Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8839
Peer-review started: April 30, 2021
First decision: June 6, 2021
Revised: June 18, 2021
Accepted: August 18, 2021
Article in press: August 18, 2021
Published online: October 16, 2021
Processing time: 168 Days and 5.3 Hours
Neurofibromatosis type 1 (NF1) is an inherited autosomal dominant disorder affecting many parts of the body with café au lait spots, skeletal deformity, and scoliosis. A familial case of NF1 with scoliosis and a painless mass had not yet been reported.
We describe the case of a 15-year-old male patient with a painless lump on the left side of his neck for 10 years and scoliosis. His right shoulder was about 5 cm lower than the left, the left side of his face was deformed, and the left submandibular skin was relaxed. The folding and drooping were obvious and movement was poor. Computed tomography revealed the involvement of the neck, upper chest wall, and surrounding left shoulder, accompanied by bone changes and scoliosis. Histological evaluation showed subepidermal pale blue mucoid degeneration, fibrous fusiform cells in the dermis in a fascicular, woven arrangement. His mother had the same medical history. The diagnosis was neurofibromatosis of the left neck. Various parts of the tumor tissue were serially resected during several visits. Eight months after surgery, there was a slight tendency to regrow.
This case of slow-progressing NF1 highlights the importance of early diagnosis and treatment to reduce its impact on the patient’s growth and development.
Core Tip: We report a familial case of slow-progressing neurofibromatosis type 1 with the presence of painless mass for 10 years and scoliosis. Histological evaluation of the mass revealed subepidermal pale blue mucoid degeneration and fibrous fusiform cells in the dermis in a fascicular, woven arrangement. Multiple operations were performed to remove the tumor.
- Citation: Mu X, Zhang HY, Shen YH, Yang HY. Familial left cervical neurofibromatosis 1 with scoliosis: A case report. World J Clin Cases 2021; 9(29): 8839-8845
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8839.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8839
Neurofibromatosis type 1 (NF1), also known as von Recklinghausen disease[1], is an inherited autosomal dominant disorder[2-5]. It is a common tumor predisposition syndrome[2,6,7] with a complex pathogenesis. Various studies have reported differing incidence rates, but the frequency of the disease is 1/(2500-4000)[2,3,8]. NF1 is characterized by prominent skeletal manifestations caused by the loss of the NF1 gene[3]. About 10%-25% of NF-1 patients develop bone deformities, including scoliosis, congenital arch, pseudarthrosis, bone cysts, cortical bone thinning, and subperiosteal bone hyperplasia, seriously reducing the patient's quality of life. Heterozygous inactivating mutations of the NF1 gene have multiple clinical manifestations, including café au lait spots, neurofibromas, Lisch nodules, and skin-fold freckles[6]. We report a rare case of an NF1 patient with a painless tumor on the left side of his neck for more than 10 years and scoliosis.
The patient was a 15-year-old boy with a painless tumor on the left side of his neck for more than 10 years.
His parents inadvertently found soybean-size masses on the left side of the neck of this boy at birth, with no pain and numbness. He also had a right leg deformity.
Two years previously, he was admitted to a hospital, suspected of having neurofib
The patient had no personal history. His mother had a history of the same disease.
