Published online Oct 6, 2021. doi: 10.12998/wjcc.v9.i28.8509
Peer-review started: April 5, 2021
First decision: July 5, 2021
Revised: July 19, 2021
Accepted: August 25, 2021
Article in press: August 25, 2021
Published online: October 6, 2021
Processing time: 175 Days and 22.9 Hours
Multiple primary malignant tumors are two or more malignancies in an individual without any relationship between the neoplasms. In recent years, an increasing number of cases have been reported. However, concomitant primary gastric and pancreatic cancer reported a relatively small incidence, involving no pancreatic acinar cell carcinoma reports. Here, we present the first case of concomitant pancreatic acinar cell carcinoma and gastric adenocarcinoma.
A 69-year-old male presented to our department with a history of vomiting, epigastric pain, and weight loss. Imaging revealed space-occupying lesions in the stomach and the tail of the pancreas, respectively. The patient underwent laparoscopic radical gastrectomy and pancreatectomy simultaneously. The pathologies of surgical specimens were completely different: The resected gastric specimen was moderate to poorly differentiated adenocarcinoma, whereas the pancreatic tumor was consistent with acinar cell carcinoma. The patient was treated with six cycles of oxaliplatin and S-1 chemotherapy. As of March 2021, the patient was healthy without any recurrence or metastasis. After thoroughly reviewing the literature on simultaneous pancreatic and gastric cancers at home and abroad, we discussed the clinical characteristics of these rare synchronous double cancers. Most of the cases had undergone surgery and adjuvant chemotherapy, and all of the cases were pathologically confirmed by the postoperative specimen.
Synchronous pancreatic acinar cells and gastric adenocarcinoma can occur and should be considered when tumors are found in these organs.
Core Tip: Acinar cell carcinoma of the pancreas is a rare form of pancreatic cancer, and the incidence of synchronous concomitant pancreatic and gastric cancer is relatively low. We report a patient with simultaneous acinar cell carcinoma of the pancreas with gastric cancer, and he underwent radical surgery for both the pancreas and the stomach. This is the first case of concomitant cancers related to pancreatic acinar cell carcinoma and gastric cancer. We also reviewed the literature on simultaneous pancreatic and gastric cancers.
- Citation: Fang T, Liang TT, Wang YZ, Wu HT, Liu SH, Wang C. Synchronous concomitant pancreatic acinar cell carcin and gastric adenocarcinoma: A case report and review of literature. World J Clin Cases 2021; 9(28): 8509-8517
- URL: https://www.wjgnet.com/2307-8960/full/v9/i28/8509.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i28.8509
Pancreatic and gastric carcinoma are the second and fifth most common digestive system tumors, respectively[1]. Pancreatic cancer is one of the deadliest malignancies and is usually diagnosed at an advanced stage, leading to poor overall survival, particularly the relatively low pancreatic acinar cell adenocarcinoma (PACC) incidence, accounting for approximately 1%–2% of exocrine pancreatic neoplasms[2]. This report describes the first case of concomitant cancers related to PACC and gastric adenocarcinoma. Furthermore, we review the literature of synchronous gastric and pancreatic tumors in the PubMed, Web of Science, CNKI, and Embase databases and discuss the principles of treatment and prognosis of concomitant gastric and pancreatic cancer.
A 69-year-old male came to our department with a history of vomiting, epigastric pain for 3 mo, and weight loss of approximately 5 kg.
The patient developed vomiting, epigastric pain for 3 mo.
The patient had no past illness.
Two younger brothers of patient had lung cancer and laryngocarcinoma, respectively.
The patient was afebrile at 36.3 ℃, the heart rate was 65 beats per min, respiration was 17 breaths per min, and blood pressure of 131/86 mmHg. Clinical abdominal examination showed tenderness in the upper abdomen without mass upon palpation, soft and relaxed, and no rebound pain.
Laboratory test results were normal, including blood, urine, and stool were within the normal ranges. However, the carcinoembryonic antigen in tumor markers was slightly elevated at 4.06 ng/mL (normal values: < 3.4 ng/mL).
