Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8616
Peer-review started: May 29, 2021
First decision: June 14, 2021
Revised: June 16, 2021
Accepted: August 6, 2021
Article in press: August 6, 2021
Published online: October 6, 2021
Processing time: 121 Days and 12.5 Hours
Primary intramedullary melanocytoma is an exceedingly rare type of primary melanocytic tumor in the central nervous system. Unfortunately, primary intramedullary melanocytoma lacks specificity in clinical symptoms and imaging features and there is currently no standard strategy for diagnosis or treatment.
A 52-year-old male patient suffered from weakness and numbness involving the bilateral lower limbs for 18 mo, and defecation and erectile dysfunction for 6 mo. Furthermore, these symptoms started to worsen for the last 3 mo. Preoperative magnetic resonance imaging (MRI) revealed an intramedullary tumor located at the T9-T10 level. In subsequently surgery, the maximal safe resection extent approached to 98%. The lesion was confirmed to be melanocytoma by pathological examination. In addition, the possibility of original melanocytoma outside the spinal cord was excluded after the examination of the whole body. Therefore, a diagnosis of primary intramedullary melanocytoma was established. The patient refused to accept radiotherapy or Gamma Knife, but MRI examination on July 28, 2020 showed no sign of development. In addition, on April 10, 2021, the recent review showed that the disorder of defecation and lower limbs improved further but erectile dysfunction benefited a little from the surgery.
After diagnosing intramedullary melanocytoma by postoperative pathology, the inspection of the whole body contributed to excluding the possibility of metastasis from other regions and further suggested a diagnosis of primary intramedullary melanocytoma. Complete resection, adjuvant radiation, and regular review are critical. In addition, maximal safe resection also benefits prognosis while the tumor is difficult to be resected totally.
Core Tip: Primary intramedullary melanocytoma is an extremely rare kind of primary melanocytic tumor within the spinal cord. The features in imaging are nonspecific depending on the degree of melanization, intra-tumoral hemorrhage, and duration of bleeding. Therefore, diagnosis confirmation consists of two key points: (1) Patho
- Citation: Liu ZQ, Liu C, Fu JX, He YQ, Wang Y, Huang TX. Primary intramedullary melanocytoma presenting with lower limbs, defecation, and erectile dysfunction: A case report and review of the literature. World J Clin Cases 2021; 9(28): 8616-8626
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8616.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8616
Primary melanocytic tumors can be generally divided into diffuse melanocytosis, melanocytoma, malignant melanoma, and meningeal melanomatosis according to the 2007 World Health Organisation (WHO) classification of tumors in the central nervous system (CNS)[1]. Besides, primary melanocytoma in the CNS derived from melanocyte of the leptomeninges instead of meningothelial cells is a rare type of neoplasm with an incidence of 1 per 10 million[2]. In general, melanocytomas are classified as intermediate grade melanocytic tumors and are usually located in the posterior fossa and spinal cord. In addition, the distribution percentage of primary intramedullary melanocytoma at the cervical, thoracic, and lumbosacral levels is 28.3%, 52.8%, and 18.9%, respectively[2].
Melanocytomas have been historically considered as benign neoplasms with mild cytology, but an increasing body of evidence has indicated the latent tendency of melanocytomas to relapse, metastasize, or transform to malignant melanocytic neoplasms[3]. Given the potential malignancy of melanocytomas, gross total resection and following radiotherapy may be the preferable treatment in an attempt to have a better prognosis of the disease[3]. Here, we describe the case of a 52-year-old man with primary intramedullary melanocytoma and furthermore discuss the diagnosis and therapy by combining the relevant background literature and the present case.
A 52-year-old male patient was admitted for weakness and numbness involving the bilateral lower limbs for 18 mo, and disorder of defecation and erectile dysfunction for 6 mo. For the last 3 mo, these symptoms started to worsen.
The patient started to present disorders of bilateral lower limbs in January 2016, and defecation and erectile dysfunction in January 2017. Subsequently, he received magnetic resonance imaging examination at a local hospital, which suggested an intramedullary mass located at the level of T9-T10. However, he did not take any cure. In April 2017, the clinical symptoms began to worsen. Therefore, the patient was admitted to our department on July 13, 2017 for further treatment.
The patient underwent an appendectomy in 1984, and had suffered from diabetes for 7 years and hypertension for 2 years. He had been taking nimodipine, metformin, and gliclazide to control the blood pressure and blood glucose levels under the supervision of local doctors.
Neither he nor anyone in his family had a history of primary intramedullary melanocytoma.
Neurologic examination presented that the myodynamia of the right lower limb was grade 3 and left lower limb was grade 4. Besides, the superficial and deep sense in the right lower limb was clearly worse than that of the left lower limb and these dysfunctions in distal lower limbs were more severe than those of proximal lower limbs as well. Moreover, achilles tendon reflex and patellar tendon reflex were brisk with absence of ankle and patellar clonus. Babinski sign was positive in the right lower limb. Anal reflex revealed a mild decrease and the Romberg sign was positive.
