Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8729
Peer-review started: June 6, 2021
First decision: June 25, 2021
Revised: July 7, 2021
Accepted: July 22, 2021
Article in press: July 22, 2021
Published online: October 16, 2021
Processing time: 130 Days and 21.9 Hours
Hypotension after the induction of anesthesia is known to be associated with various adverse events. The involvement of a series of factors makes the prediction of hypotension during anesthesia quite challenging.
To explore the ability and effectiveness of a random forest (RF) model in the prediction of post-induction hypotension (PIH) in patients undergoing cardiac surgery.
Patient information was obtained from the electronic health records of the Second Affiliated Hospital of Hainan Medical University. The study included patients, ≥ 18 years of age, who underwent cardiac surgery from December 2007 to January 2018. An RF algorithm, which is a supervised machine learning technique, was employed to predict PIH. Model performance was assessed by the area under the curve (AUC) of the receiver operating characteristic. Mean decrease in the Gini index was used to rank various features based on their importance.
Of the 3030 patients included in the study, 1578 (52.1%) experienced hypotension after the induction of anesthesia. The RF model performed effectively, with an AUC of 0.843 (0.808-0.877) and identified mean blood pressure as the most important predictor of PIH after anesthesia. Age and body mass index also had a significant impact.
The generated RF model had high discrimination ability for the identification of individuals at high risk for a hypotensive event during cardiac surgery. The study results highlighted that machine learning tools confer unique advantages for the prediction of adverse post-anesthesia events.
Core Tip: This was a retrospective study intended to develop a prediction model for hypotensive events after anesthesia during cardiac surgery. A random forest machine learning technique was used to establish a predictive algorithm using preoperative data. “Features ranked by importance” were also identified in this study. This novel prediction model can be used to predict hypotension events and help to avoid the occurrence of any potential adverse events.
- Citation: Li XF, Huang YZ, Tang JY, Li RC, Wang XQ. Development of a random forest model for hypotension prediction after anesthesia induction for cardiac surgery. World J Clin Cases 2021; 9(29): 8729-8739
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8729.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8729
Post-induction hypotension (PIH), defined as hypotension after the induction of anesthesia, is a common event that is often associated with unfavorable patient outcomes. In certain cases, it might cause adverse consequences including longer hospital stays and even death[1]. In cardiac surgery, PIH is known to be associated with postoperative adverse events[2]. During surgical procedures, anesthesiologists take all necessary precautions to ensure careful administration of medication, however, PIH can occur without warning, and is usually followed by a series of adverse events including acute kidney injury, myocardial infarction, and sometimes mortality within 30 d[3]. Therefore, devising effective strategies for early detection of intraoperative hypotension along with preventive measures could help anesthesiologists to improve the outcomes of anesthesia and surgical procedures. A variety of complex factors are known to contribute to PIH, including age, preinduction systolic blood pressure (SBP), comorbidities, and preoperative medication[4]. The existence of interlocking relationships between different factors limits the utility of simple al
In the last 30-40 years, advances in machine learning have revolutionized the field of medicine. Additionally, recent progress in the field of computing power and big-data processing ensured breakthroughs in clinical data analysis[5]. In general, machine learning approaches are focused on utilizing multidimensional data and computational methods to generalize predictions about individuals. The random forest (RF) model, first proposed by Breiman, is a robust model algorithm of machine learning that is capable of synthesizing and analyzing all kinds of datasets[6]. Complex nonlinear interactions among variables are managed by computers to minimize the inconsistencies between the observed and the predicted results, thereby ensuring increased accuracy of disease prediction[7]. The formation of multiple decision trees allows the transfer of all patient features to the trees, and a final classification is generated in terms of “voting” by decision trees. A prediction model based on an RF algorithm has been previously applied to several fields of clinical medicine. In particular, it has been used for the prediction of cardiovascular disease[8], the clinical outcome after aneurysm rupture at the time of discharge[9], diagnosis of polycystic ovary syndrome[10], and the effect of chemotherapy on patient tumors[11]. However, very few studies have reported the use of RF for the prediction of PIH in patients undergoing cardiac surgery. This study aimed to use the RF algorithm as a powerful tool to learn feature presentations and establish a prediction model for PIH in patients subject to cardiac surgery. The study involved the construction of a predictive RF model for PIH events based on patient characteristics. An RF classifier was used to identify key characteristics, and a ranking structure was established to define the importance of various features using the algorithm rules. The accuracy and superiority of the generated diagnostic RF model were evaluated by analysis of the area under the curve (AUC).