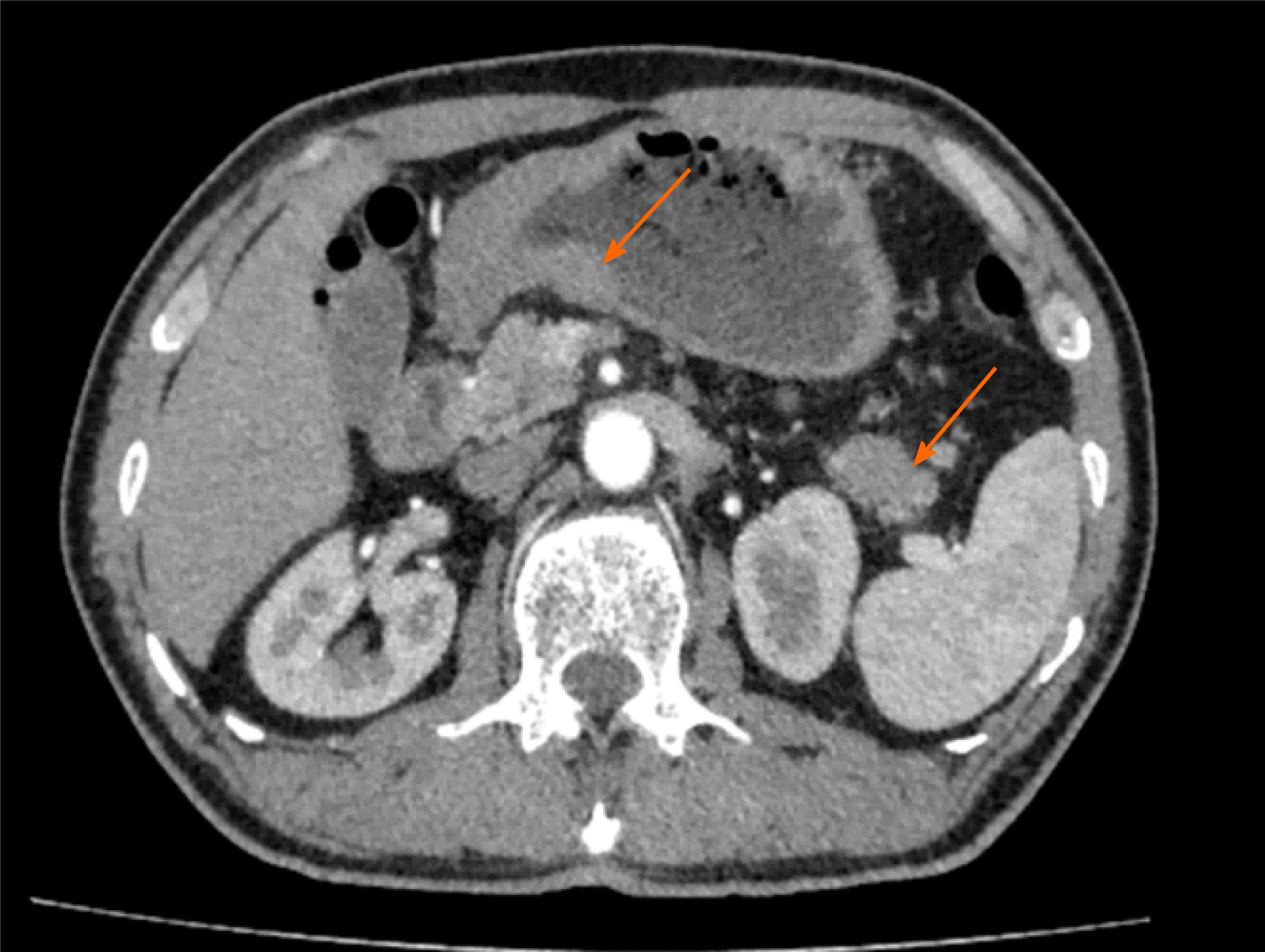
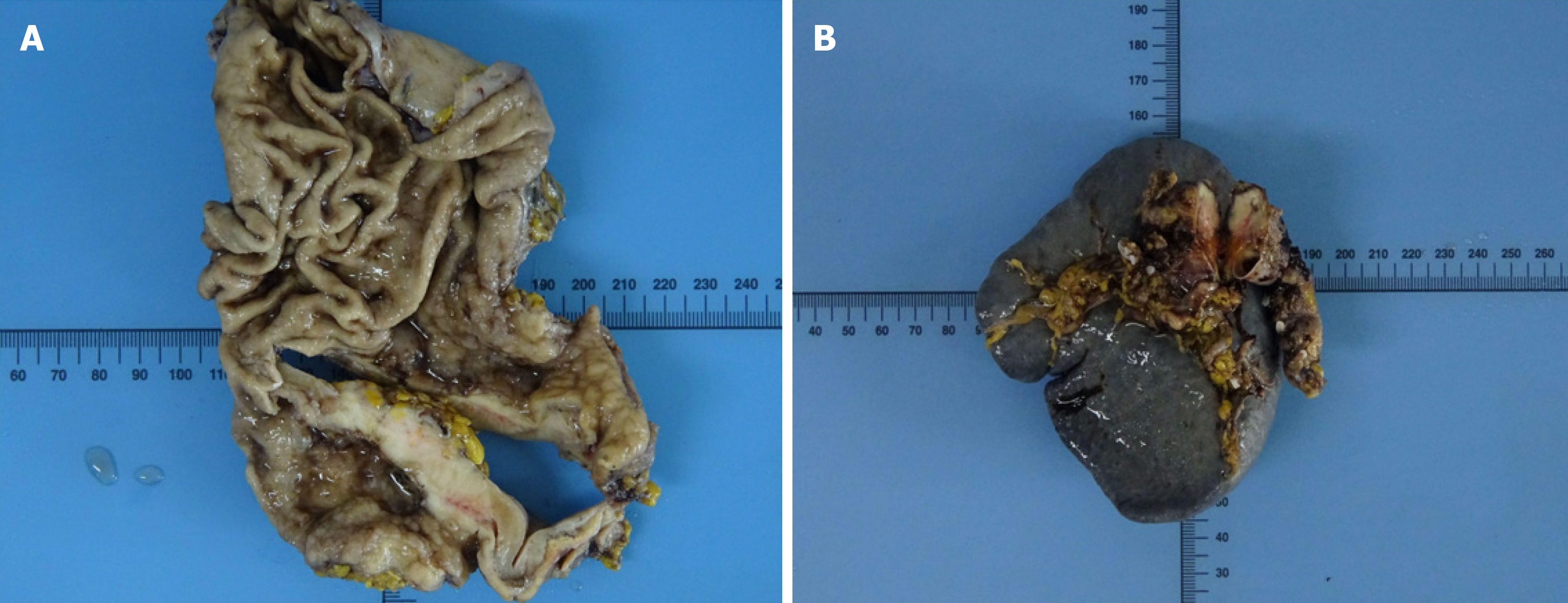
Gastroscopy revealed a large ulcer of approximately 5.5 cm × 6.6 cm × 0.5 cm originating from the gastric fundus, and pathological biopsy revealed gastric adenocarcinoma. In addition, abdominal contrast-enhanced computed tomography (CT) indicated uneven thickening in the antrum of the stomach with irregular mucosa and heterogeneous contrast enhancement on the antrum of the gastric wall and a space-occupying lesion of approximately 34 mm × 16 mm in the tail of the pancreas (Figure 1). However, there were no definite contraindications; therefore, the patient underwent laparoscopic exploration, which revealed the stomach and pancreatic masses. After evaluating the resectability of the gastric and pancreatic tumors, he underwent laparoscopic radical gastrectomy, gastric vagotomy, pancreatectomy, and splenectomy (Figure 2).