Total protein was slightly low (63.2 g/L). Triglyceride (1.75 mmol/L) and low density lipoprotein (3.47 mmol/L) were a little high. Color doppler ultrasound of the stomach and pelvis indicated a single gallbladder polyp (3 mm × 3 mm) and gallstone (5 mm × 4 mm), and benign prostate hyperplasia with multiple calcifications. The routine blood, urine, and stool tests were normal. Electrocardiogram, chest X-ray, cardiac color Doppler ultrasound, pulmonary ventilation function, and blood glucose were also normal.
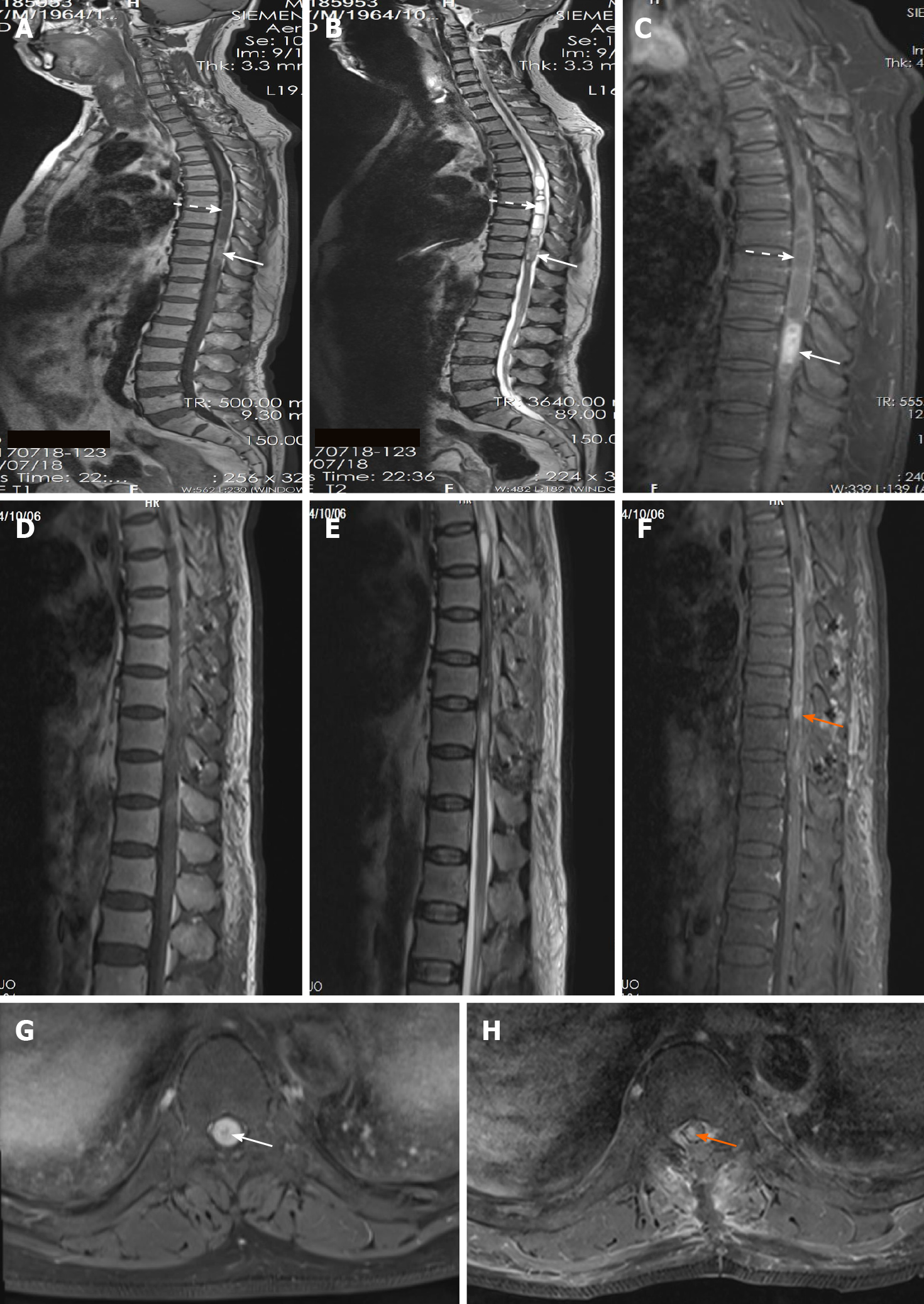
Magnetic resonance imaging (MRI) revealed an intramedullary tumor located at the T9-T10 level with oval borders and a size of 5.5 cm × 1.2 cm × 1.2 cm. The mass was slightly hyperintense on T1-weighted images (T1WI) (Figure 1A) and hypointense on T2-weighted images (T2WI) (Figure 1B). Contrast-enhanced MRI of the tumor showed mildly inhomogeneous enhancement after gadolinium administration (Figure 1C and G). The secondary lesions like syringomyelia induced by the intramedullary tumor were hypointense on T1WI (Figure 1A) and hyperintense on T2WI (Figure 1B) with mild enhancement after gadolinium management at the T5-T8 level (Figure 1C).