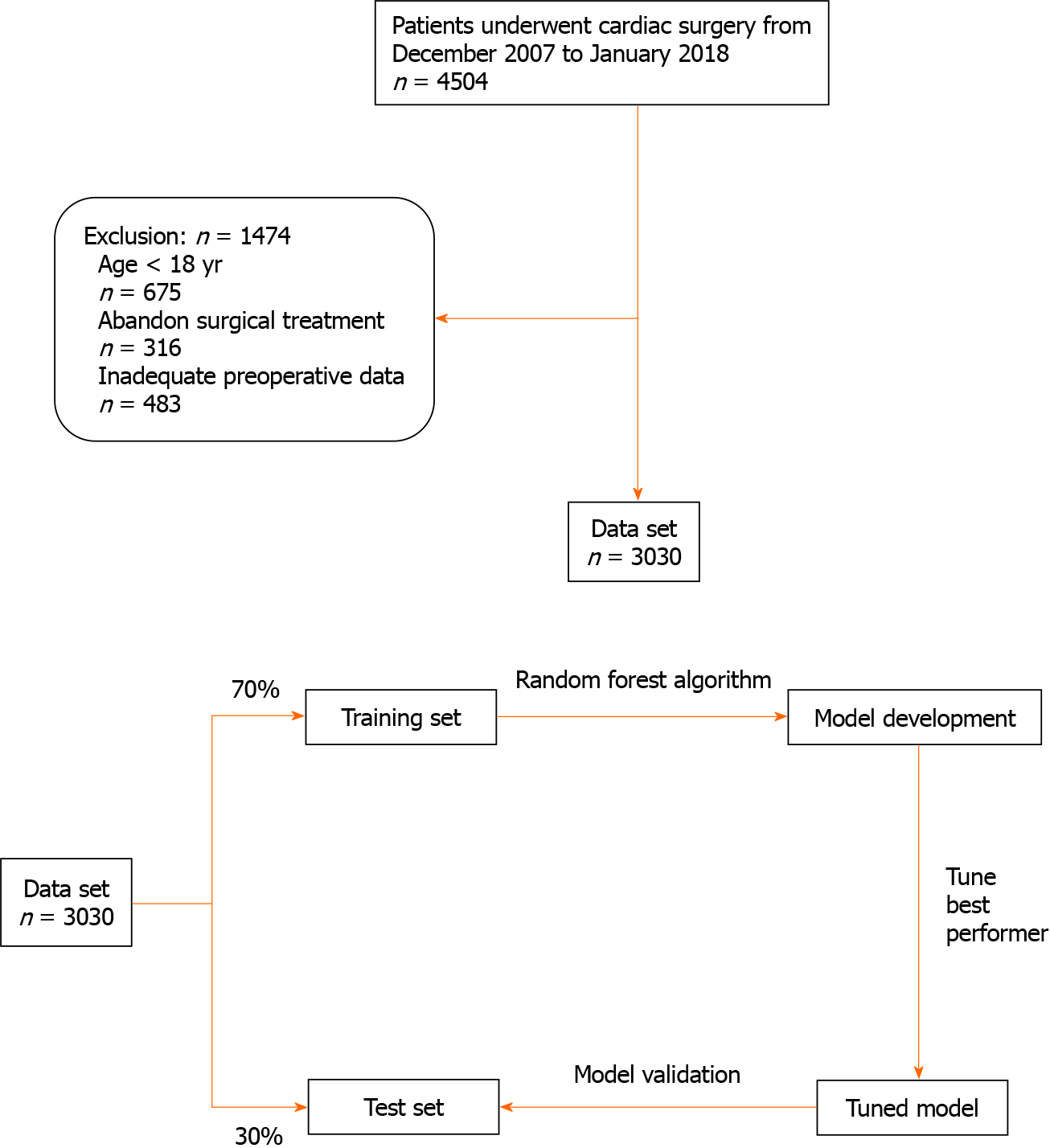
A flow chart of the study protocol is shown in Figure 1. The electronic medical records of a total of 3030 consecutive patients treated from December 2007 to January 2018 were screened. Those who were younger than 18 years of age, had abandoned surgical treatment, and those with incomplete preoperative data were excluded. The patients included in the study database were divided into two subsets, 70% were allocated to the RF model training subset and 30% were allocated to the testing subset for model validation. An RF algorithm was used to establish the PIH prediction model. The definition of PIH included an SBP threshold of < 90 mmHg or a mean blood pressure (MBP) of < 65 mmHg.
We collected preoperative and perioperative variables from electronic health records. Table 1 summarizes the data of the training and test sets. Preoperative variables included age, sex, body mass index (BMI), underlying disease, EuroSCORE I, and the American Society of Anesthesiologists (ASA) score. Experimental findings included hemoglobin, serum creatinine, and total bilirubin. Data on the patient’s preoperative medications, such as the use of beta blockers, insulin, and aspirin were also included. Intraoperative medications were introduced after entering the operating room until 10 min after the induction of anesthesia. Data on perioperative blood pressure were extracted from the anesthesia information management system. The obtained data were identical to those in the electronic health records.