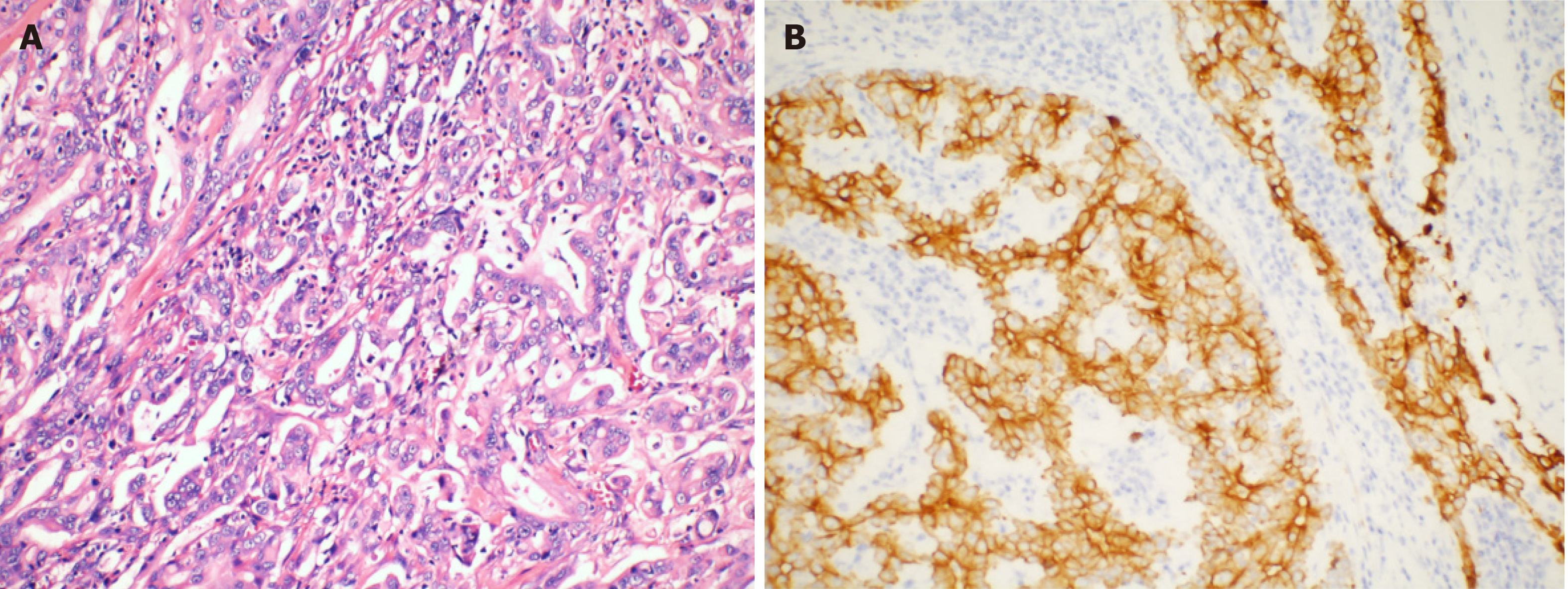
The resected stomach lesion was 5 cm × 5 cm × 1.5 cm, and the Lauren classification was the intestinal type. The pathology of the resected specimen from the stomach confirmed a moderately to poorly differentiated adenocarcinoma [pStage IIIB, T4aN2M0 per the American Joint Committee on Cancer (AJCC) eighth edition criteria] (Figure 3A). The tumor had invaded the serous membrane but did not involve the adjacent structures. Perineural and vascular infiltration were observed. Regional nodes were positive (4/32), and the resection margins were free of tumor cells. The cancer cells did not infiltrate the omentum, and there was no metastasis in the omentum lymph nodes.
