Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8552
Peer-review started: May 27, 2021
First decision: June 24, 2021
Revised: June 28, 2021
Accepted: August 18, 2021
Article in press: August 18, 2021
Published online: October 6, 2021
Processing time: 123 Days and 21.9 Hours
Spinocerebellar ataxia type 3 (SCA3) is a rare neurodegenerative disease with high genetic heterogeneity. SCA3 mainly manifests as progressive cerebellar ataxia accompanied by paralysis of extraocular muscles, dysphagia, lingual fibrillation, pyramidal tract sign, and extrapyramidal system sign. However, it rarely has clinical manifestations similar to Parkinson-like symptoms, and is even rarer in patients sensitive to dopamine. We report a patient initially diagnosed with dopamine-responsive dystonia who was ultimately diagnosed with SCA3 by genetic testing, which was completely different from the initial diagnosis.
A 40-year-old Chinese woman was admitted to hospital due to severe inflexibility. At the beginning of the disease, she presented with anxiety and sleep disorder. At the later stage, she presented with gait disorder, which was similar to Parkinson's disease. Her medical history was unremarkable, but her mother, grandmother, and uncle all had similar illnesses and died due to inability to take care of themselves and related complications. Laboratory and imaging examinations showed no abnormalities, but electromyography and electroencephalography revealed delayed somatosensory evoked potentials and slow background rhythm, respectively. Her symptoms fluctuated during the daytime, and we initially diagnosed her with dopamine-responsive dystonia. After treatment with low-dose levodopa, the patient’s symptoms were significantly improved, but the final genetic diagnosis was SCA3.
SCA3 has various clinical phenotypes and needs to be differentiated from Parkinson's syndrome and dopamine-responsive dystonia.
Core Tip: We report a female patient initially diagnosed with dopamine-responsive dystonia. After treatment with low-dose levodopa, the patient’s symptoms were significantly improved, but she was ultimately diagnosed with spinocerebellar ataxia type 3 (SCA3) by genetic testing. Sensitivity to levodopa may be a clinical feature of SCA3, and this report could add to the evidence of the SCA3 clinical phenotypes, which need to be differentiated from Parkinson's syndrome and dopamine-responsive dystonia.
- Citation: Zhang XL, Li XB, Cheng FF, Liu SL, Ni WC, Tang FF, Wang QG, Wang XQ. Spinocerebellar ataxia type 3 with dopamine-responsive dystonia: A case report. World J Clin Cases 2021; 9(28): 8552-8556
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8552.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8552
Spinocerebellar ataxia type 3 (SCA3), also known as Machado Joseph disease, is an autosomal dominant genetic disease first described by Nakano et al[1] among Portuguese immigrants in the United States in 1972. It is characterized by progressive cerebellar ataxia accompanied by paralysis of extraocular muscles, dysphagia, lingual fibrillation, pyramidal tract signs, extrapyramidal system signs, and other clinical manifestations. However, it rarely presents with symptoms of Parkinson's disease and peripheral nerve lesions and is even less common in patients sensitive to levodopa[2-5]. This phenotype has been described in cases in Singapore, but further evidence on this phenotype is needed[6]. The clinical manifestation of the patient described in this report was typical of dopamine-responsive dystonia, with symptoms fluctuating during the daytime. The effect of low-dose levodopa treatment was significant, but unexpectedly, a final diagnosis of SCA3 was confirmed by genetic testing.
A 40-year-old Chinese woman was admitted to hospital due to a 3-year history of limb inflexibility, which had become aggravated in the previous year.
Three years before presenting to our clinic, the patient developed a feeling of heaviness and inflexibility in the right lower extremity, which was aggravated after fatigue. It could be relieved after getting up in the morning or resting and was accompanied by emotional irritability and insomnia. She went to a local hospital. The doctor diagnosed her with anxiety and depression and administered paroxetine orally. After 3 mo, her symptoms were not alleviated; thus, she discontinued the drug herself. Two years ago, the patient began to gradually experience difficulties with limb movement. One year ago, the patient’s gait disorder progressively aggravated. It manifested as laborious lifting of the feet off the ground, stiffness of the lower limbs, leaning forward and a forward gait, inability to stop immediately, difficulty turning around, reduced arm swing, and other symptoms similar to Parkinson's disease. She could not take care of herself, so she visited our hospital for treatment.
The patient’s medical history was unremarkable.
The patient’s mother, grandmother, and uncle all had similar illnesses, and they eventually died because of related complications.
The patient’s vital signs were stable, and no abnormalities were found in cardiopulmonary or abdominal examinations. The patient exhibited bradykinesia, and slight horizontal nystagmus could be seen when staring left and right. There was mild lead tube-like increase in the muscle tone of extremities, a positive Romberg sign, hyperreflexia of tendons at extremities, and positive bilateral Chaddock sign and Gordon sign.