| Variable | Training set | Test set | P value |
| Patient population, n | 2121 | 909 | |
| Age (yr) | 63 (53-70) | 62 (53-69) | 0.323 |
| Male, n (%) | 1444 (68.1) | 591 (65.0) | 0.307 |
| BMI (kg/m2) | 24.8 (17.8-29.8) | 24.8 (17.3-28.9) | 0.956 |
| Smoking, n (%) | 775 (36.5) | 328 (36.1) | 0.937 |
| Surgery type, n (%) | |||
| CABG only | 750 (35.4) | 378 (41.6) | 0.128 |
| Valve surgery only | 1113 (52.5) | 455 (50.0) | 0.560 |
| CABG + valve surgery | 258 (12.2) | 76 (8.4) | 0.156 |
| Previous cardiac surgery, n (%) | 267 (12.6) | 166 (18.3) | 0.052 |
| Preoperative ventilator support, n (%) | 148 (7.0) | 54 (5.9) | 0.603 |
| Preoperative IABP, n (%) | 45 (2.1) | 40 (4.5) | 0.096 |
| Diabetes mellitus, n (%) | 687 (32.4) | 284 (31.2) | 0.756 |
| Dyslipidemia, n (%) | 800 (37.7) | 365 (40.1) | 0.564 |
| Cerebrovascular accident, n (%) | 123 (5.8) | 54 (5.9) | 0.926 |
| Myocardial infarction within 90 d, n (%) | 329 (15.5) | 135 (14.9) | 0.879 |
| Serum creatinine (mg/dL) | 0.91 (0.71-1.16) | 0.90 (0.70-1.14) | 0.128 |
| Hemoglobin (g/dL) | 13.2 (11.4-14.6) | 13.1 (11.4-14.3) | 0.403 |
| Preoperative EF (%) | 62.0 (35.0-70.0) | 61.0 (30.0-71.3) | 0.323 |
| ASA score, n (%) | |||
| Class I | 21 (1.5) | 14 (1.5) | 0.994 |
| Class II | 380 (17.9) | 157 (17.3) | 0.856 |
| Class III | 1525 (71.9) | 666 (73.3) | 0.708 |
| Class IV | 172 (8.1) | 58 (6.4) | 0.455 |
| EuroSCORE I | 3.2 (0.83-8.95) | 2.96 (0.67-9.65) | 0.355 |
| Beta blocker use, n (%) | 959 (45.2) | 383 (42.1) | 0.455 |
| Aspirin use, n (%) | 575 (27.1) | 243 (26.7) | 0.926 |
| Dobutamin use, n (%) | 19 (0.9) | 14 (1.5) | 0.460 |
| Insulin use, n (%) | 99 (4.7) | 76 (8.4) | 0.059 |
| Systolic blood pressure | 111 (88-154.8) | 119 (95-165.5) | 0.658 |
| Diastolic blood pressure | 73 (55-84) | 75 (58-89) | 0.537 |
| Mean arterial pressure | 93 (71-119) | 108 (68-121) | 0.437 |
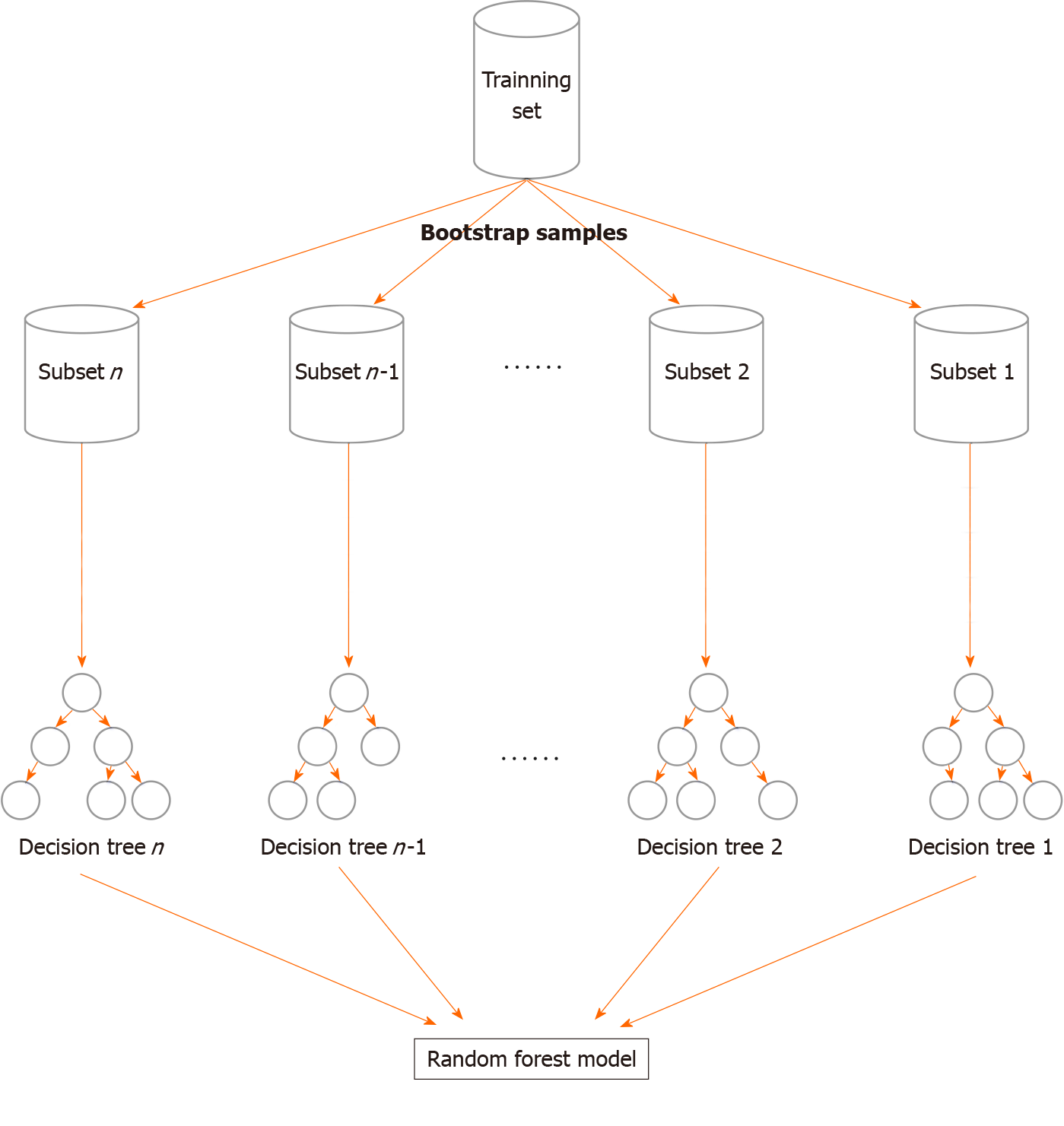
The study used an RF algorithm, which is a supervised machine learning algorithm. Briefly, RF is based on multiple decision trees that are merged to obtain a stable and accurate prediction[12]. To develop trees, RF uses a resampling technique. The classification was generated based on voting by trees, as per the specific value for the variable. Finally, the model was predicted based on majority votes[13]. Similar to a tree structure, the RF model consisted of roots, branches, and leaves that reflected the derivation of the relationship of features and events. During the construction of the RF model, the database was randomly divided into N subsets, each of which developed a tree-like structure based on the incorporated variables. Following the definition of information gain, variables were given an entropy value after being processed within the tree. As