Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8147
Peer-review started: April 8, 2021
First decision: May 11, 2021
Revised: May 24, 2021
Accepted: July 23, 2021
Article in press: July 23, 2021
Published online: September 26, 2021
Processing time: 160 Days and 19 Hours
Gastric adenomyoma (GA) is a rare submucosal benign neoplasm that occurs mostly in the gastric antrum and is often misdiagnosed. No standard treatment has been established for this disease in cases of malignancy.
A 75-year-old woman with a 10-year history of hypertension was admitted to the Emergency Department of our hospital complaining of paroxysmal exacerbation of acute abdominal pain for 1 d with no apparent cause. Enhanced computed tomography and magnetic resonance imaging indicated a mass in the caudal pancreas, cholecystitis, and cholecystic polypus. Gastrointestinal endoscopy showed a mass arising from the gastric antrum. Due to the imaging findings, pancreatic cancer (PC), gastric lesion, cholecystitis, and cholecystic polypus were our primary consideration. Radical pancreatectomy, splenectomy, and cholecystectomy were performed successfully, and the gastric tumor was locally resected. Postoperative paraffin specimens confirmed the diagnosis of caudal PC, GA, and heterotopic pancreas (HP). Unfortunately, the patient died 13 mo later due to PC metastases to the liver, lung, and adrenal glands.
GA is a rare benign disease, especially when occurring with HP. It may stem from the same origin as HP. This is the first case report to date of a patient suffering from the simultaneous occurrence of GA, HP, and PC. GA is a lesion that can mimic other benign or malignant gastrointestinal diseases; thus, a definitive diagnosis depends on postoperative pathological biopsy. Although GA and HP are both benign lesions, they should be resected because there is a chance of malignancy. Additional research should be conducted to better understand these submucosal lesions.
Core Tip: Gastric adenomyoma (GA) is a rare gastric submucosal disease that is even rarer when combined with heterotopic pancreas (HP) or pancreatic cancer (PC). HP and GA may have the same origin. This is the first case report of the simultaneous occurrence of GA, HP, and PC. In addition, 24 patients with HP and GA were investigated to identify variations among the cases and clarify the importance of surgical resection. GA can mimic other benign or malignant gastrointestinal diseases; thus, a definitive diagnosis depends on postoperative paraffin biopsy. Surgery is a vital procedure to cure GA and HP in cases of malignant transformation.
- Citation: Li K, Xu Y, Liu NB, Shi BM. Asymptomatic gastric adenomyoma and heterotopic pancreas in a patient with pancreatic cancer: A case report and review of the literature. World J Clin Cases 2021; 9(27): 8147-8156
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8147.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8147
Heterotopic pancreas (HP), also known as aberrant pancreas and accessory pancreas, is any isolated pancreatic tissue that grows outside the pancreas itself and has no anatomical connection with the normal pancreas[1]. Gastric adenomyoma (GA), also known as myoepithelial hamartoma, myoglandular hamartoma, adenomyomatous hamartoma, and adenomyosis in the stomach[2], is a rare benign tumor occurring in the submucosal layer of the gastric wall. Over 100 years after the discovery of HP in 1727 by Jean-Schultz[1], GA was first reported by Magnus-Alsleben[3] in 1903, who described it as a lesion that consists of smooth muscle fibers and circular crescent-shaped lumina that are lined with a single-layer of cylindrical epithelium. In 1909, a physician in Germany named Heinrich differentiated gastric HP into three types: Type I, with pancreatic acini and islets; type II, with acini; and type III, with only undifferentiated ducts[4]. He then named type III HP GA, with type III containing proliferated smooth muscle cells and a mucus-excreting epithelium.
GA has a low occurrence rate; only 52 cases have been reported as of 2016, according to Bhardwaj et al[5]. From 2016 on, only eight cases of GA confirmed by pathological biopsy have been reported. These cases were examined by tissue biopsy, which should contain both heterotopic pancreatic acini in the gastric wall and several undifferentiated mucus-secreting ducts lined with columnar or cubic epithelial cells surrounded by overgrown bundles of smooth muscle cells in the outermost layer. There are only 24 cases (including ours) of simultaneous HP and GA confirmed by pathological results reported in the literature. There have been no pathological biopsy reports revealing a combination of HP, GA, and pancreatic cancer (PC).
A 75-year-old woman with a diagnosis of pancreatic mass was admitted for surgery.
A 75-year-old Chinese woman was admitted because of paroxysmal exacerbation of acute abdominal pain for 1 d with no apparent cause. The pain was not responsive to changes in body position and did not radiate to the other side. The patient did not complain of nausea, sour regurgitation, or abdominal fullness but did note slight fatigue.
The patient had hypertension for the previous 10 years, which was well controlled by drug therapy.
The patient had a more than 10-year history of hypertension that was under fairly satisfactory control via medication.
Abdominal physical examination showed a flattened belly, normal borborygmus, and epigastric tenderness and rebound pain, with a negative Murphy’s sign.
Blood biochemistry tests revealed the following: Elevated blood amylase (742 U/L; chromatometry reference range: 35-135 U/L), undetectable blood lipase (chromatometry reference range: less than 79 U/L), low albumin (32.2 g/L; reference range: 40-55 g/L), low calcium (2.06 mmol/L; reference range: 2.25-2.75 mmol/L), low platelets (98 × 109/L; reference range: 100-300 × 109/L), and highly elevated CA242 (over 150 IU/mL; reference range: 0-20 IU/mL), CA50 (289.6 IU/mL; reference range: 0-20 IU/mL), and CA199 (627.4 IU/mL; reference range: 0-37 IU/mL).