Immunohistochemistry indicated positivity for pan-cytokeratin and villin and partial positivity for CK7 (Figure 3B). The tumor was negative for HER-2 (4B5) and CK20. The Ki-67 positivity was approximately 50% in a high-power field.
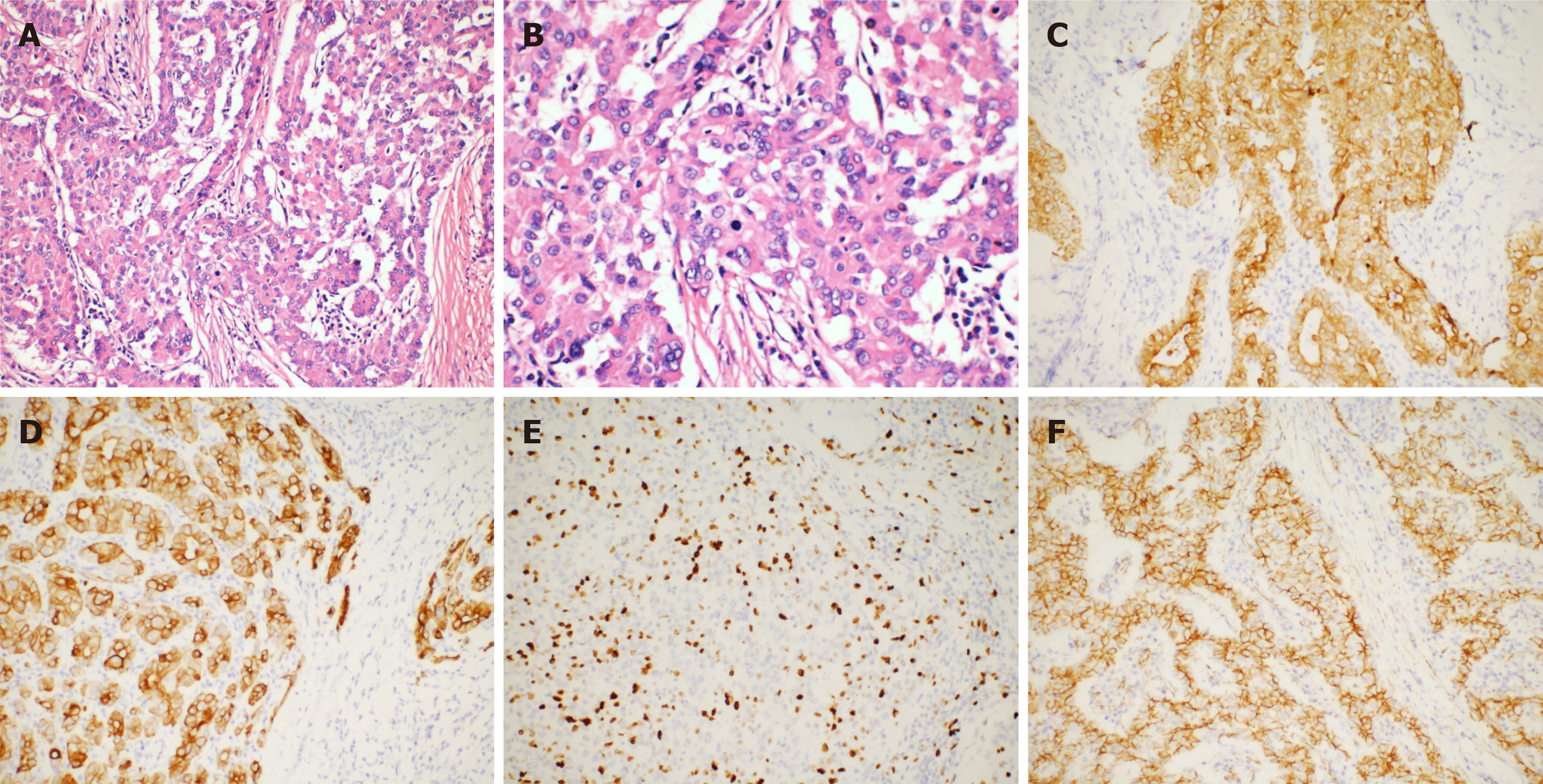
The volume of the resected pancreatic specimen was 4.1 cm × 2.2 cm × 1.5 cm. The pathology was consistent with PACC (pStage III, T3N1M0 per the AJCC eighth edition criteria) (Figure 4A and B). Perineural infiltration was observed, but there was no vascular infiltration. Regional nodes were negative, and the resection margins were free of tumor cells. Immunohistochemistry indicated positivity for CAM5.2, CK19, CK7, and membranous expression of beta-catenin and scattered positivity for carcinoembryonic antigen. The Ki-67 positivity was 30% in one high-power field (Figure 4C-F). The tumor was negative for vimentin, chromogranin A, synaptophysin, CD10, and CD56.
