kim JH, Kim JC. Comparison of recurrence patterns between ≤5 years and >5 years after curative operations in colorectal cancer patients. J Surg Oncol. 2013;108:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Brierley JD, Gospodarowicz MK, Wittekind C. CTNM classification of malignant tumours. 8th ed. Wiley-Blackwell, Chichester, 2017. |
| 8. | Tsunematsu S, Chuma M, Kamiyama T, Miyamoto N, Yabusaki S, Hatanaka K, Mitsuhashi T, Kamachi H, Yokoo H, Kakisaka T, Tsuruga Y, Orimo T, Wakayama K, Ito J, Sato F, Terashita K, Nakai M, Tsukuda Y, Sho T, Suda G, Morikawa K, Natsuizaka M, Nakanishi M, Ogawa K, Taketomi A, Matsuno Y, Sakamoto N. Intratumoral artery on contrast-enhanced computed tomography imaging: differentiating intrahepatic cholangiocarcinoma from poorly differentiated hepatocellular carcinoma. Abdom Imaging. 2015;40:1492-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Cho YB, Chun HK, Yun HR, Lee WS, Yun SH, Lee WY. Clinical and pathologic evaluation of patients with recurrence of colorectal cancer five or more years after curative resection. Dis Colon Rectum. 2007;50:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Frontali A, Benichou B, Valcea I, Maggiori L, Prost À la Denise J, Panis Y. Is follow-up still mandatory more than 5 years after surgery for colorectal cancer? Updates Surg. 2020;72:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kozaka K, Sasaki M, Fujii T, Harada K, Zen Y, Sato Y, Sawada S, Minato H, Matsui O, Nakanuma Y. A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: 'bile ductular carcinoma'. Histopathology. 2007;51:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:669-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 351] [Article Influence: 31.9] [Reference Citation Analysis (2)] |
| 13. | Eisenberg B, Decosse JJ, Harford F, Michalek J. Carcinoma of the colon and rectum: the natural history reviewed in 1704 patients. Cancer. 1982;49:1131-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhao GH S-Editor: Yan JP L-Editor: Filipodia P-Editor: Wu RR