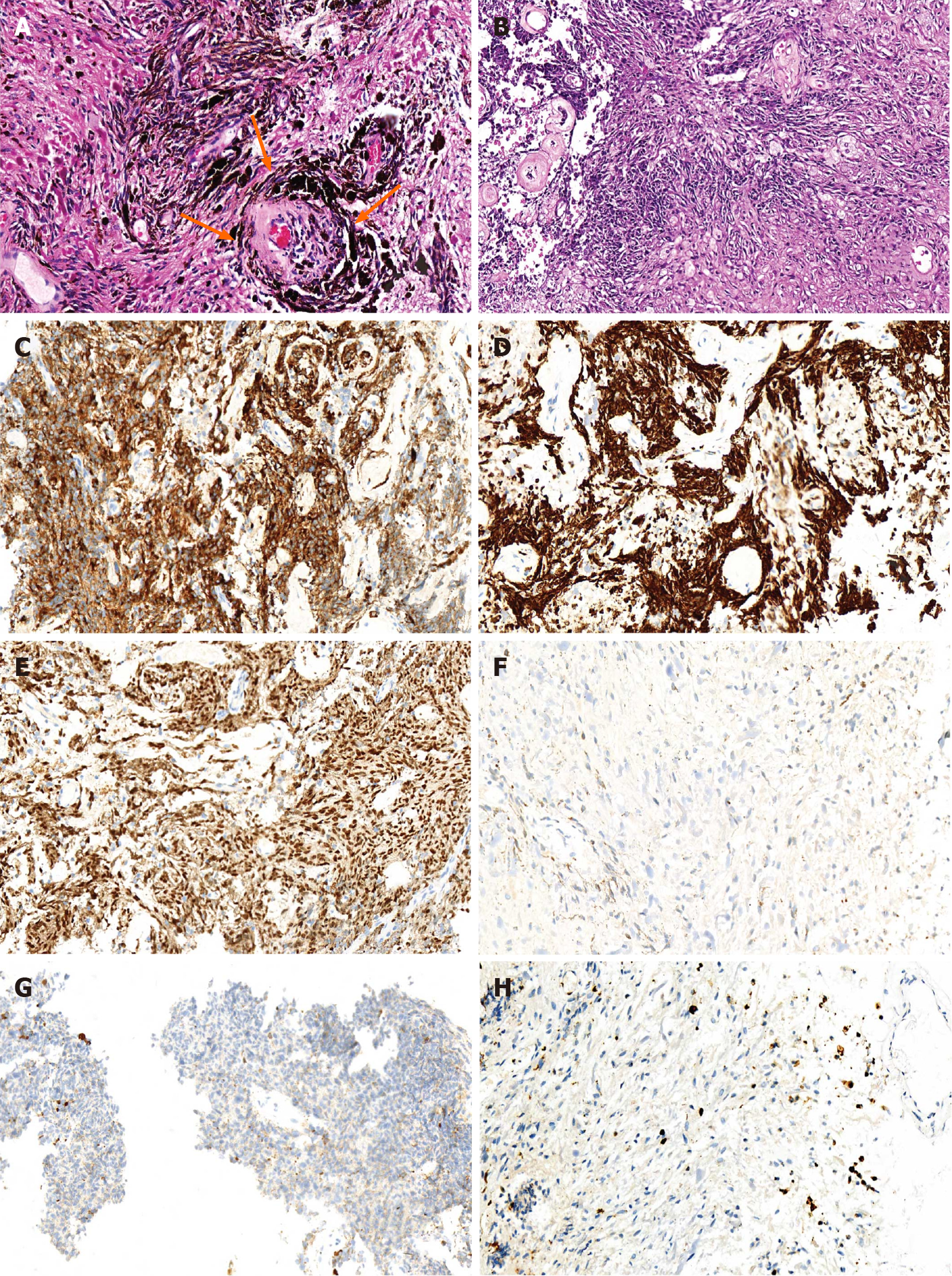
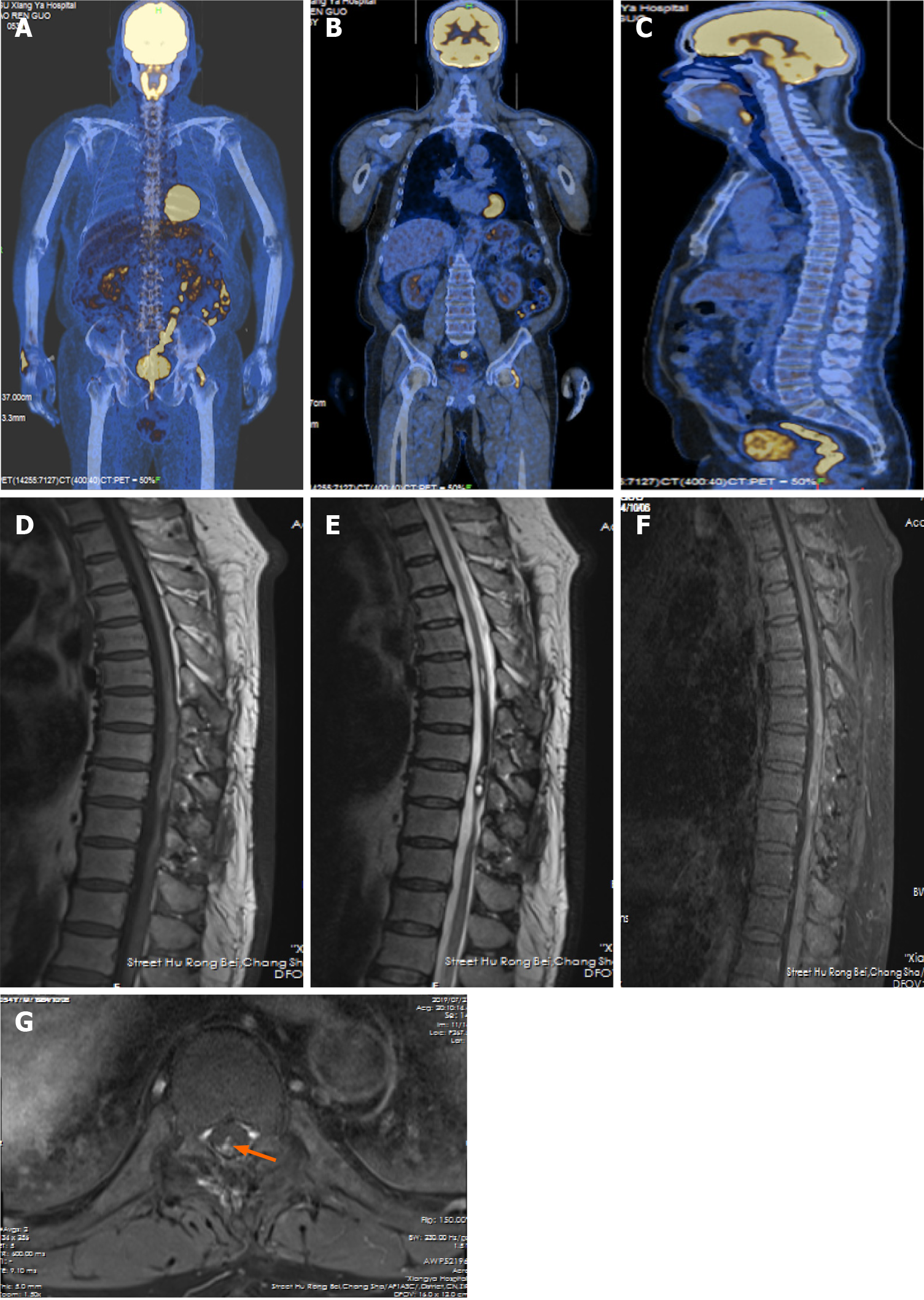
The lesion was confirmed to be melanocytoma by histopathological examination with typical characteristics of primary melanocytic tumors like positive manifestation of sex determinant region Y box 10 (SOX10) protein, human melanoma black 45 (HMB-45), and antimelanoma antibody, and negative presence of epithelial membrane antigen (EMA) and glial fibrillary acidic protein (GFAP). The proliferative index (Ki 67) was almost equal to 3% (Figure 2). The possibility of original melanocytoma outside the spinal cord was excluded after the examinations of postoperative positron emission tomography–computed tomography (PET-CT) scanning, ophthalmological test, and gastrointestinal and dermatological inspection (Figure 3A-C). Accordingly, the final diagnosis of the patient was primary and intramedullary intermediate grade melanocytoma at the T9-T10 level.
The patient underwent surgical operation with approximately gross total resection on July 19, 2017. The resection extent reached to 98%. During the operation, clear cerebrospinal fluid was visible in the intramedullary cavity with golden yellow substance adhering to the wall of chamber at the T5-T8 level. At T9-T10, some stale blood clots were observed. After removing these blood clots, the mass was found in the right of T9-T10 without capsule. The mass was well-circumscribed, but it possessed abundant vascularity. Furthermore, a tiny part adhered to the normal spinal cord closely, so we only performed the maximal safe resection with minimal residual (1.5 cm × 0.4 cm × 0.3 cm) to ensure patient’s life quality. The duration of surgery lasted for 5 h and the blood loss was 300 mL. The patient did not receive blood transfusion.