shown in Figure 2, the variables that achieved peak values were placed at the top node location, closest to the root of the tree[8]. The value of a variable was indicated by the mean decrease in the Gini (MDG) index[14]. It has been established that the higher the index value, the more important is the value given by the variable. To evaluate the performance of the RF model, the AUC of the receiver operating characteristic (ROC) curve was calculated. The Scikit-learn package (https://scikit-learn.org/stable/) was used for the development of the RF model.
Python 2.7 was used for the data analysis. Continuous variables were reported as means ± SD, and between-group differences were evaluated by a t-tests. Categorical variables were reported as n (%) with and differences were evaluated by Fisher’s exact tests. P < 0.05 was considered to be the threshold of statistical significance.
The records of 3030 patients who underwent cardiac surgery between December 2007 and January 2018, were obtained from the electronic medical record system. The patients were 62 ± 8.9 years of age, 2035 (67.2%) were men, 1103 (36.4%) were smokers, 818 (27.0%) took aspirin, 971 (32.0%) had diabetes, and 175 (5.8%) used insulin. Coronary artery bypass graft surgery was performed in 1128 patients (37.2%), valve replacement in 1568 (51.7%), and coronary artery bypass plus valve surgery in 334 (11.0%) patients. The patients were randomly assigned to training and testing subsets in a ratio of 7:3. The baseline characteristics for the training and testing sets are summarized in Table 1. Importantly, no statistically significant differences between the two sets were recorded (P > 0.05).
The data obtained from the anesthesia information management system revealed that PIH was reported in 1578 patients (52.1%) during anesthesia before surgery. The incidence of PIH was 51.3% in the training set (1088/2121) and 53.9% in the test set.
For the prediction of PIH, an RF model was generated using a total of 2121 training samples, with all the variables used as input variables. A schema of the construction of the RF model is shown in Figure 2. Briefly, the RF model was an integrated algorithm composed of multiple decision trees. It was characterized by anti-overfitting and anti-noise abilities. During training, a 10-fold cross-validation strategy was adopted. The RF model adopted the bootstrap sampling method in which N participants were randomly selected from the dataset and included in the training subset. The remaining participants were categorized as the testing subset. Each split was forced to consider only a subset of predictors, which allowed all predictors to reveal their importance. The final determination of the model predictions was based on the majority votes.