One month after the operation, chemotherapy consisting of oxaliplatin and S-1 (SOX) was initiated and the patient was then treated with six chemotherapy cycles.
As of March 2021, the patient was healthy without any recurrence or metastasis.
Gastric cancer is characterized by a synchronous second primary cancer in 1.0%–5.0% of cases[3-5]. This is the fourth most common cancer associated with pancreatic carcinoma, comprising approximately 5% of all cases of gastric carcinoma associated with carcinoma of other organs[3]. Gastric cancer is the most common synchronous tumor associated with pancreatic cancer[6]. Patients with pancreatic and stomach cancers demonstrated significantly better OS (33.9 mo) than patients with only pancreatic cancer (17.0 mo)[6]. This may be because pancreatic cancer is generally early-stage when synchronous concomitant cancers are diagnosed.
A review of the literature on simultaneous pancreatic and gastric cancers at home and abroad revealed that synchronous concomitant tumors involving the two organs are rare, and PACC is more uncommon. Details of reported cases are shown in Table 1[7-20], including our case. The average age at diagnosis is 67 years (42–77 years), and men are twice as likely to be diagnosed with synchronous pancreatic and gastric cancer than women. Pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic tumor in these cases. Among the 17 synchronous concomitant cancer cases, PDAC accounted for 70.6% (12/17) and PACC accounted for 11.1% (2/17). The pathological type was not mentioned in the remaining three cases. The most common tumor location was the head of the pancreas, accounting for 66.7% of cases (10/15). Two cases of tumors in the body of the pancreas and three cases of tumors located in the tail of the pancreas have been described. In two cases, the tumor location was not reported. Eleven patients (64.7%) underwent surgery for pancreatic and gastric tumors. All were diagnosed pathologically after surgery—which is consistent with our case—and none were diagnosed before surgery. These patients underwent curative resection; this may indicate that these patients were diagnosed at earlier stages and are likely to have better prognoses than patients with only pancreatic cancer. Nevertheless, concomitant cancers exist, and a second tumor should not necessarily be considered as a metastasis from another organ, leading to misdiagnosis and the abandonment of surgical resection.
| Ref. | Age | Gender | Gastric tumorlocation | Gastric histology | Pancreatic tumor location | Pancreatic histology | Treatment |
| Eriguchi et al[7], 2000 | 76 | Male | Upper gastric angle | Moderately differentiated tubular adenocarcinoma | Not mentioned | Well to moderately differentiated tubular adenocarcinoma | Surgically treated |
| Kubota et al[8], 2009 | 67 | Male | Not mentioned | Moderately differentiated adenocarcinoma | Not mentioned | Absence of pancreatic histology | Chemotherapy: S-1, paclitaxel and lentinan |
| Meng et al[9], 2011 | 42 | Male | Gastric antrum | Gastric GIST | Pancreatic head | Pancreatic GIST | Surgically treated |
| Shen et al[10], 2010 | 72 | Female | Major gastric curvature | Gastric GIST | The head of the pancreas | Poorly differentiated PDAC; malignant fibrous histiocytoma | Surgically treated |
| Muroni et al[11], 2010 | 73 | None | Gastric antrum and pyloric portion | Moderately differentiated adenocarcinoma | Uncinate portion of the pancreas | Poorly differentiated PDAC | Surgically treated |
| Dasanu et al[12], 2011 | 75 | Male | Not mentioned | GIST | The head of the pancreas | Moderately to poorly differentiated carcinoma | Surgically treated |
| Kourie et al[13], 2013. case 1 | 56 | Male | Anterior part of the antrum | Poorly differentiated adenocarcinoma with independent mucus-secreting cells | The head of the pancreas | Necrotic ductal adenocarcinoma | Chemotherapy: Folfirinox |
| Kourie et al[13], 2013. case 2 | 62 | Male | Gastric wall of the greater curvature | Gastric adenocarcinoma with mucinous component | Tail of the pancreas | Tubular adenocarcinoma (ck7+; ck20-; ck19+) | Chemotherapy: Folfirinox |
| Ohtsubo et al[14], 2013 | 77 | Male | In the middle of stomach | Adenocarcinoma stage IB, T2bN0M0 | Pancreatic head | Adenocarcinoma stage IIA, T3N0M0 | Treated with chemotherapy: S-1 |
| Baba et al[15], 2015 | 70 | Male | The fundal region and greater curvature of the stomach | Low grade gastric calcified stromal tumor (GIST) | The head of the pancreas | Adenocarcinoma | Surgically treated |
| Ghothim et al[16], 2015. case 1 | 73 | Male | The antrum of the stomach | Adenocarcinoma (pT1N1M0 stage IB, G2) | The head of the pancreas | Ductal pancreatic cancer. (pT2N1M0, stage IIB, G3) | Surgically treated; gemcitabine in six cycles |
