Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8274
Peer-review started: June 13, 2021
First decision: June 25, 2021
Revised: June 28, 2021
Accepted: July 28, 2021
Article in press: July 28, 2021
Published online: September 26, 2021
Processing time: 94 Days and 22.3 Hours
With rapid and extensive administration of inactivated coronavirus disease 2019 (COVID-19) vaccine to the general population in China, it is crucial for clinicians to recognize neurological complications or other side effects associated with COVID-19 vaccination.
Here we report the first case of Bell’s palsy after the first dose of inactivated COVID-19 vaccine in China. The patient was a 36-year-old woman with a past history of Bell’s palsy. Two days after receiving the first dose of the Sinovac Life Sciences inactivated COVID-19 vaccine, the patient developed right-side Bell’s palsy and binoculus keratoconjunctivitis. Prednisone, artificial tears and fluorometholone eye drops were applied. The patient’s symptoms began to improve by day 7 and resolved by day 54.
As mRNA COVID-19 vaccine trials reported cases of Bell’s palsy as adverse events, we should pay attention to the occurrence of Bell’s palsy after inactivated COVID-19 vaccination. A history of Bell’s palsy, rapid increase of immunoglobulin M and immunoglobin G-specific antibodies to severe acute respiratory syndrome coronavirus 2 may be risk factors for Bell‘s palsy after COVID-19 vaccination.
Core Tip: Bell’s palsy has been reported as an adverse event in coronavirus disease 2019 (COVID-19) mRNA vaccine trials, but no cases have been seen following administration of inactivated COVID-19 vaccines. Here we report a case of Bell’s palsy in a patient with a history of recurrent Bell’s palsy following one dose of inactivated COVID-19 vaccine. Because of a rapid increase of immunoglobin M- and immunoglobin G-specific antibodies to severe acute respiratory syndrome coronavirus 2 and keratoconjunctivitis of both eyes after vaccination, we assumed that the humoral immune system was intensively activated, causing local inflammation of the facial nerve and cornea. A history of Bell’s palsy and rapid increase of specific antibodies may be risk factors for Bell’s palsy after COVID-19 vaccination.
- Citation: Yu BY, Cen LS, Chen T, Yang TH. Bell’s palsy after inactivated COVID-19 vaccination in a patient with history of recurrent Bell’s palsy: A case report. World J Clin Cases 2021; 9(27): 8274-8279
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8274.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8274
The ongoing coronavirus disease 2019 (COVID-19) pandemic has had a huge impact on people’s health, daily life, and on the economy worldwide. To control the spread of the epidemic and to meet the coming opening of China, COVID-19 vaccination was initiated for the public from the end of 2020. Currently three types of COVID-19 vaccines have been granted emergency use and marketing authorization by National Medical Products Administration of China. According to the different techniques used for vaccine design, they can be divided into inactivated, live-vectored mRNA, and recombinant COVID-19 vaccines. Of those vaccines, the inactivated vaccine has been the most widely administered in China and is manufactured by two companies, Sinopharm China National Biotec Group and Sinovac Life Sciences. Phase III clinical trials of the inactivated vaccines are underway[1]. Initial efficacy and safety data on the inactivated vaccine have been reported[2,3]. To the best of our knowledge, there is no mention of facial paralysis in the literature describing the efficacy and safety of the inactivated vaccine.
Bell’s palsy is an acute, unilateral facial paralysis. In the general population, the incidence ranges from 11.5-53.3 per 100000[4]. The cause of facial palsy is still unclear. It is reported that the incidence of Bell’s palsy increased in vaccine trials[5,6]. The correlation between Bell’s palsy and vaccination should receive attention. Here we report a case of 36-year-old Chinese woman with a previous history of Bell’s palsy, who developed Bell’s palsy 2 d after receiving inactivated COVID-19 vaccine.
A 36-year-old woman presented at our outpatient department 2 d after receiving inactivated COVID-19 vaccine, with the chief complaints of eye discomfort and right-side facial weakness.
She received the first dose of Sinovac Life Sciences (Beijing, China) COVID-19 vaccine, which contains 3 μg/0.5 mL of inactivated severe acute respiratory syndrome co
The patient suffered from left-side Bell’s palsy in 2003. She recovered after 1 mon of treatment with prednisone and acupuncture. She denied any other nervous disease or other chronic diseases.
The patient had no particular individual or family history.