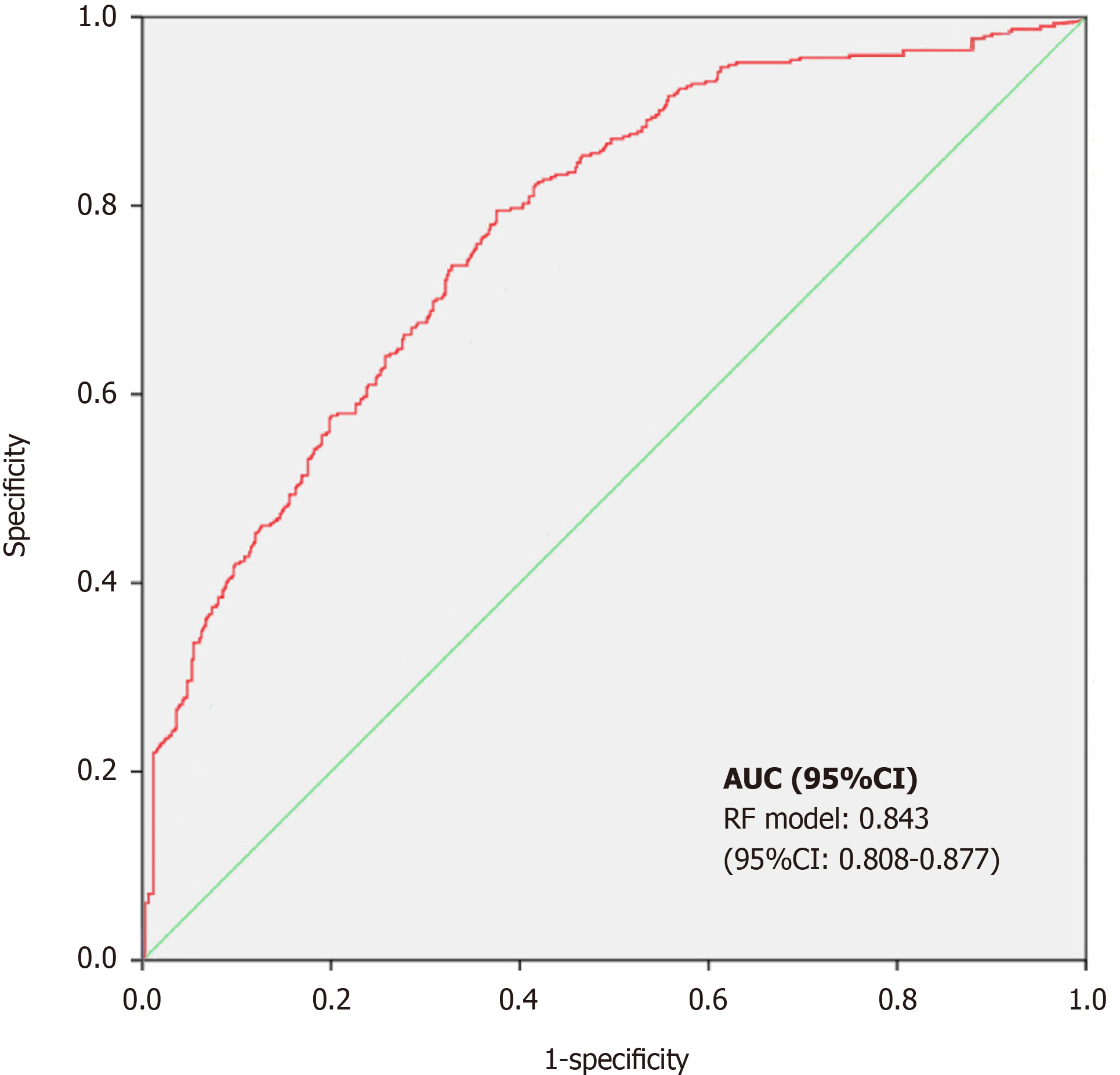
To evaluate the effectiveness of the prediction model, 909 participants from the testing subset were used. The accuracy of the RF model was 83.1%, the sensitivity was 78.8%, and the specificity was 85.6%. ROC curve analysis was used to evaluate the effectiveness of the prediction model. The horizontal and vertical coordinates of this curve represented the sensitivity and 1 − specificity, respectively. For the ROC curve, the AUC is 0.50 when calculated by random prediction. An AUC value of 1 represents 100% identification, and an AUC > 0.8 indicates that the model is characterized by a high degree of discrimination. As a general rule, a higher value of AUC is associated with better performance of the model. In this study, an RF-based risk assessment model was developed for PIH prediction in patients, during cardiac surgery. The RF model could assist the doctors in the identification of patients at increased risk of experiencing PIH during surgery. The ROC-AUC coupled with sensitivity and specificity is shown in Figure 3. In terms of the effectiveness of the model to predict PIH, RF showed a high discrimination capacity (AUC = 0.843; 95%CI: 0.808-0.877).