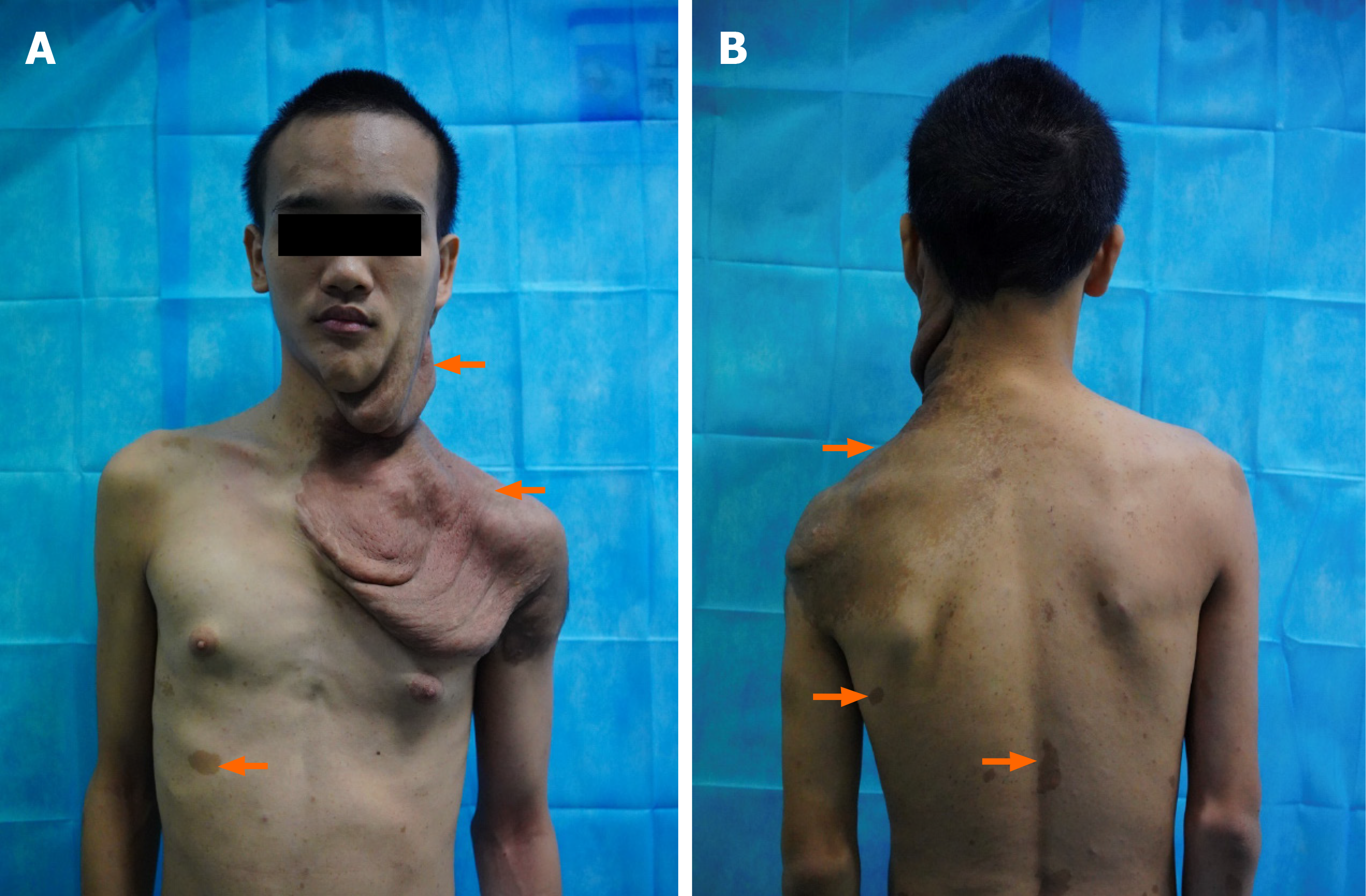
The patient came to our hospital on July 24, 2020. Physical examination showed that the right shoulder was about 5 cm lower than the left one, and the left side of the face was deformed. The left submandibular skin was relaxed, soft, and incompressible with obvious folding and drooping. Multiple superficial brown masses of varying sizes were seen in the submandibular region, the neck, and the posterior mastoid process. They were incompressible and felt like rosary nodules. The skin temperature was normal. The left earlobe adhered to the sternocleidomastoid at 2 cm, and skin pigmentation was visible on the shoulder and 1 cm behind the armpit. The skin between the left shoulder and chest was visibly relaxed, folded, and drooping. Café au lait spots of different sizes were present on the back and lower limbs. A soft, incompressible bump with a clear boundary, no adhesion, and poor motility was seen between the index finger and the middle finger on the back of the left hand (Figure 1).