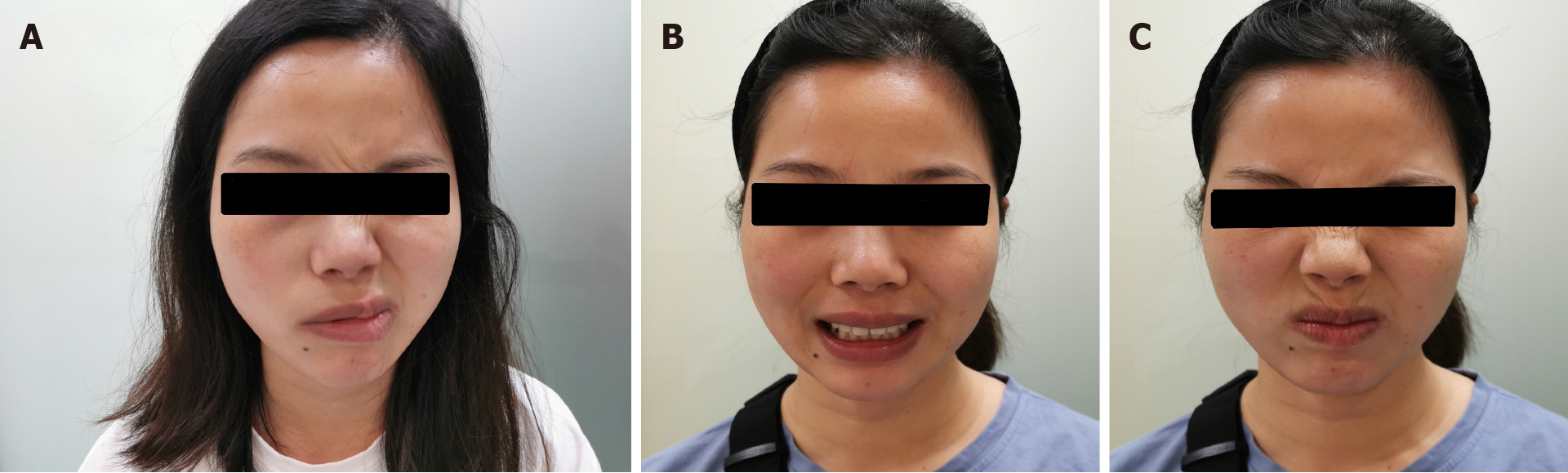
Her body weight was 52 kg. She was oriented and coherent. Cranial nerve (CN) examination was significant for House-Brackmann (H-B) grade Ⅲ isolated right CN 7 palsy (Figure 1). Her motor, sensory, and cerebellar examinations were normal.
Blood immunoglobin M (IgM) and immunoglobin G (IgG)-specific antibodies to SARS-CoV-2 whole-virion were positive. In addition, there were no positive findings in routine, blood biochemistry, serum immunoglobin A, IgM, and IgG.
There was no positive finding in a computed tomography scan of the brain.
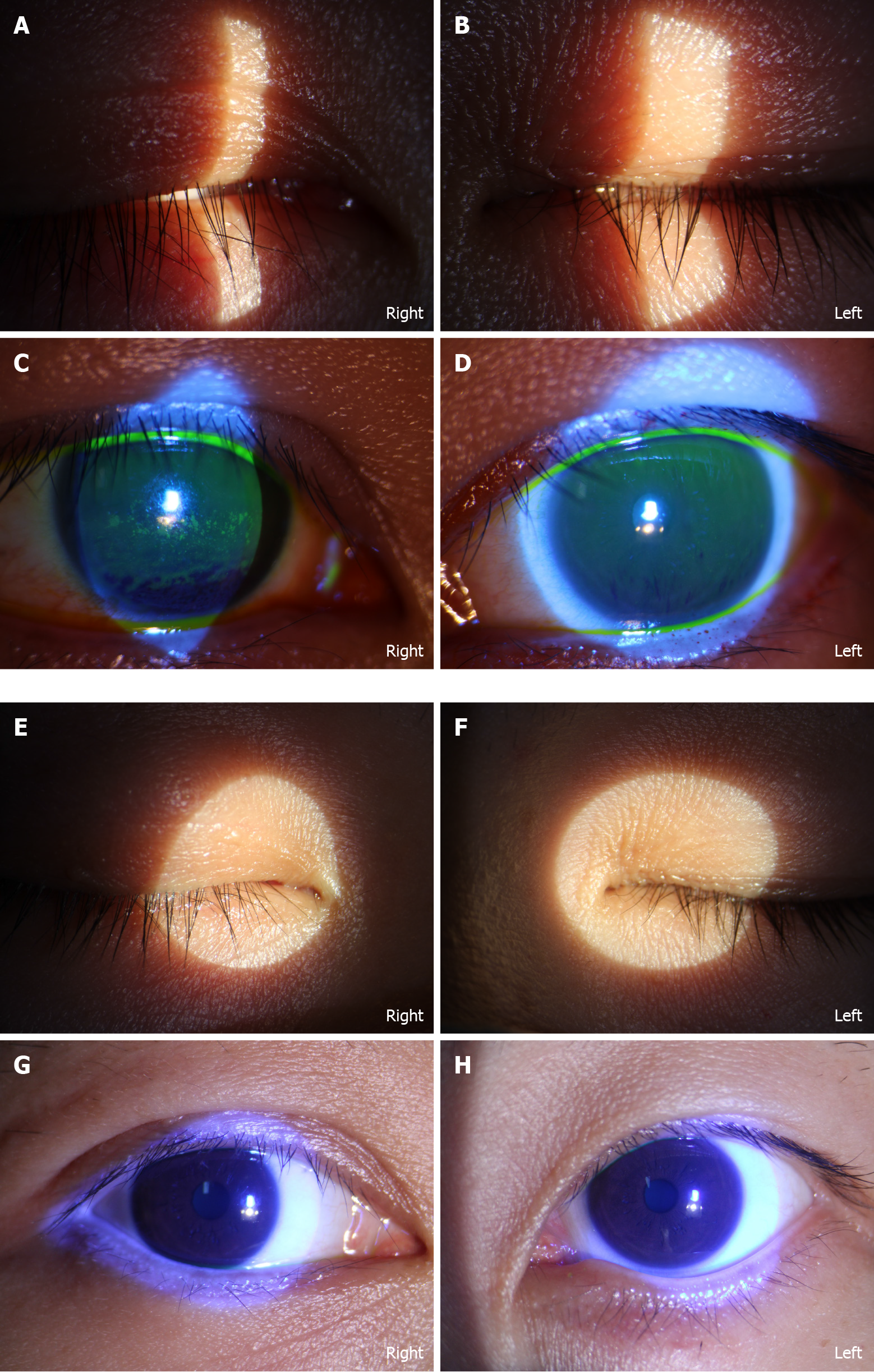
She was diagnosed with Bell’s palsy and keratoconjunctivitis.