The hospital stay was 15 d and there was no hospital stay related issues. Postoperative MRI revealed that a small piece of tumor remained with heterogeneous enhancement before discharge (Figure 1D-F and H). The patient refused to accept radiotherapy or Gamma Knife radiosurgery. Follow-up examination on July 28, 2020 showed no sign of further growth of the lesion with heterogeneous enhancement, compared to the previous MRI scans (Figure 3D-G). On April 10, 2021, the patient received regular review at a local hospital without performing MRI scan. As for clinical symptoms, the weakness involving the bilateral lower limbs got significant improvement from rehabilitation, as the myodynamia of the right lower limb was grade 4 and left lower limb was grade 5. Furthermore, the disorder of defecation obtained relatively significant improvement but erectile dysfunction only acquired mild benefit from the resection. According to the latest review, the progression free survival had approached to 45 mo.
The designation “meningeal melanocytoma” in the primary melanocytic tumors of the CNS was confirmed for the first time in 1972, which frequently tended to occur in the veutro of medulla or the upper segments of the spinal cord, but the term “melanocytoma” defining the subtype of neoplasms was adopted by the WHO classification[1,4]. The age of onset ranged from 5 years to 79 years with a peak around the fifth decade[5]. The ratio of male to female was 2.2:1, and the tumors are mainly located at the level of cervical vertebra[6]. Benign histology and favorable clinical course are the key factors to differentiate melanocytomas from malignant melanomas. Hence, the clinical symptoms of melanocytoma are usually caused by expanding extrusion instead of infiltrative growth, which was also verified in the present case during operation, and there are various neurologic deficits depending on the locations of tumors[4]. However, some literature reported potential aggressive behaviour of melanocytomas such as metastasis, recurrence, and malignant transformation[3,7]. Therefore, it is crucial to parse primary melanocytomas from metastasis melanocytomas. However, there has been no standard strategy of diagnosis and treatment for primary melanocytomas in the CNS so far due to the insufficient data available. Nonetheless, the criteria proposed by Hayward might be helpful to diagnose primary CNS melanocytic tumors: (1) No malignant melanoma tumor outside the CNS; (2) Involvement of the leptomeninges (spinal or cranial); (3) Intramedullary spinal lesions; (4) Hydrocephalus; (5) Tumor in the pituitary or pineal gland; and (6) a single intracerebral lesion[8].
Preoperative diagnosis of melanocytomas in the CNS on radiologic examinations is also a dilemma, as the imaging features are nonspecific depending on the degree of melanization, intra-tumoral hemorrhage, and duration of bleeding. Besides, paramagnetic free radicals in melanin were considered to be responsible for shortening the relaxation times of T1 and T2 by the proton-electron dipole-dipole proton relaxation enhancement mechanism[9]. The MRI appearance mainly was hyper-intensity on T1WI and hypo-intensity on T2WI with homogenous enhancement after gadolinium enhancement[10]. As for this case, the presence of inhomogeneous enhancement may be caused by intratumoral hemorrhage.
Pathological examination is an accurate and indispensable way to confirm the explicit diagnosis of melanocytomas in the CNS. The characteristics of primary melanocytic tumors are spindle or epithelioid cells arranged in the formation of sheets, bundles, nests, or whorls surrounded by reticulin fibres, which contain various degrees of melanin pigment in the cytoplasm. The appearance of melanocytomas includes low mitotic activity and absence of necrosis, nuclear atypia, and microvascular invasion. By contrast, the presence of primary malignant melanocytic tumors such as melanomas generally reveals a high proliferation index (Ki 67 > 5%)[11,12]. The mean rate of soluble protein-100 (S-100), HMB-45, and antimelanoma antibody to diagnose melanocytic tumors was 95%, 86%, and 84% respectively. In addition, HMB-45 was considered to be more specific but less sensitive than S-100[13]. SOX10 protein, a transcription factor, encoded by a gene located on chromosome 22q13.1, could regulate the differentiation of neural crest-derived melanocytes by affecting the expression of microphthalmia transcription factor[14]. Compared with S-100, SOX10 was recognized as having a higher sensitivity and/or specificity for melanocytic tumors, as S-100 protein was positively expressed in multiple types of tumors in spite of high sensitivity[14]. Therefore, SOX10 identification was adopted instead of S-100 in this case. The negative expression of GFAP and EMA is helpful to differentiate melanocytic tumors from gliomas and meningiomas[15,16]. In combination with previous studies, the diagnostic criteria for melanocytomas were proposed on the basis of positive manifestation of S-100 and/or SOX10 protein, Vimentin, HMB- 45, and antimelanoma antibody and negative presence of EMA, GFAP, and neuron specific enolase[11,12,14]. As for our case, the PET-CT and physical examination of whole body helped to exclude the malignant melanoma tumor outside the spinal cord, and the confirmation of pathological examination further validated the diagnosis of primary intramedullary melanocytoma.