Routine laboratory tests showed no abnormalities, and thyroid function, blood ammonia, and ceruloplasmin were all within the normal range.
There were no obvious abnormalities in brain or spinal cord imaging.
In an electromyogram, the differentiation of the P15 and N20 somatosensory evoked potentials in both lower extremities was acceptable, with roughly normal incubation periods and reduced amplitudes. The incubation period of the P40 somatosensory evoked potential in both lower extremities was slightly prolonged, the waveform differentiation was poor, and the repeatability was also poor. The patient’s background rhythm in an electroencephalogram was slightly slower than normal.
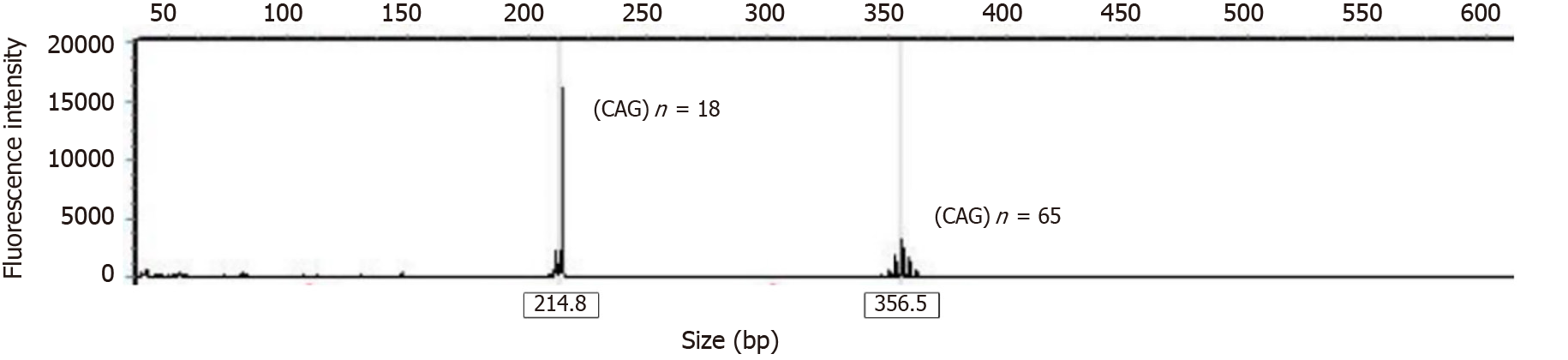
Genetic test showed 18 and 65 CAG repeat units of the ATXN3 gene (Figure 1).
The final diagnosis was SCA3.
According to the clinical symptoms, the patient was administered a small oral dose (one quarter of a pill) of madopar once a day (each pill contains 200 mg levodopa and 50 mg benserazide). After 14 d of treatment, the patient’s limb mobility improved significantly, and she was able to take care of herself and work normally. The final diagnosis was SCA3 by genetic testing in this patient who was sensitive to dopamine, which is extremely rare[7].
After discharge, the patient continued to take the same daily dose of oral madopar. She was able to work and perform daily activities normally.
The present patient had a long clinical course of the disease. The initial manifestation was one-sided limb inflexibility with perceived heaviness, which did not affect the patient’s daily life. She went to a local hospital for medical treatment and was diagnosed with somatization caused by anxiety. Although the symptoms were gradually aggravated in the later stage, she did not comply with the treatment. SCA is characterized by progressive cerebellar ataxia and pyramidal signs[8] and is sometimes accompanied by extrapyramidal symptoms and muscle atrophy caused by peripheral nerve damage. In addition, gaze, dystonia, and facial and lingual atrophy are characteristics of SCA3. It has been reported[9] that gait ataxia is the first symptom in 92.4% of patients with SCA3. Our patient did not have these manifestations in the early stage of the disease. The initial presentation was mild anxiety symptoms and sleep disorder. In the later stage, it gradually progressed and manifested as dopamine-responsive dystonia, but Parkinson's disease could not be ruled out. Therefore, the differential diagnosis of this case was complex.