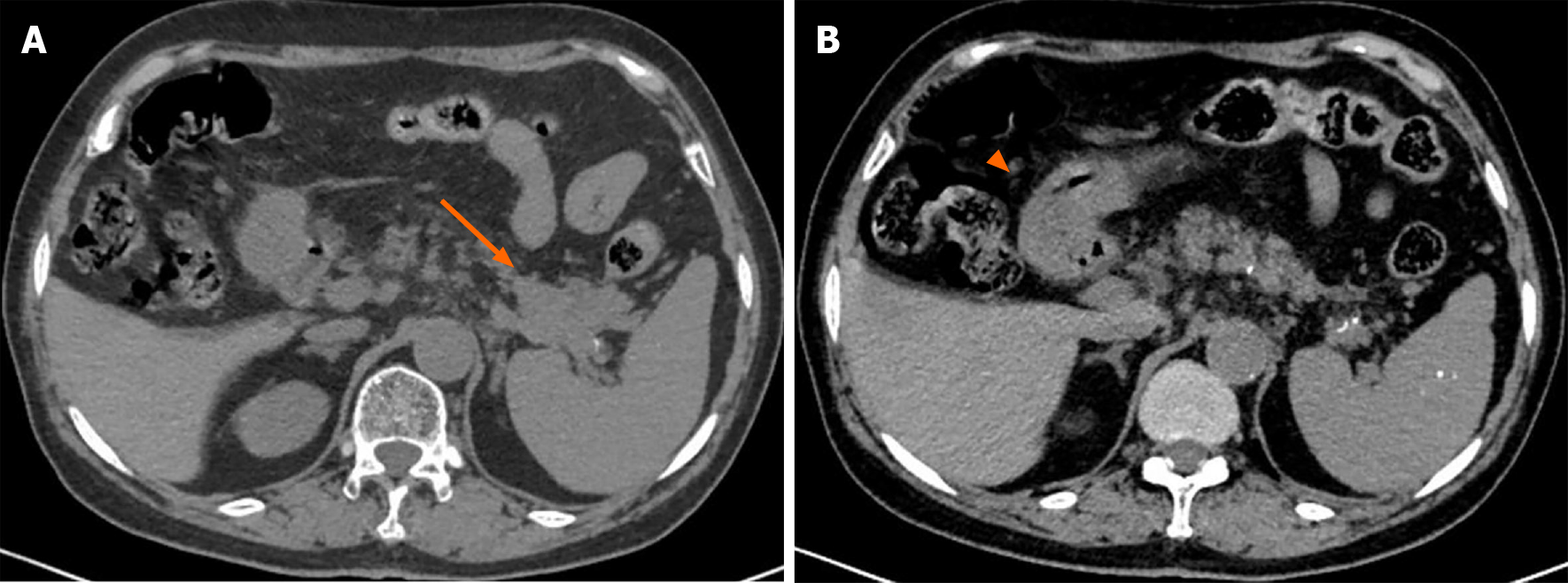
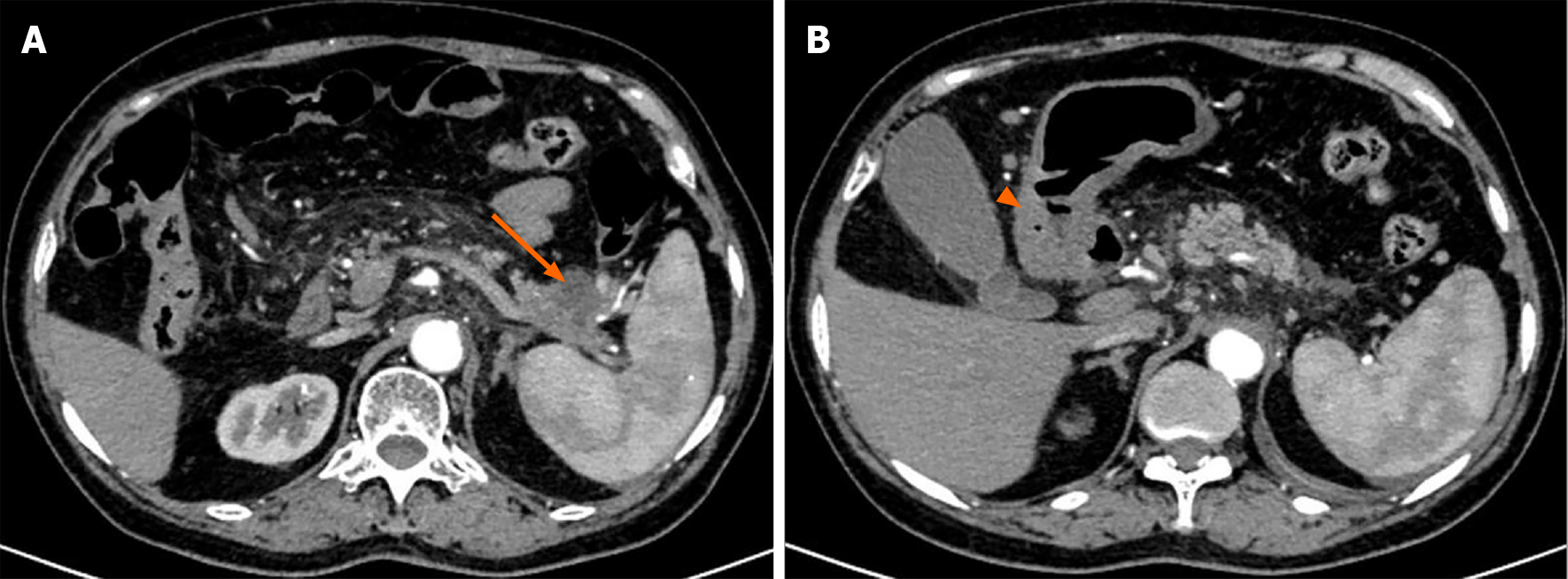
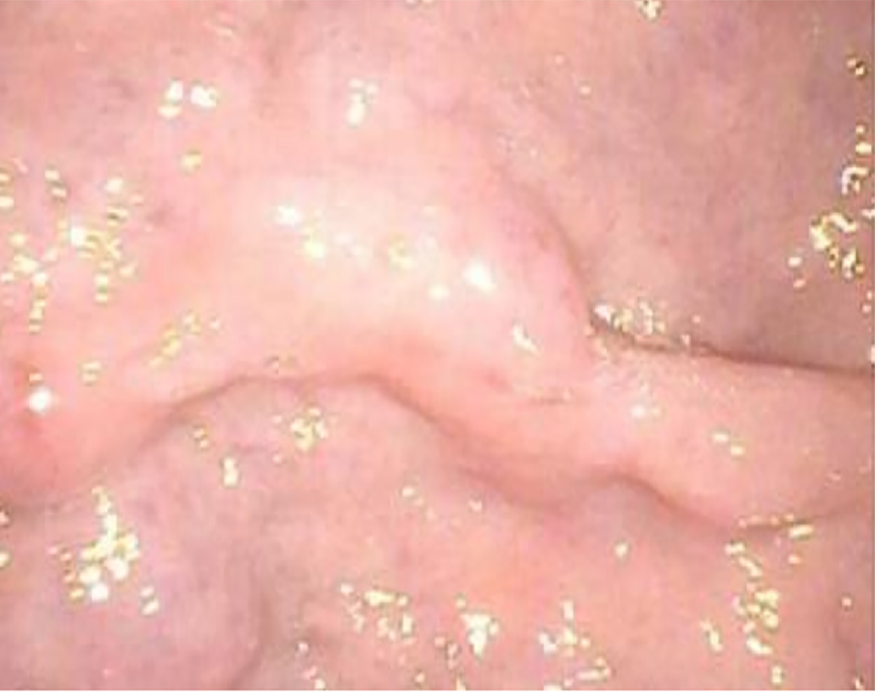
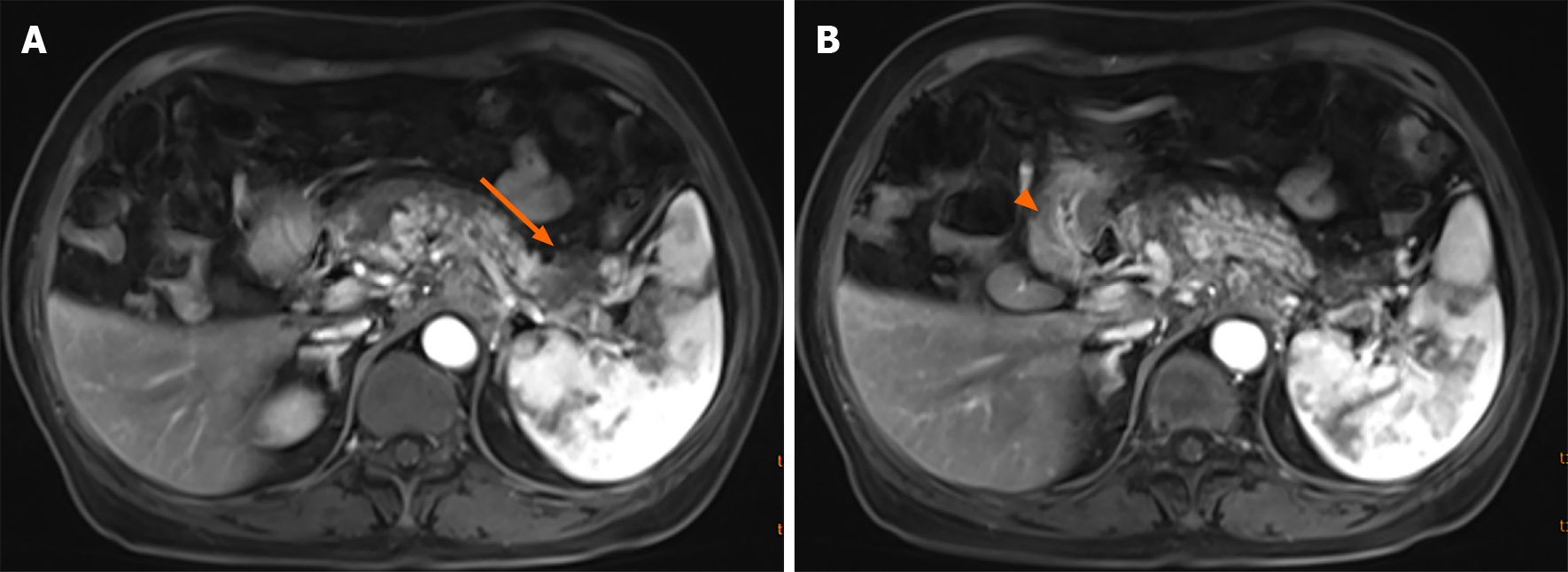
A plain CT scan showed a mass in the body and tail of the pancreas, with little exudation (Figure 1A). The morphology of the pancreas was disrupted, indicating edema, hyperemia, and fat liquefaction. The stomach appeared normal (Figure 1B). Two days later, contrast-enhanced CT showed a cystic solid mass surrounding splenic vessels in the caudal pancreas, indicating a pancreatic malignant tumor (Figure 2A). Multiple hepatic cysts, cholecystitis, and cholecystic polypus were also detected. The gastric antral wall was found to be thickened (Figure 2B). Gastric endoscopy was performed 3 d