| Ghothim et al[16], 2015. case 3 | 74 | Male | The antrum of the stomach | Gastric adenocarcinoma diffuse type (pT2bN2M0, G3) | Pancreatic head | Papillary mucinous carcinoma (pT2N0M0, stage IB, G1) | Surgically treated; Radiotherapy and chemotherapy |
| Fiore et al[17], 2015. case 1 | 63 | Male | Not mentioned | Gastric GIST (T2N0) | Pancreatic head | Adenocarcinoma (T2N0) | Surgically treated |
| Santos-Fernández et al[18], 2015 | 64 | Female | Prepiloric antral ulcer | Well differentiated gastric adenocarcinoma | Pancreatic tail | Pancreatic adenocarcinoma | Not mentioned |
| Arabadzhieva et al[19], 2016 | 60 | Female | In the pyloric area | Gastric GIST | Pancreatic body | Pancreatic neuroendocrine tumor | Surgically treated |
| Yonenaga et al[20], 2016 | 63 | Male | Antrum of the stomach | Poorly differentiated adenocarcinoma. | The body of the pancreas | PACC | Chemotherapy |
| Our case, 2021 | 69 | Male | Antrum of the stomach | Gastric adenocarcinoma | The tail of the pancreas | PACC | Surgically treated; Chemotherapy |
The clinical manifestations of PACC are related to the location and size of the tumor. Unlike patients with PDAC, patients with PACC present with nonspecific symptoms, including abdominal discomfort, weight loss, weakness, nausea, vomiting, melena, and diarrhea[21]. However, the clinical symptoms of PDAC, such as painless obstructive jaundice, are uncommon in PACC[22].
Endoscopic ultrasonography (EUS) and imaging findings such as CT and magnetic resonance imaging (MRI) help achieve a correct preoperative diagnosis for concomitant cancers[23]. CT is a valuable tool for the accurate preoperative evaluation of the local extent of gastric cancer; EUS can be used for histopathological confirmation[24]. PACC tumors tend to be solid when small and contain cystic or necrotic areas when large. These tumors generally lack dilatation of the biliary or pancreatic ducts on CT[25]. However, PACC can be difficult to diagnose based on radiological findings alone. EUS-guided fine-needle aspiration (EUS-FNA) has a very high sensitivity (> 85%) and specificity (> 95%) for diagnosis of malignancy in a solid pancreatic mass compared to cross-sectional imaging (CT/MRI)[26]. Whereas the position of the pancreas is relatively deep and EUS-FNA is difficult. An experienced radiologist can give a preliminary imaging diagnosis of PDAC, which tends to be hypovascular, appearing hypoechoic on imaging[27]. However, it is difficult to distinguish whether or not the primary tumor has metastasized to other organs in imaging, because tumors can also metastasize through the hematogenous or the lymphatic pathway in addition to direct invasion. If necessary, preoperative pathology must be performed to opt for the correct surgical approach. The present case of abdominal CT revealed a 41-mm heterogenous mass with a clear boundary in the tail of the pancreas, which is suggestive of a primary tumor.
The prevalence of pancreatic metastasis of gastric cancer is extremely rare with, only 12 cases of isolated pancreatic metastasis in gastric cancer have been reported in the literature[28]. Correspondingly, metastatic gastric tumor secondary to pancreatic carcinoma is clinically unusual, with only seven cases of gastric metastasis of pancreatic cancer have been reported in the literature[29-35]. In all these cases, the histopathology and immunohistochemical of primary cancer and metastatic cancer are consistent. However, this is completely different from our case. The histopathology of the two resected specimens was different, showing adenocarcinoma in the stomach and acinar cell carcinoma in the pancreas. Moreover, immunohistochemical studies showed differences in staining at the two sites. Finally, we concluded that both of them were primary tumors and not metastatic tumors.
PACC is associated with a better prognosis than PDAC but a worse prognosis than pancreatic neuroendocrine tumors[36]. Metastatic PACCs are generally not curable and are treated with systemic chemotherapy[37]. The treatment regimens have not yet been standardized. Takahashi et al[38] reported that platinum-containing regimens exhibited some potential efficacy in patients with advanced PACC. The response to platinum-containing regimens was 40%, and the overall survival tended to be better in patients who had received a platinum-containing regimen[38]. Simultaneous removal of concomitant primary carcinomas should be attempted; radiotherapy and chemotherapy should also be considered for patients who need adjuvant treatment decided by both disease stages[39]. If adjuvant treatment is required, the physician should select an antineoplastic therapy that considers both cancers. In our case—whether gastric adenocarcinoma or PACC—the optimal chemotherapy regimen was SOX.