Surgical resection plays a crucial role in the treatment of melanocytomas in the CNS. Complete tumor resection remarkably decreased the recurrence rates at 3 years and 5 years compared to incomplete tumor resection[17]. Owing to the extensive growth of melanocytomas, surgical resection could relieve pain symptoms immediately, but neurological deficits might need more time to recover. To date, the standard adjuvant therapy for melanocytomas has not yet proposed due to its rarity. Adjuvant radiation therapy and Gamma Knife radiosurgery for incomplete tumor resection should be taken into account, as these strategies were helpful to control tumor growth and improve the clinical outcome[18]. Even for the patients who underwent complete tumor resection, adjuvant radiation therapy could diminish the recurrence rates to 22% at 5 years[19,20]. Moreover, irradiation could be used to treat these tumors which are otherwise difficult to be resected[21,22]. Verma et al[23] treated a patient suffering from recurrent melanocytoma in the spinal cord after first surgical operation (approximate 95%) and then radiation therapy followed by a second partial excision (approximate 90%) and adjuvant therapy with corynebacterium parvum, dactinomycin, dacarbazine, and the progression free survival was 15 mo. Koch et al[24] treated a patient with recurrent melanocytoma in the cerebello-pontine angle with intracerebral and spinal meningeal seeding after first resection by radiotherapy in combination with chemotherapy (oral temozolomide), but the patient died after 5 mo. The two cases indicated that the recurrent tumor might not respond to a combined radiotherapy and chemotherapy. The same result was also reported by Roser et al[25]. In fact, the role of radiochemotherapy in the treatment of melanocytomas needs further research. In terms of the present case, after approximately complete resection, the clinical symptoms induced by extensive growth of tumor was ameliorated remarkably and no relapse or aggression was discovered in spite of not getting radiochemotherapy. In addition, to now, the patient has been receiving regular review every 6 to 12 mo in order to avoid neglecting potential malignant transformation and metastasis. Some previous studies about primary melanocytomas in the spinal cord are summarized in Table 1.
| Case No. | Ref. | Time | Age (yr) | Sex | Location | EOR | RT | Relapse | MT | Metastasis | Treatment | Follow-up | Comment |
| 1 | Verma et al[23] | 1979 | 71 | F | T2-T3 | Subtotal | Yes | Yes | NG | No | Re-resection and chemotherapy | 53 mo | PFS is 15 mo after the second therapy |
| 2 | Litofsky et al[26] | 1992 | 32 | M | Clivus - C5 | Total | No | No | - | - | - | 40 mo | |
| 3 | Ibáñez et al[22] | 1997 | 44 | F | T11 | Total | No | No | - | - | - | 54 mo | |
| 4 | Matsumoto et al[27] | 1998 | 48 | M | T8-T9 | Total | No | No | - | - | - | 12 mo | |
| 5 | Rades et al[17] | 2001 | 23 | F | T4-T7 | Subtotal | No | Yes | Yes | Yes | Re-resection and RT | 54 mo | Died from brain metastases |
| 6 | Das et al[28] | 2001 | 50 | M | T10 | NG | Yes | Yes | NG | Yes | - | 30 mo | Died from aspiration pneumonia |
| 7 | Iida et al[29] | 2002 | 42 | M | T8-T10 | NG | No | No | - | - | - | 4 mo | Died from a urinary tract infection |
| 8 | Iida et al[29] | 2002 | 52 | M | C2 | Subtotal | No | No | - | - | - | 24 mo | |
| 9 | Turhan et al[7] | 2004 | 64 | M | T12-L2 | Total | No | No | - | - | - | 24 mo | |
| 10 | Turhan et al[7] | 2004 | 19 | F | T8 | Total | No | No | - | - | - | 36 mo | |
| 11 | Wang et al[30] | 2007 | 57 | M | L5-S1 | Total | No | Yes | NG | No | Re-resection and RT | 17 mo | 5 mo after the second surgery, metastases were found in the liver and the left ninth rib |
| 12 | Chacko et al[31] | 2008 | 22 | M | T6-T11 | Total | No | No | - | - | - | 96 mo | |
| 13 | Karikari et al[10] | 2009 | 32 | F | T10 | Total | No | No | - | - | - | 3 mo | |
| 14 | Karikari et al[10] | 2009 | 20 | M | T12 | Total | No | No | - | - | - | 6 W | |
| 15 | Caruso et al[32] | 2009 | 62 | M | T11 | Total | No | No | - | - | - | 24 mo | |
| 16 | Perrini et al[3] | 2010 | 79 | F | T10-T11 | Subtotal | No | Yes | Yes | No | Re-resection | 30 mo | |
| 17 | Eskandari et al[33] | 2010 | 45 | M | T11 | Total | No | Yes | NG | No | RT | 36 mo | |
| 18 | Wagner et al[34] | 2015 | 63 | M | C2-C3 | Total | No | Yes | NG | No | RT | 18 mo | Neurological stabilization for 15 mo after radiotherapy |
| 19 | Wang et al[35] | 2016 | 60 | M | T1; T3-T4 | Total | No | No | - | - | - | 19 mo | |
| 20 | Reutov et al[36] | 2016 | 28 | F | C1-C2 | Total | No | No | - | - | - | 24 mo | |
| 21 | Lee et al[12] | 2017 | 45 | M | C1 | Total | No | No | - | - | - | 6 mo | |
| 22 | Gupta et al[37] | 2017 | 20 | M | C1-C2 | Total | Yes | No | - | - | - | 12 mo |
In summary, the 5-year survival rate of primary melanocytomas in the spinal cord is more than 90% after surgical operation, but it is vital for doctors and patients to pay more attention to the potential malignance of primary melanocytomas such as local recurrence, adjacent structure invasion, cerebrospinal fluid spread, and distant metastases[6].