later. The images showed chronic superficial and atrophic gastritis, a protruding lesion in the submucosal area of the antrum (Figure 3), and no Helicobacter pylori infection. Contrast MRI was performed the following day and indicated PC (Figure 4A) and uniform thickening of the antral wall (Figure 4B). Due to the aforementioned imaging findings, we decided to perform operations to relieve PC and tried to figure out the nature of the gastric mass intraoperatively.
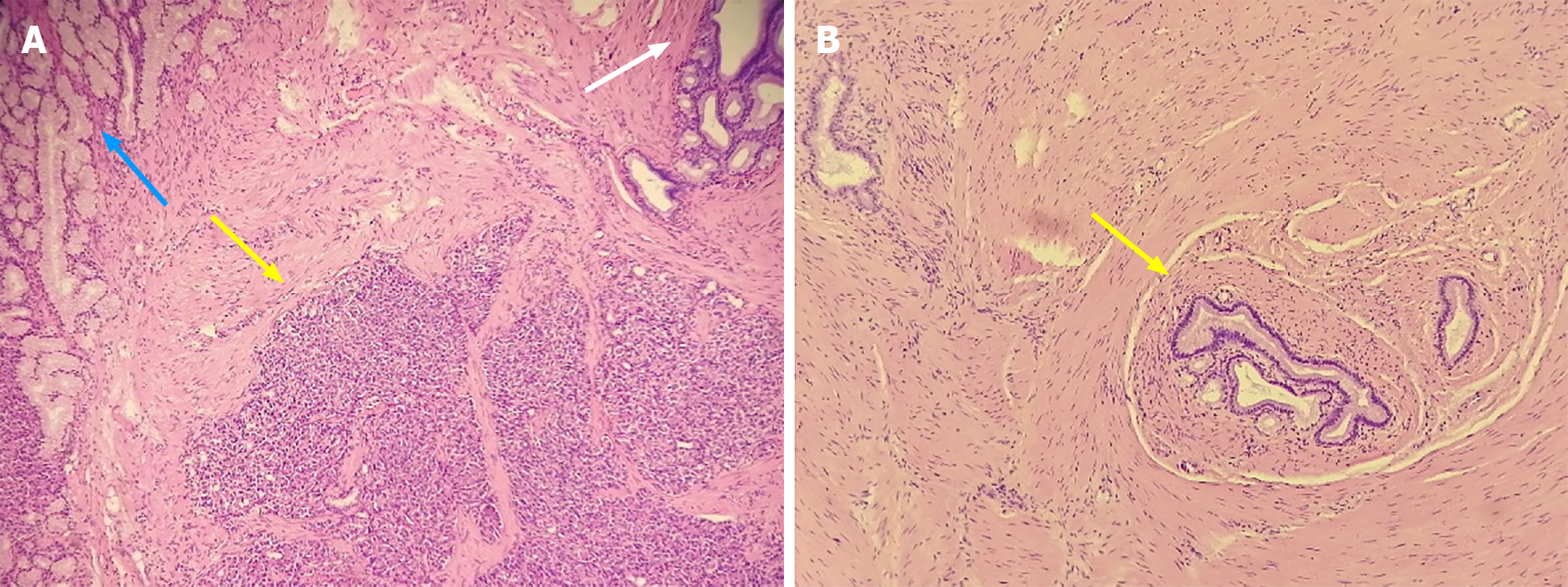
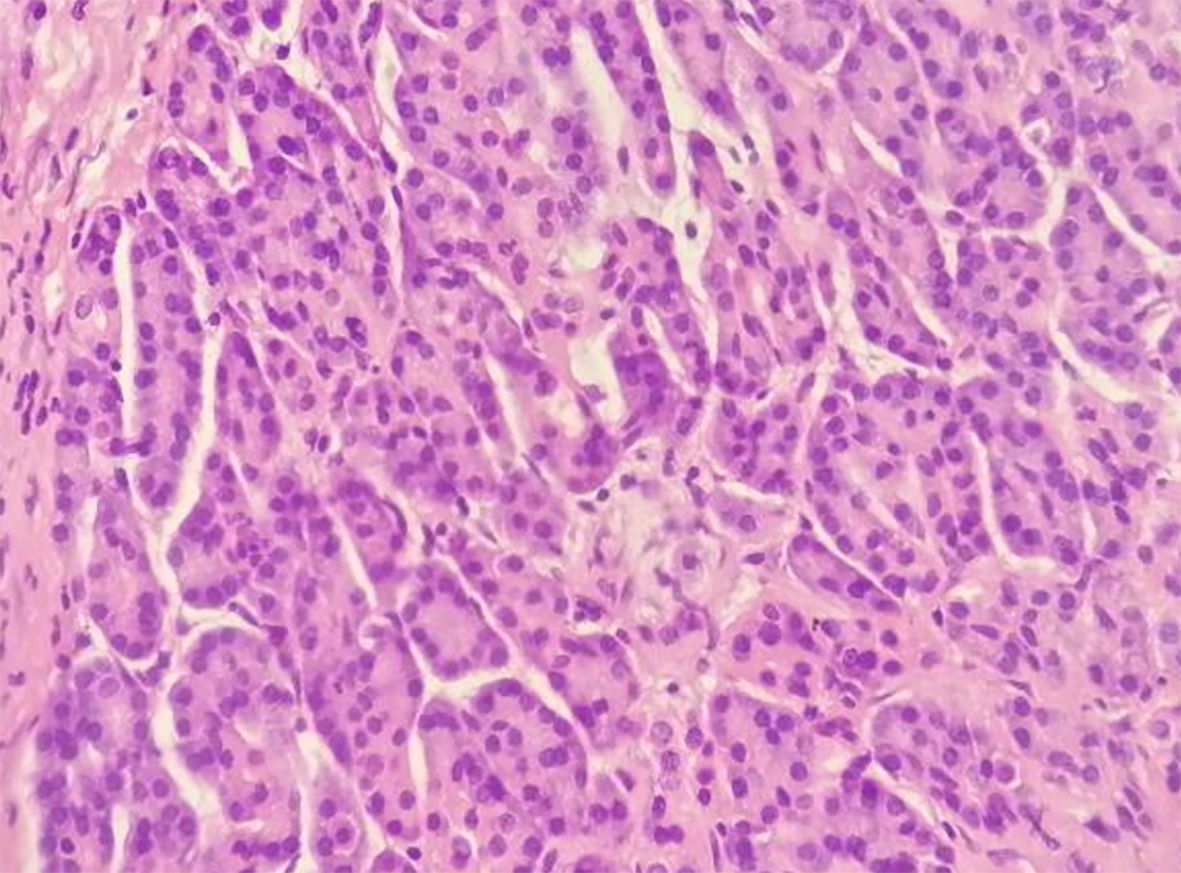
The gastric mass under pathological examination post operation was a gray, solid, and tough nodule measuring 2 cm × 2.2 cm × 1 cm in the pylorus. Immunohistochemical staining showed that the tumor cells were positive