The presence of synchronous multiple primary malignancies does not necessarily signify an unfavorable prognosis as long as adequate diagnosis and effective treatment are performed. In the future, well-powered clinical trials will be needed to augment our understanding of these processes.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15530] [Article Influence: 2588.3] [Reference Citation Analysis (6)] |
| 2. | Sridharan V, Mino-Kenudson M, Cleary JM, Rahma OE, Perez K, Clark JW, Clancy TE, Rubinson DA, Goyal L, Bazerbachi F, Visrodia KH, Qadan M, Parikh A, Ferrone CR, Casey BW, Fernandez-Del Castillo C, Ryan DP, Lillemoe KD, Warshaw AL, Krishnan K, Hernandez-Barco YG. Pancreatic acinar cell carcinoma: A multi-center series on clinical characteristics and treatment outcomes. Pancreatology. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Ha TK, An JY, Youn HG, Noh JH, Sohn TS, Kim S. Surgical outcome of synchronous second primary cancer in patients with gastric cancer. Yonsei Med J. 2007;48:981-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Lee JH, Bae JS, Ryu KW, Lee JS, Park SR, Kim CG, Kook MC, Choi IJ, Kim YW, Park JG, Bae JM. Gastric cancer patients at high-risk of having synchronous cancer. World J Gastroenterol. 2006;12:2588-2592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Buyukasik O, Hasdemir AO, Gulnerman Y, Col C, Ikiz O. Second primary cancers in patients with gastric cancer. Radiol Oncol. 2010;44:239-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Shin SJ, Park H, Sung YN, Yoo C, Hwang DW, Park JH, Kim KP, Lee SS, Ryoo BY, Seo DW, Kim SC, Hong SM. Prognosis of Pancreatic Cancer Patients with Synchronous or Metachronous Malignancies from Other Organs Is Better than Those with Pancreatic Cancer Only. Cancer Res Treat. 2018;50:1175-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Eriguchi N, Aoyagi S, Hara M, Okuda K, Tamae T, Fukuda S, Hashino K, Hashimoto M, Sato S, Furukawa S, Fujiki K, Jimi A. A case of synchronous double cancers of the pancreas and stomach. Kurume Med J. 2000;47:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Kubota E, Kataoka H, Hayashi K, Kamiya T, Sasaki M, Ogasawara N, Yamada T, Wada T, Mori Y, Mizoshita T, Shimura T, Mizushima T, Okamoto Y, Ohara H, Joh T. Advanced stomach and pancreas cancer successfully treated with combination chemotherapy with S-1/paclitaxel/lentinan. Hepatogastroenterology. 2009;56:106-110. [PubMed] |
| 9. | Meng L, Fang SH, Jin M. An unusual case of pancreatic and gastric neoplasms (2010: 12b). Malignant GISTs originating from the pancreas and stomach. Eur Radiol. 2011;21:663-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Shen ZL, Wang S, Ye YJ, Wang YL, Sun KK, Yang XD, Jiang KW. Carcinosarcoma of pancreas with liver metastasis combined with gastrointestinal stromal tumour of the stomach: is there a good prognosis with the complete resection? Eur J Cancer Care (Engl). 2010;19:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 11. | Muroni M, D'Angelo F, Pezzatini M, Sebastiani S, Noto S, Pilozzi E, Ramacciato G. Synchronous gastric adenocarcinoma and pancreatic ductal adenocarcinoma. Hepatobiliary Pancreat Dis Int. 2010;9:97-99. [PubMed] |
| 12. | Dasanu CA, Mesologites T, Trikudanathan G. Synchronous tumors: adenosquamous carcinoma of pancreas and GIST of stomach. J Gastrointest Cancer. 2011;42:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 13. | Kourie HR, Markoutsaki N, Roussel H, Rahmi G, Van der Stiegel M, Palazzo L, Fabre M, Cuenod CA, Dubreuil O, Landi B, Rougier P, Taieb J. Double pancreatic and gastric adenocarcinomas: a rare association. Clin Res Hepatol Gastroenterol. 2013;37:e137-e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Ohtsubo K, Ishikawa D, Nanjo S, Takeuchi S, Yamada T, Mouri H, Yamashita K, Yasumoto K, Gabata T, Matsui O, Ikeda H, Takamatsu Y, Iwakami S, Yano S. Synchronous triple cancers of the pancreas, stomach, and cecum treated with S-1 followed by pancrelipase treatment of pancreatic exocrine insufficiency. JOP. 2013;14:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Baba H, Elfahssi M, Belhamidi MS, Elhjouji A, Bounaim A, Ali AA, Sair K, Zentar A. An exceptional collision tumor: gastric calcified stromal tumor and pancreatic adenocarcinoma. Pan Afr Med J. 2015;22:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 16. | Ghothim M, Havlík R, Skalický P, Klos D, Vrba R, Strážnická J, Skopal L, Neoral Č, Loveček M. [Synchronous cancer duplicities of pancreas and stomach/kidney and their surgical treatment]. Rozhl Chir. 2015;94:251-255. [PubMed] |
| 17. | Fiore M, de Stefano G, Coppola N, Giorgio A. Synchronous and metachronous gastric gist with pancreatic adenocarcinoma: report of 2 cases and a review of literature. Gastroenterol Hepatol Bed Bench. 2015;8:298-301. [PubMed] |