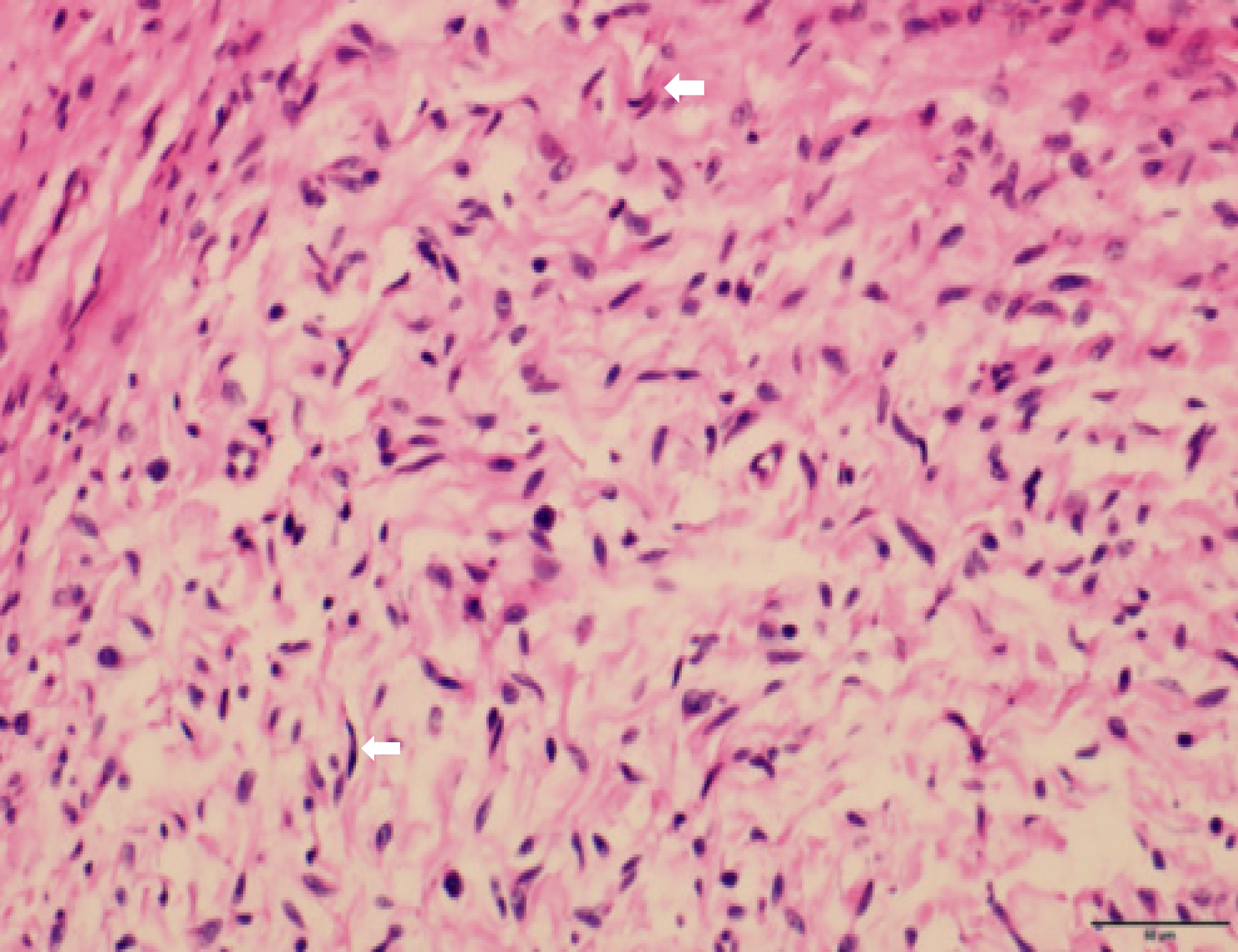
Histological examination showed subepidermal pale blue mucoid degeneration. The fibrous fusiform cells in the dermis had a fascicular and woven arrangement. The focus was the palisaded obvious boundary, wavy nucleus, and focal pigmentation. Based on the histopathological findings, the patient was diagnosed with left cervical neurofibromatosis, with short sleeves on both sides and tumor cells in the long sleeve and base margin (Figure 2). The diagnosis was confirmed through the clinical manifestations, imaging and histopathological examination, and comprehensive discussion by clinicians. The tumor tissues in the face, neck, and chest were resected under local anesthesia.