for CK8, partially positive for Ki-67, and negative for CD147. Micrography showed multiple multifocal heterotopic pancreatic acini (Figure 5A), mucus-excreting glands such as Brunner’s glands, and undifferentiated ducts surrounded by proliferative smooth muscles in the submucosal layer, which typically indicate GA with HP. The tissue in the other slice showed undifferentiated epithelial cells covered with thickened smooth muscle, indicating GA alone (Figure 5B), with no signs of malignancy. Regarding the 3.5 cm × 2 cm × 3 cm pancreatic mass, distinct dysplasia of the acini and cells arranged in a mass without polarity indicated cancer cells. The nuclei were deeply basophilic, with common karyokinesis, enlarged in size, and increased in number compared to those in normal tissue (Figure 6).
The patient was diagnosed with caudal PC, gastric HP, and GA, accompanied by acute pancreatitis, cholecystic polypus, and hypertension.
The patient underwent radical pancreatectomy, splenectomy, and cholecystectomy, and the gastric tumor was locally resected. During the operation, a mass measuring 4 cm × 4 cm × 3 cm was excised from the tail of the pancreas, and the antral mass was excavated 1 cm away from its rim. Microscopy analysis revealed that the frozen biopsy sample was a benign lesion.
Postoperatively, the patient went through sequential chemotherapy and regular follow-ups. However, she unfortunately died 13 mo postoperatively due to PC metastases to the liver, lung, and adrenal glands (April 2017).