| 18. | Santos-Fernández J, Arenal-Vera JJ, Cítores-Pascual MÁ, Fernández-Orcajo P, de-Benito-Sanz M, Benito-Fernández C, Tinoco-Carrasco C. Synchronic gastric and pancreatic ductal adenocarcinoma. A case report. Rev Esp Enferm Dig. 2015;107:642-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Arabadzhieva E, Yonkov A, Bonev S, Bulanov D, Taneva I, Vlahova A, Dikov T, Dimitrova V. A rare case with synchronous gastric gastrointestinal stromal tumor, pancreatic neuroendocrine tumor, and uterine leiomyoma. World J Surg Oncol. 2016;14:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Yonenaga Y, Kurosawa M, Mise M, Yamagishi M, Higashide S. Pancreatic-type Acinar Cell Carcinoma of the Stomach Included in Multiple Primary Carcinomas. Anticancer Res. 2016;36:2855-2864. [PubMed] |
| 21. | Wisnoski NC, Townsend CM Jr, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Zhou W, Han X, Fang Y, Han S, Cai Y, Kuang T, Lou W, Wang D. Clinical Analysis of Acinar Cell Carcinoma of the Pancreas: A Single-Center Experience of 45 Consecutive Cases. Cancer Control. 2020;27:1073274820969447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Tatli S, Mortele KJ, Levy AD, Glickman JN, Ros PR, Banks PA, Silverman SG. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol. 2005;184:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, Jung SH, Kim NY, Kim YH, Lee KH, Kim HH, Park DJ, Lee HS, Jung HC, Song IS. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010;25:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Liu K, Peng W, Zhou Z. The CT findings of pancreatic acinar cell carcinoma in five cases. Clin Imaging. 2013;37:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Narkhede RA, Desai GS, Prasad PP, Wagle PK. Diagnosis and Management of Pancreatic Adenocarcinoma in the Background of Chronic Pancreatitis: Core Issues. Dig Dis. 2019;37:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Wangermez M. Endoscopic ultrasound of pancreatic tumors. Diagn Interv Imaging. 2016;97:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Yokoyama Y, Sakata H, Uekusa T, Tajima Y, Ishimaru M. Solitary pancreatic metastasis of gastric cancer with synchronous pancreatic ductal carcinoma: A case report. Int J Surg Case Rep. 2020;70:164-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Umezaki N, Hashimoto D, Nakagawa S, Yamao T, Tsukamoto M, Kitano Y, Arima K, Yamamura K, Miyata T, Okabe H, Chikamoto A, Matsumura F, Baba H. Cystic gastric metastasis from pancreatic cancer. Surg Case Rep. 2018;4:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Takahashi M, Yoshitomi H, Kato A, Furukawa K, Takayashiki T, Kuboki S, Takano S, Sugiura K, Kawasaki K, Miyazaki M, Ohtsuka M. A case of successfully resected metachronous gastric and gallbladder metastases from pancreatic body cancer. Surg Case Rep. 2019;5:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Sasajima J, Okamoto K, Taniguchi M. Hematogenous Gastric Metastasis of Pancreatic Cancer. Case Rep Gastroenterol. 2016;10:75-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Nakazuru S, Sakakibara Y, Ishida H, Mori K, Mita E. Gastric metastasis from pancreatic neuroendocrine tumor. Gastrointest Endosc. 2018;88:559-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Rothermel LD, Strosberg C, Centeno BA, Malafa MP. Case Report of Isolated Gastric Metastasis of Pancreatic Cancer From a Diagnostic Biopsy: Management of a Rare Oncologic Entity. Cancer Control. 2020;27:1073274820904042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Ito T, Inokuma T. Gastric metastasis of pancreatic neuroendocrine tumor 5 years after surgical resection of the primary lesion. Dig Endosc. 2015;27:781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Takamori H, Kanemitsu K, Tsuji T, Kusano S, Chikamoto A, Okuma T, Iyama K. Metastatic gastric tumor secondary to pancreatic adenocarcinoma. J Gastroenterol. 2005;40:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Huang X, Li M, Zhang L, Xiong J, Lu H, Tian B. Clinical characteristics and treatment analysis of pancreatic acinar cell carcinoma: A single institutional comparison to pancreatic ductal adenocarcinoma. Surg Oncol. 2021;37:101528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Al-Hader A, Al-Rohil RN, Han H, Von Hoff D. Pancreatic acinar cell carcinoma: A review on molecular profiling of patient tumors. World J Gastroenterol. 2017;23:7945-7951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 38. | Takahashi H, Ikeda M, Shiba S, Imaoka H, Todaka A, Shioji K, Yane K, Kojima Y, Kobayashi S, Asagi A, Ozaka M, Takada R, Nagashio Y, Horiguchi S, Kasuga A, Suzuki E, Terashima T, Ueno M, Morizane C, Furuse J. Multicenter Retrospective Analysis of Chemotherapy for Advanced Pancreatic Acinar Cell Carcinoma: Potential Efficacy of Platinum- and Irinotecan-Containing Regimens. Pancreas. 2021;50:77-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Fontenot J, Spieler B, Hudson C, Boulmay B. Pancreatic acinar cell carcinoma--literature review and case report of a 56-year-old man presenting with abdominal pain. Radiol Case Rep. 2020;15:39-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fusaroli P S-Editor: Fan JR L-Editor: A P-Editor: Li JH