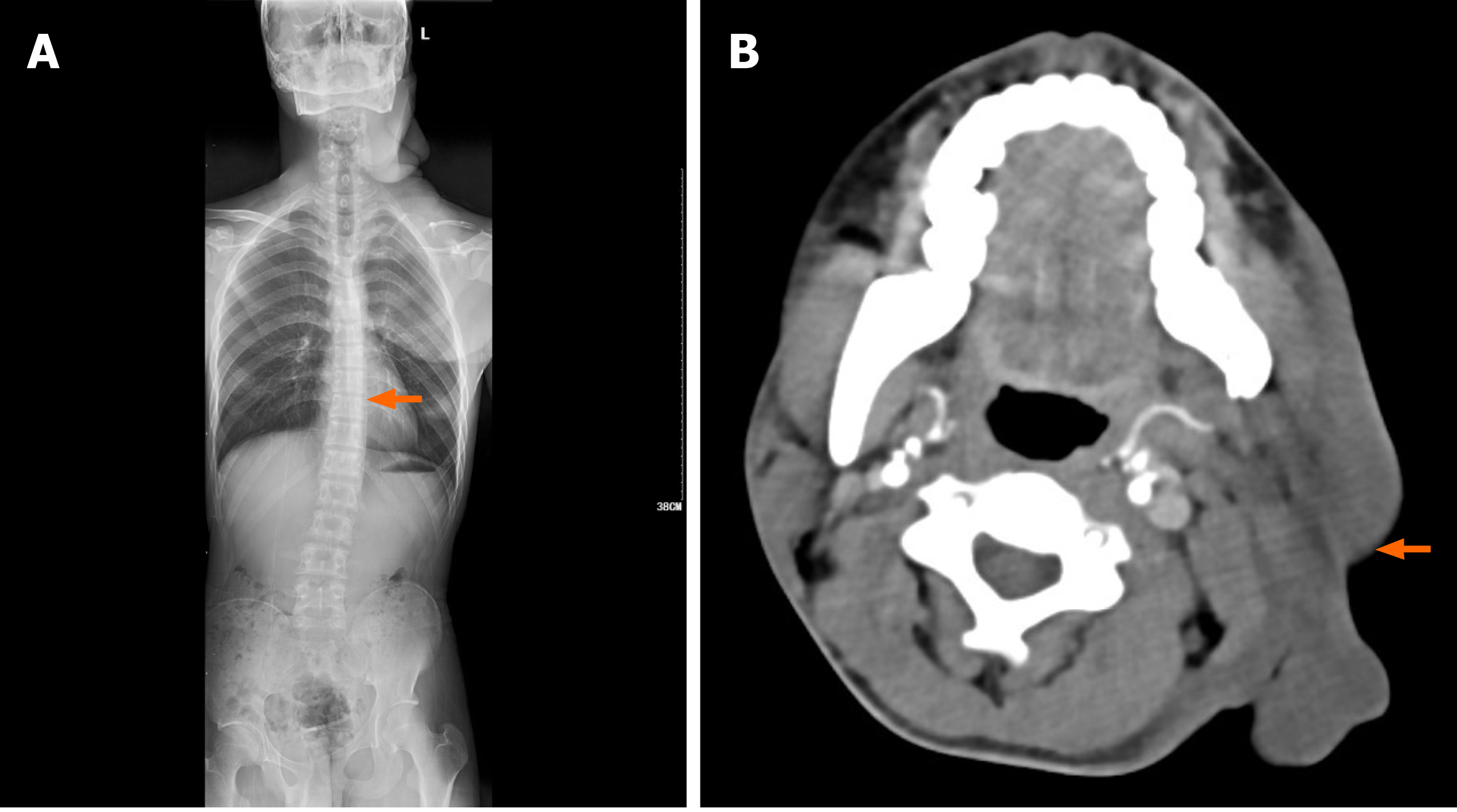
In this patient, digital radiology confirmed a diagnosis of scoliosis and an abnormal left clavicle. The CT report showed soft tissue changes of the left side of the face (Figure 3). We performed head and neck artery angiography, left thyroid trunk and intrathoracic arteriography, digital-subtraction angiography, cerebral angiography, and cerebral artery malformation embolization under local anesthesia on July 29, 2020. Arteriography was done to show the blood supply to the tumor and its characteristics to accurately evaluate the difficulty of the operation and to reduce intraoperative bleeding after embolization. The left common carotid artery, occipital artery, and branches of the facial artery supplied blood to the mass. Left subclavian arteriography showed the superior scapular, middle cerebral, and internal mammary arteries supplying blood to the chest mass. Multiple operations were planned. The left cervical neurofibroma mass was completely resected under local anesthesia on July 30, 2020 and sent for routine pathology.
The final diagnosis was left cervical neurofibromatosis.
Tumor tissues in the face, neck, and chest were resected under local anesthesia.
Eight months after surgery, follow-up showed that the patient had recovered well, but that there had been minor recurrence.
NF1 is a common neurocutaneous condition with an autosomal dominant pattern of inheritance[9]. It is caused by a germline microdeletion of the NF1 gene at 17 q11.2.29[2,8]. This large gene (60 exons and 300 kilobases of genomic DNA) has one of the highest rates of spontaneous mutation of the human genome[10]. About half the cases are familial[10,11]. A systematic genetic study by Thompson in 1900, showed that the disease was familial in 30 out of 77 reported cases[2]. NF1 cases were first reported by Wouter et al[12] in 1991. In 1994, Zhang et al[13] first reported neurofibromatosis in China. Neurofibromas are more common in children and youth but can also be seen in adults. We here report a rare familial case of NF1 with a mass on the left side of the neck for more than 10 years and scoliosis. The earliest historical evidence of NF1 appeared in the 13th century[10]. Still, it was not until Friedrich Daniel von Recklinghausen published his landmark paper (in German) on multiple fibromas of the skin and their relationship to the multiple neuromas in 1882 that neurofibromatosis gained recognition as a distinct disorder.