As described by Choi et al[6] in 2000, gastric submucosal tumors are uncommon lesions (< 2% of surgically resected gastric masses). The most common types are gastrointestinal stromal tumor (GIST), leiomyoma, and gastric lipoma[7]. GA is one of the rarest diseases among gastric submucosal tumors, and its definition is based on pathological classifications. As mentioned before, there have been only 60 cases of GA up to now. In 2017, Emerson et al[7] have reported that 17 of the 571 cases that could not be fully resected by endoscopic submucosal dissection were GA, while these data contained no pathological images. Even if these lesions are indeed GA, as well as those who did not undergo operations and thus had no definitive diagnosis, a consensus has been reached that GA is an uncommon disease[8-10]. GA has occurred in all age groups, from 7 d[8] to 84 years[9]. To some extent, its low occurrence rate is because the majority of these cases are asymptomatic[10-12]; however, some GA patients experience epigastric pain and discomfort, with/without nausea[13], vomiting[14], dyspepsia[15], and melena[16]. Concerning small children or infants, the first symptom may be nonbilious vomiting[8,13-14,17-19] or esophageal reflux[20]. Some GA cases are detected by autopsy[20]. Meaningful diagnosis methods include radiographical methods such as barium meal X-ray[21], in which a filling defect in the gastric antrum or pylorus region provides an imaging diagnosis[22,23], CT[4], ultrasound (US)[13], gastrointestinal endoscopy ultrasound[24], and MRI[17]. How
Because of its rare occurrence, GA is typically not considered and can be easily misdiagnosed since it is similar to other benign or malignant gastrointestinal lesions, such as GIST[15,21,25-27], leiomyoma[5,28], gastric carcinoma[29], gastric adenocarcinoma[30], and pylorus stenosis[13-14,17], making the differential diagnosis cha
Although GA is indeed a rare submucosal disease, other diseases can accompany GA, such as HP. As previously mentioned, there have been only 20 known cases of the simultaneous occurrence of HP and GA. Among these cases (Table 1), no reports have identified any differences in the occurrence rate by sex, age, or district. Most of these patients experienced pain or discomfort, and only a few cases were detected by physical examination. Although most of the patients had benign lesions, two experienced malignant transformation. All of the biopsy tissues were collected from the pyloric antrum (either the lesser or greater curvature). There are several discussions about the source of both lesions, apart from Heinrich’s 1909 definition.
| Ref. | Year | Age | Sex | Main complaint | Diagnostic method1 | Clinical diagnosis2 | Surgery |
| Bedir et al[10] | 2018 | 26 yr | F | Occasionally detected3 | Radiography | GA | Mini-gastric bypass; subtotal gastrectomy |
| Campbell[35] | 1949 | 37 yr | F | Indigestion and discomfort | Radiography | Gastric benign tumor | Partial gastrectomy |
| Eisenberger et al[21] | 2001 | 21 yr | F | Emesis, heartburn, mid/epigastric pain, and weight loss | Radiography endoscopy | Benign GIST | Mini-laparotomy; mass dissection |
| Emerson et al[7] | 2004 | 52 yr | M | Epigastric and left upper quadrant pain, postprandial emesis, bloating, abdominal distention | Endoscopy | N/A4 | 50% gastrectomy with vagotomy |
| Faigel et al[36] | 2001 | 34 yr | F | Dyspepsia and nausea | Radiography endoscopy | N/A | Endoscopic mucosal resection |
| Floros et al[37] | 1982 | 29 yr | M | Intermittent epigastric pain | Radiography | N/A | Endoscopic wedge resection |
| Haubrich[16] | 1955 | 60 yr | F | Melena | Radiography | Early malignant neoplasm | Subtotal gastrectomy |
| Kagawa et al[38] | 2007 | 26 yr | F | Intermittent severe abdominal pain, high fever | Radiography endoscopy | Benign submucosal tumor | Laparoscopic wedge resection; pyloroplasty |
| Kamrani et al[39] | 2019 | 15 yr | F | Nausea and vomiting | Radiography endoscopy | N/A | Distal gastrectomy with a gastroduodeno-stomy |
| Keshgegian et al[28] | 1978 | 30 yr | F | Left upper quadrant pain | Radiography | Leiomyoma | Mass dissection |
| Kerkez et al[15] | 2011 | 5 yr | F | Intermittent epigastric pain | Radiography | N/A | Distal gastrectomy, cyst excision with gastroduodenostomy |
| Nabi et al[26] | 2012 | 35 yr | M | Intermittent epigastric pain and vomiting | Radiography endoscopy | GIST | Hemigastrectomy followed by gastrojejunal anastomosis |
| Portale et al[29] | 2007 | 71 yr | M | Recurrent duodenal ulcer | Radiography endoscopy | Gastric carcinoma | Subtotal gastrectomy with BII reconstruction |
| Reardon et al[40] | 1999 | 31 yr | F | Acute epigastric pain | Radiography endoscopy | Leiomyoma or a lipoma | Laparoscopic resection with an end-to-end gastroduodenostomy |
| Rhim et al[8] | 2013 | 1 wk | M | Persistent vomiting | Radiography | N/A | Antrectomy with Billroth I anastomosis |
| Song et al[27] | 2004 | 35 yr | M | Occasionally detected | Radiography endoscopy | GIST | Wedge resection |
| Zarling[41] | 1981 | 25 yr | F | Epigastric discomfort and early satiety | Radiography endoscopy | N/A | Distal antrectomy |
| Vandelli et al[42] | 1993 | 42 yr | F | Intermittent postprandial epigastric pain, nausea, and vomiting | Endoscopy | N/A | Polypectomy and Billroth II gastrectomy |
| Erberich et al[2] | 2000 | 48 yr | M | Epigastric pain and vomiting | Radiography endoscopy | Gastric polyps | Local excision of both lesions |
| Janota et al[23] | 1966 | 27 yr | M | Epigastric pain | N/A | Peptic ulcer | Partial gastrectomy; closure of the duodenal stump |
| Lasser et al[22] | 1977 | 35 yr | M | Discomfort | Radiography | N/A | Subtotal gastrectomy |
| Stewart et al[20] | 1984 | 81 yr | M | Renal failure, anemia, and melena | N/A | N/A | N/A |
| 53 yr | M | Discomfort | Radiography | N/A | Endoscopic resection |
As proposed by Erberich et al[2] in 2000, HP in the stomach is thought to be derived from the dorsal anlage. As shown in the pathological images, HP appears to be located on the surface of the polyp, accompanied by Brunner’s glands and GA in the lower layers, as if the deep-seated lesion is triggered by exocrine pancreatic acini. However, even if HP secretes hormones and fluid, these substances can only be released into the gastric lumen, without affecting smooth muscle cells and undifferentiated mucus-secreting cells in the submucosa. In another position described by Takeyama et al[18] in 2007, GA was found to be a component of hamartoma without well-differentiated pancreatic tissue and an independent lesion rather than a part of HP. However, we found that GA can accompany HP, but it can also develop alone, as depicted in Figure 5. In summary, GA can be described as an independent lesion (i.e., without HP) or with local inflammatory changes caused by HP.