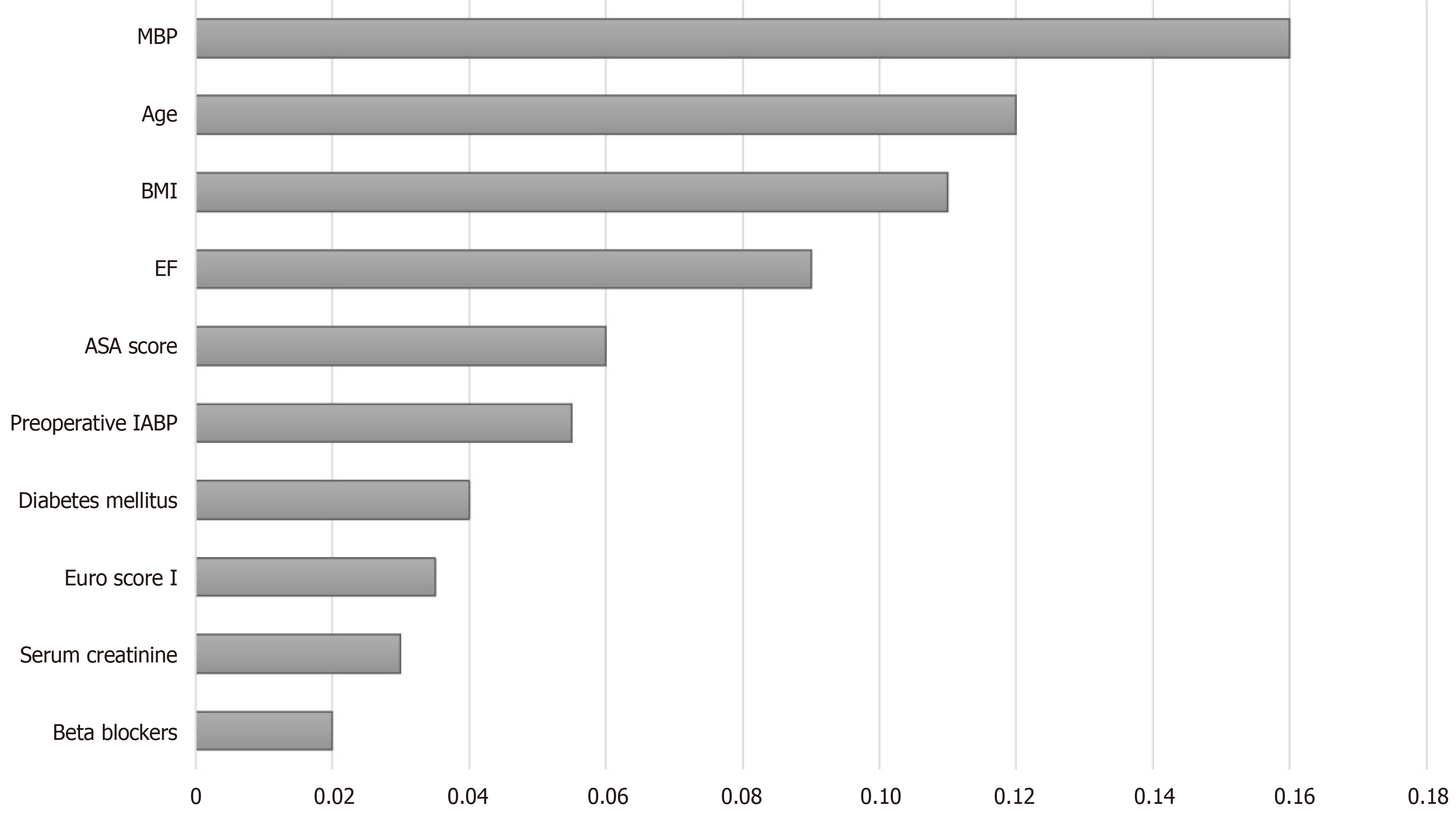
The ranked importance of features in the RF model was determined by the MDG index. The variables were ranked both by the mean decrease in accuracy and the Gini index. As shown in Figure 4, the 10 most important variables were MBP, age, BMI, ejection fraction, ASA score, preoperative use of an intra-aortic balloon pump, diabetes mellitus, EuroSCORE I, serum creatinine, and beta blocker use. Among the variables, MBP had the most significant impact, followed by age. Interestingly, the indicator of the pumping capacity of the heart ranked fourth.