During the treatment period, the patient mainly presented with inflexibility and stiffness in the limbs, a toe-first and forward-leaning gait, small strides, difficulty turning around, reduced arm swing, and other symptoms similar to Parkinson’s disease. Her symptoms fluctuated during the daytime and could be relieved after rest. Based on the family history and the lack of obvious signs of spinal cord or cerebellar atrophy on craniocerebral and spinal cord imaging, we diagnosed the patient with dopamine-responsive dystonia. After treatment with low-dose levodopa, her symptoms were significantly improved and controlled. However, the final genetic test confirmed a diagnosis of SCA3, which was quite different from the initial diagnosis. SCA3 has a wide range of clinical phenotypes, and many researchers believe that there are six clinical subtypes[10-12]. Our patient presented with slow progressive Parkinsonism and was sensitive to low-dose levodopa. Her cerebellar function defect was mild, and gene testing showed short CAG repeats. These are rare in patients with SCA3, so this patient can be classified as SCA3 type IV. The patient had late onset and mild clinical symptoms. Genetic testing showed 18 and 65 CAG repeat units. This is consistent with the fewer CAG repeats reported in the literature in patients with late onset and mild symptoms. SCA3 can present with Parkinsonian symptoms and has characteristics of diurnal fluctuation, which are mainly related to disease progression in the dopaminergic system in the substantia nigra. Some scholars believe that the pathological mechanism of SCA3 is related to lesions of the substantia nigra and dentate nucleus in the cerebellum[5]. This may be the main pathological mechanism of SCA3 in the Parkinsonian/ataxia phenotype. Therefore, dopamine is clinically effective in the treatment of this disease.
The onset of this disease was relatively late in the present patient, and there were no obvious signs of spinocerebellar injury. There was no apparent ataxia throughout the clinical course. The clinical manifestation of the patient was dopamine-responsive dystonia, which made the detection of spinocerebellar signs and diagnosis difficult. It is challenging to distinguish between dopamine-responsive dystonia, SCA3, and Parkinson’s disease. Clinicians should carefully enquire about the medical and family history of such patients and conduct a physical examination. Genetic testing is an important technique for the diagnosis of genetic diseases. Clinicians should carry out genetic testing earlier for suspected genetic diseases that are difficult to identify. The patient’s levodopa treatment was delayed for 3 years, and she remained sensitive to madopar. The treatment was well-tolerated, and she was followed up for 21 mo with good symptom control.
This case suggests that SCA3 has various clinical phenotypes which must be differentiated from Parkinson’s disease and dopamine-responsive dystonia. For patients with atypical SCA3 with anxiety symptoms, sleep disorders, and a relevant family history, clinicians should perform genetic testing as soon as possible. Therefore, sensitivity to levodopa may be a clinical feature of SCA3, and this report could add to the evidence on the clinical phenotype of SCA3.
| 1. | Nakano KK, Dawson DM, Spence A. Machado disease. A hereditary ataxia in Portuguese emigrants to Massachusetts. Neurology. 1972;22:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Xu HL, Su QN, Shang XJ, Sikandar A, Lin MT, Wang N, Lin H, Gan SR. The influence of initial symptoms on phenotypes in spinocerebellar ataxia type 3. Mol Genet Genomic Med. 2019;7:e00719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Rosenberg RN. Machado-Joseph disease: an autosomal dominant motor system degeneration. Mov Disord. 1992;7:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Matilla T, McCall A, Subramony SH, Zoghbi HY. Molecular and clinical correlations in spinocerebellar ataxia type 3 and Machado-Joseph disease. Ann Neurol. 1995;38:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Suite ND, Sequeiros J, McKhann GM. Machado-Joseph disease in a Sicilian-American family. J Neurogenet. 1986;3:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Berthier R, Douady F, Marcille G, Sotto JJ, Schaerer R, Hollard D. [Study of colony forming cells and aggregates (CFCA) in vitro in blood and bone marrow of patients with chronic myeloid leukemia: simplified bovine serum albumin gradient centrifugation]. Nouv Rev Fr Hematol. 1975;15:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Lee WW, Jeon B, Kim R. Expanding the Spectrum of Dopa-Responsive Dystonia (DRD) and Proposal for New Definition: DRD, DRD-plus, and DRD Look-alike. J Korean Med Sci. 2018;33:e184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Coutinho P, Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978;28:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 206] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Dahl G, Schudt C, Gratzl M. Fusion of isolated myoblast plasma membranes. An approach to the mechanism. Biochim Biophys Acta. 1978;514:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Méndez-Guerrero A, Uriarte-Pérez de Urabayen D, Llamas-Velasco S. Spinocerebellar ataxia type 3 presenting with writer's cramp without ataxia. Int J Neurosci. 2018;128:684-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | de Gaetano G, Donati MB, Bonaccorsi A, Franco R, Dejana E, Buczko W. Letter: Cyclic A.M.P. and arrhythmias. Lancet. 1976;1:864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 12. | Dong Y, Sun YM, Ni W, Gan SR, Wu ZY. Chinese patients with spinocerebellar ataxia type 3 presenting with rare clinical symptoms. J Neurol Sci. 2013;324:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Nucci G S-Editor: Liu M L-Editor: A P-Editor: Li X