NF1 is a familial genetic disorder characterized by a benign tumor. NF1 patients with tumors on the body surface can be easily diagnosed by family history, clinical manifestations, and imaging findings. Café au lait spots are seen in 95% of the patients[8]. NF1 usually manifests as isolated multiple café au lait spots that appear in childhood and are highly suggestive of but not specific for NF1[5]. Other clinical manifestations of NF1 include multiple cutaneous neurofibromas, brown maculae, Lisch nodules (i.e. iris pigment hamartoma), and axillary or inguinal freckles. Some cases of neurofibromatosis have been reported with bone lesions, such as spinal, skull, and vertebral deformities[14]. Scoliosis has been reported in 10% to 26% of individuals affected with NF1 in various clinic-based series. There are two different forms, dystrophic and non-dystrophic. The dystrophic form, which is progressive and associated with vertebral scalloping and wedging, almost always develops before 10 years of age, whereas the milder non-dystrophic form of scoliosis typically occurs during adolescence[15]. A familial case of NF1 with scoliosis and a painless mass in the neck has not been reported to date. A typical tissue manifestation of neurofi
NF1 tumors are typically benign, and the main treatment is surgery. However, surgery does not provide a radical cure as even with complete surgical resection, recurrence is expected in approximately 20% of cases[17]. Recent clinical trials have evaluated mitogen-activated protein kinase inhibitors for symptomatic plexiform neurofibroma[7]. Additionally, based on cell screening with a library of known bioactive compounds, we found that protein phosphatase 2, a plaque inhibitor and the calcium channel blocker nifedipine are potential therapeutic agents for NF1[18]. Identification of additional NF1 targeting molecules and good preclinical mouse models can provide a better understanding of the clinical features of NF1 and its treatment[19]. Genetically engineered mice have additional limitations in the study of cancer, as reviewed by Watson et al[20]. Swine (Sus scrofa) models provide solutions to many of the issues. Swine have great genetic homology with humans and are more anatomically representative[21]. Genetically engineered swine models of NF1 have been established. These minipigs phenotypically display clinical features of NF1 present in patients, which is unique compared with other NF1 models. The pigs develop café au lait macules, neurofibromas, and optic pathway gliomas[21]. Importantly, tumor cells undergo spontaneous loss of heterozygosity mimicking the second-hit phenomenon that occurs in humans[21]. Holstein cattle have also been used as preclinical model for human NF[22]. Large, comparative genomic studies in human, canine, and rodent models of NF1 would be of value to help identify commonly affected and targetable pathways that may serve as drug targets or potential biomarkers for NF1 patients[22].