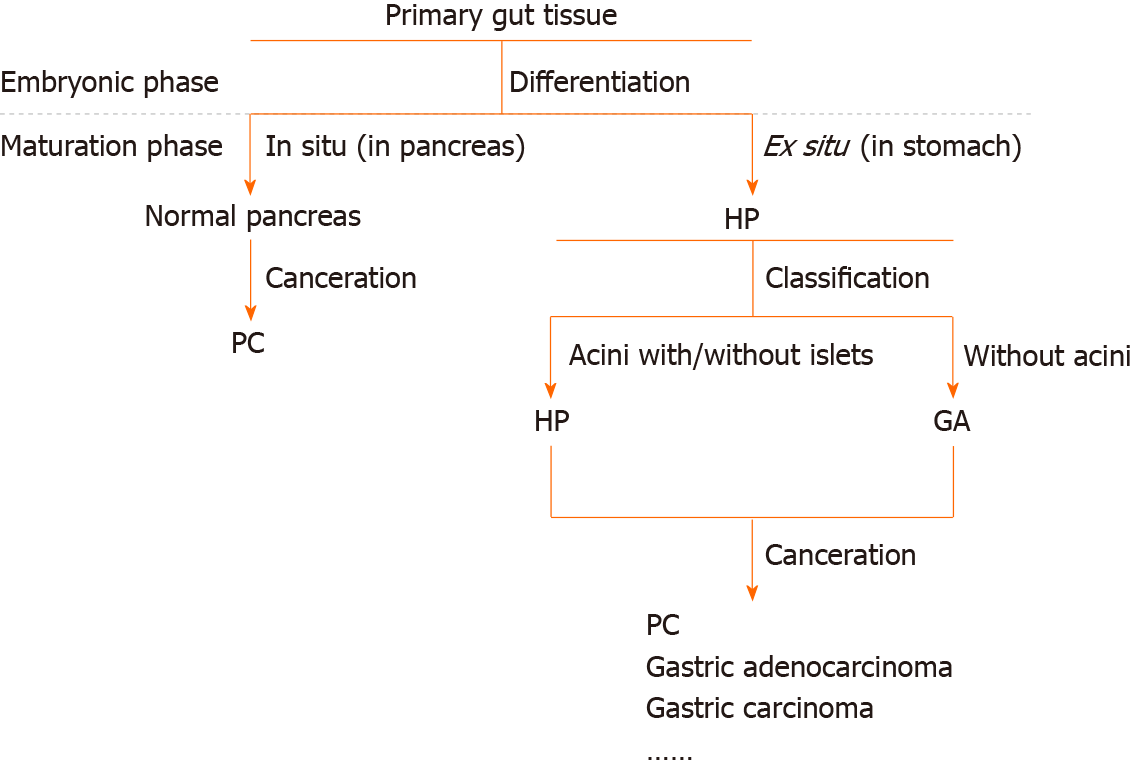
Thus, we approve Heinrich’s definition. GA and HP are, in fact, the same lesion. Differences in cytology, histology, and biological behavior exist because different parts of the primary gut remain in situ (Figure 7). In the fourth week of embryonic development, the pancreas derives from both the ventral and dorsal antrum[31], and some parts of the primary pancreatic cells that remain are affected by the gastric local microenvironment and develop into different tissues, including the original pancreatic glands, gastric glands, Brunner’s glands, undifferentiated glands, and proliferating smooth muscle cells. Different tissues exist because of the different phases at which these cells leave from the original site and the different microenvironments. The proliferating smooth muscle and undifferentiated mucus-secreting epithelial cells converge, perhaps because they are in the same differentiation state. In a word, HP and GA stem from the same origin.
A standard treatment for GA and HP has not been established. In the 20 patients with GA and HP, except for those with cancerous lesions, the operative methods included endoscopic or laparoscopic mass resection, partial gastrectomy, or subtotal and total gastrectomy depending on the severity of the disease and the general condition of the patient. However, all lesions were successfully removed, and symptoms were relieved.