Several machine learning approaches have been previously developed and used in the field of anesthesia, particularly for postoperative pain management[15,16] and assessment of patient use of analgesic pumps[17]. In this retrospective study, the application of the RF model in predicting PIH events during cardiac surgery was investigated using preoperative and perioperative variables. The final model, ge
PIH is a common event, with a prevalence of approximately 20%[21]. PIH is known to be associated with significant postoperative adverse events. In a previous study, Maheshwari et al[22] reported the occurrence of PIH after induction of anesthesia, which resulted in an increased postoperative hospital stay and even death. In
Ranking of the importance of various features showed that MBP was the most significant contributor to the prediction of PIH in cardiac surgery. The importance of MBP in this study seems reasonable, as the anesthesiologist usually tends to predict the blood pressure via monitoring of circulatory system dynamics during anesthesia induction. Patient age was found to be the second most important predictor, which might have reflected the high vulnerability of old individuals to hypotensive events compared with young patients. Among the other characteristics, EF ranked fourth in the impact on PIH prediction. It is physiologically plausible for patients who un
The RF model integrated various characteristic features of individuals, and thus displayed an effective ability to identify patients at increased risk for PIH events. It is well established that effective monitoring of vital signs, maintenance of circulatory stability, and rapid response along with intervention are effective measures that help to reduce adverse complications associated with surgery. However, despite the use of sophisticated modern equipment by experienced anesthesiologists, the monitoring of hemodynamic changes in blood flow and early detection of poor performance is not easy. Therefore, it is clinically important to develop a diagnostic alternative tool that leverages the available information to provide accurate judgments that assist in clinical decision making. In the past few years, machine learning has gained immense attention in the field of clinical and medical research. Recent studies that utilized machine learning, reported a relationship between neural networks and hemodynamic changes[23-25]. The widely established electronic medical record system includes a large amount of clinically relevant information about each individual and provides a rich database for the development of a prediction model. The application of an RF-based model ensures effective processing and analysis of such large datasets. Fur
This study had some limitations. The retrospective nature of the study makes it vulnerable to selection bias, and also makes it impossible to identify a causal link. As the quality of data obtained from the database might affect the prediction performance of the RF model, some factors might have been excluded from the analysis, which might have changed the prediction results. The study only included patients who underwent cardiac surgery with total intravenous anesthesia administered by an infusion pump. There might thus be some discrepancies in the results obtained from patients with noncardiac surgery. The study did not include external validation of the generated model. Future research should focus on the integration of the model with patient electronic health data, medical information, and imaging examination to construct a real-time clinical decision-support system.
Several previous studies have reported better performance of RF models compared with traditional models, particularly in the prediction of mortality in cardiac surgery[27]. In this study, RF modeling had a high competence in predicting PIH in cardiac surgery. The incorporation of perioperative variables into machine learning modeling might improve the ability to identify patients at increased risk of PIH during cardiac surgery. In this era of personalized medicine, precise machine learning modeling based on accessible individual characteristics might provide an opportunity for early intervention for PIH management by anesthesiologists.
Hypotension, which most often occurs on the induction of anesthesia, is known to lead to the development of adverse events and poor outcomes in patients following surgery. However, risk scores based on conventional logistic regression analysis have a low ability to discriminate characteristics that influence the development of post-induction hypotension (PIH) events.
Recently, a model based on machine learning techniques was reported to effectively predict or actively monitor events of interest by using variables in medical records datasets.
We attempted to construct a random forest (RF) model for the prediction of PIH events by using electronic information in a patient records dataset.
Data were acquired from the electronic dataset of the Second Affiliated Hospital of Hainan Medical University. A RF model based on an up-to-date machine learning algorithm was used to predict post-anesthesia hypotension in patients during cardiac surgery.
Of the 3030 patients analyzed, 1578 (52.1%) experienced hypotensive events after anesthesia. The RF model had a high predictive performance, with an AUC of 0.843 (0.808-0.877). The most important variable attributing to the accuracy of hypotension prediction after anesthesia in the RF model was the mean blood pressure, followed by age and body mass index.
RF technology can accurately predict PIH in patients following cardiac surgery.
In the era of individualized medicine, precise machine learning modeling based on accessible patient information may offer anesthesiologists an opportunity for early intervention in PIH events.