At present, preclinical models of genetically engineered mouse type 1 neurocytoma-related malignancies serve as a platform for evaluating rational targets and are useful in designing and implementing human clinical trials[23]. To date, the Neurofi
Clinical manifestations have always been the main diagnostic criteria for NF1. Two striking aspects of neurofibromatosis 1 are its progressive nature and its extreme variability[28]. Nf1 regulation of metabolism may affect other tissues, like bones[29]. There is an increased risk of osteoporosis and an abnormal bone turn over in NF1. Clinicians need to pay attention to the physical examination results for early diagnosis and treatment to reduce the impact of NF1 on growth and development. Additionally, about 40% of the affected patients show skeletal pathology. Scoliosis significantly affects growth and development, and its early diagnosis and treatment, including surgery and drug therapy, are important. However, more studies are required to evaluate the genetics of NF1 and its effects on angiogenesis.
Clinical manifestations have always been the main diagnostic criteria for NF1. Clinicians need to pay attention to the physical examination results for an early diagnosis and treatment to reduce the impact of NF1 on the growth and development of patients. Additionally, about 40% of the affected patients show skeletal pathology. Scoliosis significantly affects the growth and development of the patients, and therefore, its early diagnosis and treatment, including surgery and drug therapy, are important. However, more studies are required to evaluate the genetics of NF1 and its effects on angiogenesis.
We are grateful to the patient, who gave his informed consent for publication.
| 1. | Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575-578. [PubMed] |
| 2. | Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989;26:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 363] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Bok S, Shin DY, Yallowitz AR, Eiseman M, Cung M, Xu R, Li N, Sun J, Williams AL, Scott JE, Su B, Shim JH, Greenblatt MB. MEKK2 mediates aberrant ERK activation in neurofibromatosis type I. Nat Commun. 2020;11:5704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 462] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Ferrari F, Masurel A, Olivier-Faivre L, Vabres P. Juvenile xanthogranuloma and nevus anemicus in the diagnosis of neurofibromatosis type 1. JAMA Dermatol. 2014;150:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Armstrong AE, Brossier NM, Hirbe AC. Neurofibromatosis type 1-related tumours in paediatrics: an evolving treatment landscape. Lancet Child Adolesc Health. 2020;4:488-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Williams KB, Largaespada DA. New Model Systems and the Development of Targeted Therapies for the Treatment of Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Reynolds RM, Browning GG, Nawroz I, Campbell IW. Von Recklinghausen's neurofibromatosis: neurofibromatosis type 1. Lancet. 2003;361:1552-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Ferner RE, Huson SM, Thomas N, Moss C, Willshaw H, Evans DG, Upadhyaya M, Towers R, Gleeson M, Steiger C, Kirby A. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 610] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 10. | Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14; quiz 15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 307] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 11. | Ratner N, Miller SJ. A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer. 2015;15:290-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 12. | Schievink WI, Piepgras DG. Cervical vertebral artery aneurysms and arteriovenous fistulae in neurofibromatosis type 1: case reports. Neurosurgery. 