Prednisone (40 mg/d) was administered for 1 wk. Artificial tears and fluorometholone eye drops (Santen, Osaka, Japan) were prescribed four times daily. Acupuncture therapy was applied three times weekly beginning of April 24, 2021 (Figure 2).
The patient’s symptoms began to improve by day 14, and by July 10, 2021, the pa
This is the first case of Bell’s palsy in a patient with a previous history of Bell’s palsy following one dose of inactivated COVID-19 vaccine. Bell’s palsy has been reported after administration of a COVID-19 mRNA vaccine[7,8] and patients with a history of Bell’s palsy had a three and a half to seven times higher morbidity than the general population[5]. The incidence of Bell’s palsy may also be increased following injection of other inactivated vaccines including quadrivalent meningococcal conjugate[5], H1N1, and other seasonal influenza vaccines[6].
Inactivated vaccines are the classic form used to protect against viral infection by inducing specific T cell and neutralizing antibody responses[5]. A clinical trial of inactivated COVID-19 vaccine indicated that the immune responses were induced after two doses of vaccine[3]. However, IgM- and IgG-specific antibodies to the SARS-CoV-2 whole virion tested positive after first dose of vaccine in our case. Keratoconjunctivitis is a typical manifestation of COVID-19 infection[9]. The patient’s left eye could close completely, but keratoconjunctivitis was present. We assumed that her humoral immune system was intensively activated, causing local inflammation of the facial nerve and cornea. Repajic et al[8] also reported a case of Bell’s palsy after mRNA COVID-19 vaccination in a patient with a history of Bell’s palsy. The association between Bell’s palsy history and COVID-19 vaccination could be of importance, and pathophysiological evidence needs further investigation.
Based on the analysis of this case and other COVID-19 related cases, we consider that patients with a history of Bell’s palsy may be at risk of recurrence after COVID-19 vaccination by mRNA or inactivated vaccines and physicians need to be vigilant about that. The absence of cerebrospinal fluid examination may be a limitation for this case as it is necessary to make it clear whether there was any infection in the cerebrospinal fluid. The rapid increase of IgM and IgG-specific antibodies to SARS-CoV-2 after vaccination may be a related observable factor.
| 1. | Palacios R, Patiño EG, de Oliveira Piorelli R, Conde MTRP, Batista AP, Zeng G, Xin Q, Kallas EG, Flores J, Ockenhouse CF, Gast C. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac - PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 2. | Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, Huang W, Xu W, Huang B, Wang W, Zhang W, Li N, Xie Z, Ding L, You W, Zhao Y, Yang X, Liu Y, Wang Q, Huang L, Xu G, Luo B, Liu P, Guo W. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 832] [Article Influence: 166.4] [Reference Citation Analysis (12)] |
| 3. | Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, Chen X, Liu X, Jiang C, Li J, Yang M, Song Y, Wang X, Gao Q, Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 986] [Article Influence: 164.3] [Reference Citation Analysis (0)] |
| 4. | Zhang W, Xu L, Luo T, Wu F, Zhao B, Li X. The etiology of Bell's palsy: a review. J Neurol. 2020;267:1896-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 5. | Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21:450-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 6. | Lee GM, Greene SK, Weintraub ES, Baggs J, Kulldorff M, Fireman BH, Baxter R, Jacobsen SJ, Irving S, Daley MF, Yin R, Naleway A, Nordin JD, Li L, McCarthy N, Vellozzi C, Destefano F, Lieu TA; Vaccine Safety Datalink Project. H1N1 and seasonal influenza vaccine safety in the vaccine safety datalink project. Am J Prev Med. 2011;41:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Colella G, Orlandi M, Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Repajic M, Lai XL, Xu P, Liu A. Bell's Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell's palsy. Brain Behav Immun Health. 2021;13:100217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Douglas KAA, Douglas VP, Moschos MM. Ocular Manifestations of COVID-19 (SARS-CoV-2): A Critical Review of Current Literature. In Vivo. 2020;34:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Syahputra DA S-Editor: Wang LL L-Editor: Filipodia P-Editor: Yuan YY