| 1. | Wong GTC, Irwin MG. Post-induction hypotension: a fluid relationship? Anaesthesia. 2021;76:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Maheshwari A, McCormick PJ, Sessler DI, Reich DL, You J, Mascha EJ, Castillo JG, Levin MA, Duncan AE. Prolonged concurrent hypotension and low bispectral index ('double low') are associated with mortality, serious complications, and prolonged hospitalization after cardiac surgery. Br J Anaesth. 2017;119:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Khan AI, Fischer M, Pedoto AC, Seier K, Tan KS, Dalbagni G, Donat SM, Arslan-Carlon V. The impact of fluid optimisation before induction of anaesthesia on hypotension after induction. Anaesthesia. 2020;75:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Südfeld S, Brechnitz S, Wagner JY, Reese PC, Pinnschmidt HO, Reuter DA, Saugel B. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H. eDoctor: machine learning and the future of medicine. J Intern Med. 2018;284:603-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 585] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 6. | Shen W, Guo Y, Wang Y, Zhao K, Wang B, Yuille A. Deep Differentiable Random Forests for Age Estimation. IEEE Trans Pattern Anal Mach Intell. 2021;43:404-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Rahman SA, Walker RC, Maynard N, Trudgill N, Crosby T, Cromwell DA, Underwood TJ; NOGCA project team AUGIS. The AUGIS Survival Predictor: Prediction of Long-term and Conditional Survival after Esophagectomy Using Random Survival Forests. Ann Surg. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Yang L, Wu H, Jin X, Zheng P, Hu S, Xu X, Yu W, Yan J. Study of cardiovascular disease prediction model based on random forest in eastern China. Sci Rep. 2020;10:5245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 9. | Xia N, Chen J, Zhan C, Jia X, Xiang Y, Chen Y, Duan Y, Lan L, Lin B, Chen C, Zhao B, Chen X, Yang Y, Liu J. Prediction of Clinical Outcome at Discharge After Rupture of Anterior Communicating Artery Aneurysm Using the Random Forest Technique. Front Neurol. 2020;11:538052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Xie NN, Wang FF, Zhou J, Liu C, Qu F. Establishment and Analysis of a Combined Diagnostic Model of Polycystic Ovary Syndrome with Random Forest and Artificial Neural Network. Biomed Res Int. 2020;2020:2613091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Liu D, Zhang X, Zheng T, Shi Q, Cui Y, Wang Y, Liu L. Optimisation and evaluation of the random forest model in the efficacy prediction of chemoradiotherapy for advanced cervical cancer based on radiomics signature from high-resolution T2 weighted images. Arch Gynecol Obstet. 2021;303:811-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Sun J, Zhong G, Huang K, Dong J. Banzhaf random forests: Cooperative game theory based random forests with consistency. Neural Netw. 2018;106:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Pavey TG, Gilson ND, Gomersall SR, Clark B, Trost SG. Field evaluation of a random forest activity classifier for wrist-worn accelerometer data. J Sci Med Sport. 2017;20:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Su X, Xu Y, Tan Z, Wang X, Yang P, Su Y, Jiang Y, Qin S, Shang L. Prediction for cardiovascular diseases based on laboratory data: An analysis of random forest model. J Clin Lab Anal. 2020;34:e23421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Tighe PJ, Lucas SD, Edwards DA, Boezaart AP, Aytug H, Bihorac A. Use of machine-learning classifiers to predict requests for preoperative acute pain service consultation. Pain Med. 2012;13:1347-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Tighe PJ, Harle CA, Hurley RW, Aytug H, Boezaart AP, Fillingim RB. Teaching a Machine to Feel Postoperative Pain: Combining High-Dimensional Clinical Data with Machine Learning Algorithms to Forecast Acute Postoperative Pain. Pain Med. 2015;16:1386-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Hu YJ, Ku TH, Jan RH, Wang K, Tseng YC, Yang SF. Decision tree-based learning to predict patient controlled analgesia consumption and readjustment. BMC Med Inform Decis Mak. 2012;12:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Shi HY, Lee KT, Lee HH, Ho WH, Sun DP, Wang JJ, Chiu CC. Comparison of artificial neural network and logistic regression models for predicting in-hospital mortality after primary liver cancer surgery. PLoS One. 2012;7:e35781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 19. | Wise ES, Hocking KM, Brophy CM. Prediction of in-hospital mortality after ruptured abdominal aortic aneurysm repair using an artificial neural network. J Vasc Surg. 2015;62:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Wang H, Jin Y. A Random Forest-Assisted Evolutionary Algorithm for Data-Driven Constrained Multiobjective Combinatorial Optimization of Trauma Systems. IEEE Trans Cybern. 2020;50:536-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 1066] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 22. | Maheshwari K, Turan A, Mao G, Yang D, Niazi AK, Agarwal D, Sessler DI, Kurz A. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73:1223-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 23. | Kendale S, Kulkarni P, Rosenberg AD, Wang J. Supervised Machine-learning Predictive Analytics for Prediction of Postinduction Hypotension. Anesthesiology. 2018;129:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 24. | Hatib F, Jian Z, Buddi S, Lee C, Settels J, Sibert K, Rinehart J, Cannesson M. Machine-learning Algorithm to Predict Hypotension Based on High-fidelity Arterial Pressure Waveform Analysis. Anesthesiology. 2018;129:663-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 380] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 25. | Kang AR, Lee J, Jung W, Lee M, Park SY, Woo J, Kim SH. Development of a prediction model for hypotension after induction of anesthesia using machine learning. PLoS One. 2020;15:e0231172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Lee HC, Jung CW. Vital Recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Sci Rep. 2018;8:1527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 27. | Allyn J, Allou N, Augustin P, Philip I, Martinet O, Belghiti M, Provenchere S, Montravers P, Ferdynus C. A Comparison of a Machine Learning Model with EuroSCORE II in Predicting Mortality after Elective Cardiac Surgery: A Decision Curve Analysis. PLoS One. 2017;12:e0169772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Birtolo LI S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yuan YY