1991;29:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Zhang BL, Gu LH, Bai ZX. A report of giant neurofibromatosis with deformity of right lower extremity. Zhongguo Dianzixue Chubanshe. 1994;381. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Stevenson DA, Yan J, He Y, Li H, Liu Y, Zhang Q, Jing Y, Guo Z, Zhang W, Yang D, Wu X, Hanson H, Li X, Staser K, Viskochil DH, Carey JC, Chen S, Miller L, Roberson K, Moyer-Mileur L, Yu M, Schwarz EL, Pasquali M, Yang FC. Multiple increased osteoclast functions in individuals with neurofibromatosis type 1. Am J Med Genet A. 2011;155A:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 16. | Lü D, Li MX, Ma LZ, Zhang XY, Xiao H, Chen F, Liu J, Li Z. Neurofibromatosis of the head and neck involving the mediastinum: two cases report. Shandong Daxue Erbihouyan Xuebao. 2018;32:82-86. |
| 17. | Washington EN, Placket TP, Gagliano RA, Kavolius J, Person DA. Diffuse plexiform neurofibroma of the back: report of a case. Hawaii Med J. 2010;69:191-193. [PubMed] |
| 18. | Semenova G, Stepanova DS, Deyev SM, Chernoff J. Medium throughput biochemical compound screening identifies novel agents for pharmacotherapy of neurofibromatosis type 1. Biochimie. 2017;135:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 557] [Article Influence: 61.9] [Reference Citation Analysis (1)] |
| 20. | Watson AL, Carlson DF, Largaespada DA, Hackett PB, Fahrenkrug SC. Engineered Swine Models of Cancer. Front Genet. 2016;7:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Isakson SH, Rizzardi AE, Coutts AW, Carlson DF, Kirstein MN, Fisher J, Vitte J, Williams KB, Pluhar GE, Dahiya S, Widemann BC, Dombi E, Rizvi T, Ratner N, Messiaen L, Stemmer-Rachamimov AO, Fahrenkrug SC, Gutmann DH, Giovannini M, Moertel CL, Largaespada DA, Watson AL. Genetically engineered minipigs model the major clinical features of human neurofibromatosis type 1. Commun Biol. 2018;1:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Osum SH, Watson AL, Largaespada DA. Spontaneous and Engineered Large Animal Models of Neurofibromatosis Type 1. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 358] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 24. | Gu YH, Ren JY, Li QF, Wang ZC. Angiogenesis and anti‐angiogenesis targeted therapy in neurofibroma. Zhongguo Linchuang Yixue. 2019;26:931-935. |
| 25. | Markham A, Keam SJ. Selumetinib: First Approval. Drugs. 2020;80:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Riccardi C, Perrone L, Napolitano F, Sampaolo S, Melone MAB. Understanding the Biological Activities of Vitamin D in Type 1 Neurofibromatosis: New Insights into Disease Pathogenesis and Therapeutic Design. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Friedman JM. Neurofibromatosis 1: clinical manifestations and diagnostic criteria. J Child Neurol. 2002;17:548-54; discussion 571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Brunetti-Pierri N, Doty SB, Hicks J, Phan K, Mendoza-Londono R, Blazo M, Tran A, Carter S, Lewis RA, Plon SE, Phillips WA, O'Brian Smith E, Ellis KJ, Lee B. Generalized metabolic bone disease in Neurofibromatosis type I. Mol Genet Metab. 2008;94:105-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Jalabert M, Ferkal S, Souberbielle JC, Sbidian E, Mageau A, Eymard F, Le Corvoisier P, Allanore L, Chevalier X, Wolkenstein P, Guignard S. Bone Status According to Neurofibromatosis Type 1 Phenotype: A Descriptive Study of 60 Women in France. Calcif Tissue Int. 2021;108:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Velikova TV S-Editor: Wang LL L-Editor: Filipodia P-Editor: Xing YX