We report a case of primary intramedullary melanocytoma at the T9-T10 level presenting with lower limbs, defecation, and erectile dysfunction. In addition, the postoperative progression free survival had reached to 45 mo till the latest follow-up. The main therapy strategy includes gross total resection and adjuvant radiation. This case proves evidence that maximal safe resection can provide benefits to prognosis and improve the quality of life, when complete resection is difficult to achieve. Considering the potential malignancy, postoperative examination of whole body regions, after the pathological diagnosis of intramedullary melanocytoma, can help exclude the probability of metastasis from other regions. Therefore, it is reasonable to confirm the diagnosis of primary intramedullary melanocytoma. Based on this case, we would recommend patients to receive adjuvant radiation, which can prolong their progression free survival. Notably, regular follow-up is crucial, as physical examination and MRI scan can help find early progression or relapse.
| 1. | Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7079] [Cited by in RCA: 8144] [Article Influence: 428.6] [Reference Citation Analysis (0)] |
| 2. | Liubinas SV, Maartens N, Drummond KJ. Primary melanocytic neoplasms of the central nervous system. J Clin Neurosci. 2010;17:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (3)] |
| 3. | Perrini P, Caniglia M, Pieroni M, Castagna M, Parenti GF. Malignant transformation of intramedullary melanocytoma: case report. Neurosurgery. 2010;67:E867-9; discussion E869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 4. | Limas C, Tio FO. Meningeal melanocytoma ("melanotic meningioma"). Its melanocytic origin as revealed by electron microscopy. Cancer. 1972;30:1286-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 5. | Salah El-Din AM, Aboul-Ela HM, Alsawy MF, Koheil A, Ashry AH. Spinal meningeal melanocytoma in a 5-year-old child: a case report and review of literature. Egypt J Neurol Psychiatr Neurosurg. 2018;54:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 6. | Yang C, Fang J, Li G, Jia W, Liu H, Qi W, Xu Y. Spinal meningeal melanocytomas: clinical manifestations, radiological and pathological characteristics, and surgical outcomes. J Neurooncol. 2016;127:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 7. | Turhan T, Oner K, Yurtseven T, Akalin T, Ovul I. Spinal meningeal melanocytoma. Report of two cases and review of the literature. J Neurosurg. 2004;100:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 8. | Hayward RD. Malignant melanoma and the central nervous system. A guide for classification based on the clinical findings. J Neurol Neurosurg Psychiatry. 1976;39:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Czarnecki EJ, Silbergleit R, Gutierrez JA. MR of spinal meningeal melanocytoma. AJNR Am J Neuroradiol. 1997;18:180-182. [PubMed] |
| 10. | Karikari IO, Powers CJ, Bagley CA, Cummings TJ, Radhakrishnan S, Friedman AH. Primary intramedullary melanocytoma of the spinal cord: case report. Neurosurgery. 2009;64:E777-8; discussion E778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 11. | Reddy R, Krishna V, Sahu BP, Uppin M, Sundaram C. Multifocal spinal meningeal melanocytoma: an illustrated case review. Turk Neurosurg. 2012;22:791-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Lee JK, Rho YJ, Jeong DM, Rhim SC, Kim SJ. Diagnostic Clue of Meningeal Melanocytoma: Case Report and Review of Literature. Yonsei Med J. 2017;58:467-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Yu CH, Chen HH, Liu CM, Jeng YM, Wang JT, Wang YP, Liu BY, Sun A, Chiang CP. HMB-45 may be a more sensitive maker than S-100 or Melan-A for immunohistochemical diagnosis of primary oral and nasal mucosal melanomas. J Oral Pathol Med. 2005;34:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Ordóñez NG. Value of SOX10 immunostaining in tumor diagnosis. Adv Anat Pathol. 2013;20:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Tichy J, Spechtmeyer S, Mittelbronn M, Hattingen E, Rieger J, Senft C, Foerch C. Prospective evaluation of serum glial fibrillary acidic protein (GFAP) as a diagnostic marker for glioblastoma. J Neurooncol. 2016;126:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 16. | Menke JR, Raleigh DR, Gown AM, Thomas S, Perry A, Tihan T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. 2015;130:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 17. | Rades D, Heidenreich F, Tatagiba M, Brandis A, Karstens JH. Therapeutic options for meningeal melanocytoma. Case report. J Neurosurg. 2001;95:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ali Y, Rahme R, Moussa R, Abadjian G, Menassa-Moussa L, Samaha E. Multifocal meningeal melanocytoma: a new pathological entity or the result of leptomeningeal seeding? J Neurosurg. 2009;111:488-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Rades D, Schild SE. Dose-response relationship for fractionated irradiation in the treatment of spinal meningeal melanocytomas: a review of the literature. J Neurooncol. 2006;77:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Rades D, Schild SE, Tatagiba M, Molina HA, Alberti W. Therapy of meningeal melanocytomas. Cancer. 2004;100:2442-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Chow M, Clarke DB, Maloney WJ, Sangalang V. Meningeal melanocytoma of the planum sphenoidale. Case report and review of the literature. J Neurosurg. 2001;94:841-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 22. | Ibáñez J, Weil B, Ayala A, Jimenez A, Acedo C, Rodrigo I. Meningeal melanocytoma: case report and review of the literature. Histopathology. 1997;30:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Verma DS, Spitzer G, Legha S, McCredie KB. Chemoimmunotherapy for meningeal melanocytoma of the thoracic spinal cord. Report of a case. JAMA. 1979;242:2435-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 24. | Koch HJ, Roeber S, Zimmermann UW, Schäfer C, Villarrubia V, Kuchelmeister K, Schachenmayr W, Bogdahn U, Steinbrecher A. [Spinal and cerebral leptomeningeal seeding from a melanocytoma of the cerebello-pontine angle]. Wien Med Wochenschr. 2005;155:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Roser F, Nakamura M, Brandis A, Hans V, Vorkapic P, Samii M. Transition from meningeal melanocytoma to primary cerebral melanoma. Case report. J Neurosurg. 2004;101:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Litofsky NS, Zee CS, Breeze RE, Chandrasoma PT. Meningeal melanocytoma: diagnostic criteria for a rare lesion. Neurosurgery. 1992;31:945-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 27. | Matsumoto S, Kang Y, Sato S, Kawakami Y, Oda Y, Araki M, Kawamura J, Uchida H. Spinal meningeal melanocytoma presenting with superficial siderosis of the central nervous system. Case report and review of the literature. J Neurosurg. 1998;88:890-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Das A, Ratnagopal P, Puvanendran K, Teo JG. Spinal meningeal melanocytoma with hydrocephalus and intracranial superficial siderosis. Intern Med J. 2001;31:562-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 29. | Iida M, Llena JF, Suarez MA, Malik S, Weidenheim KM, LaSala P, Hirano A. Two cases of spinal meningeal melanocytoma. Brain Tumor Pathol. 2002;19:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Wang F, Li X, Chen L, Pu X. Malignant transformation of spinal meningeal melanocytoma. Case report and review of the literature. J Neurosurg Spine. 2007;6:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Chacko G, Rajshekhar V. Thoracic intramedullary melanocytoma with long-term follow-up. J Neurosurg Spine. 2008;9:589-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Caruso R, Marrocco L, Wierzbicki V, Salvati M. Intramedullary melanocytoma: case report and review of literature. Tumori. 2009;95:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (2)] |
| 33. | Eskandari R, Schmidt MH. Intramedullary spinal melanocytoma. Rare Tumors. 2010;2:e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 34. | Wagner F, Berezowska S, Wiest R, Gralla J, Beck J, Verma RK, Huber A. Primary intramedullary melanocytoma in the cervical spinal cord: Case report and literature review. Radiol Case Rep. 2015;10:1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Wang YB, Wang WJ, Zhao HT, Li W, Peng T, Zhang XF. Multiple melanocytoma of the thoracic spine: a case report and literature review. Spine J. 2016;16:e59-e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 36. | Reutov AA, Ryzhova MV, Kushel' YV. [Intramedullary melanocytoma: a clinical case report and literature review]. Zh Vopr Neirokhir Im N N Burdenko. 2016;80:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Gupta PK, Misra S, Verma R, Soni N, Lamin JC, Mishra RK, Behari S, Kumar S. Primary intradural cervical spine melanocytoma: A rare tumor and review of literature. Neurol India. 2017;65:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bains L S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Ma YJ