Currently, more precise methods aided by the innovation of surgical instruments, such as those used for endoscopic submucosal dissection[7], are used in the differential diagnosis of gastric submucosal tumors, reducing the need for subtotal and total gastrectomy in treating benign disease[13,17]. In 2017, Sinan Wang and colleagues studied 571 patients and found 17 with GA, all of whom underwent endoscopic submucosal dissection and recovered uneventfully[7]. However, the results were not confirmed by pathological biopsy, as the pathological images were not included in that paper. In addition, some surgeons have pointed out that GA can transform into a malignant tumor[7,11,32,33]. Although not clearly depicted in previous reports, unless this benign tumor transforms into a malignant tumor or causes severe complications such as intestinal obstruction or upper gastrointestinal bleeding, the patient may only experience slight increases in white blood cell and neutrophil counts and abnormal inflammatory biomarkers, such as procalcitonin and C-reactive protein[10-12,34]. Consequently, surgery is the gold standard for diagnosing and treating patients with GA, especially those with malignant transformation[7,32]. For a patient with malignant PC, such as ours, it is important to identify the traits of the accidentally detected mass by postoperative pathological biopsy to evaluate whether it is a metastasis, a malignant tumor in situ, or a benign gastrointestinal tumor. However, the surgical method should be chosen cautiously, avoiding further harm to the patient. Thus, we performed intraoperative pathology, identified the benign characteristics of the lesion, and carried out local resection.
Nevertheless, the prognosis depends on the malignant tumor on the caudal pancreas. If it spreads to other organs and reoccurs, the patient’s chances of survival could be extremely low.
GA is a rare submucosal benign lesion that typically occurs in the gastric antrum and seldom occurs with HP. This report describes a patient diagnosed with PC along with asymptomatic GA and HP. HP and GA are actually the same lesion, but differences between the two exist because of the various differentiation statuses and local microenvironments. We investigated a previous report of 20 patients with simultaneous HP and GA and found that the surgical method used is important in the diagnosis and treatment of GA.
We would like to thank Yang GL, Liao JR, and Wang ZW for contributing to the data collection and image analysis.
| 1. | Rezvani M, Menias C, Sandrasegaran K, Olpin JD, Elsayes KM, Shaaban AM. Heterotopic Pancreas: Histopathologic Features, Imaging Findings, and Complications. Radiographics. 2017;37:484-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 2. | Erberich H, Handt S, Mittermayer C, Tietze L. Simultaneous appearance of an adenomyoma and pancreatic heterotopia of the stomach. Virchows Arch. 2000;436:172-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Magnus-Alsleben E. Adenomyome des Pylorus. hysiologie Und Für Klinische Medizin. 1903;173:137-155. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 4. | Duran Álvarez MA, Gómez López JR, Guerra Garijo T. Gastric Adenomyoma: The Unexpected Mimicker. GE Port J Gastroenterol. 2017;24:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Bhardwaj S, Sinha S, Kundu R, Kaur R. Gastric adenomyoma: a case report. Int Surg J. 2018;5:1587. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Choi YB, Oh ST. Laparoscopy in the management of gastric submucosal tumors. Surg Endosc. 2000;14:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Emerson L, Layfield LJ, Rohr LR, Dayton MT. Adenocarcinoma arising in association with gastric heterotopic pancreas: A case report and review of the literature. J Surg Oncol. 2004;87:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Rhim JH, Kim WS, Choi YH, Cheon JE, Park SH. Radiological findings of gastric adenomyoma in a neonate presenting with gastric outlet obstruction. Pediatr Radiol. 2013;43:628-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Delvaux S, Ectors N, Geboes K, Desmet V. Gastric gland heterotopia with extensive lymphoid stroma: a gastric lymphoepithelial cyst. Am J Gastroenterol. 1996;91:599-601. [PubMed] |
| 10. | Bedir R, Yılmaz R, Kalcan S, Özdemir O. Gastric adenomyoma determined incidentally during sleeve gastrectomy: A case report. J Clin Anal Med. 2018;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Park SH, Kim J, Kim M, Jung EH, Kim HK, Kim SS, Cho YS. Adenomyoma in the Body of Stomach Presenting as a Pedunculated Polyp Treated by Endoscopic Mucosal Resection. Korean J Helicobacter Upper Gastrointestinal Res. 2016;16:31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Lee H, Jang YJ, Heo J. A Rare Case of Cystic Subepithelial Tumor in the Stomach: Gastric Adenomyoma. J Korean Soc Radiol. 2015;73:389-392. [DOI] [Full Text] |
| 13. | Oviedo Gutiérrez M, Amat Valero S, Gómez Farpón A, Montalvo Ávalos C, Fernández García L, Lara Cárdenas DC, Barnes Marañón S, Granell Suárez C, Vega Mata N, López López AJ, González Guerrero M, Álvarez Muñoz V. [Infantile hypertrofic pyloric stenosis or gastric adenomyoma? Cir Pediatr. 2015;28:153-155. [PubMed] |
| 14. | Aljahdali A, Oviedo A, Blair GK. Gastric hamartoma of the pylorus in an infant. J Pediatr Surg. 2012;47:E29-E31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kerkez MD, Lekić NS, Culafić DM, Raznatović ZJ, Ignjatović II, Lekić DD, Mijac DD. Gastric adenomyoma. Vojnosanit Pregl. 2011;68:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Haubrich WS. Myo-epithelial hamartoma of the stomach. Gastroenterology. 1955;28:1027-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 17. | Sánchez García S, Rubio Solís D, Anes González G, González Sánchez S. [Gastric adenomyoma clinically simulating hypertrophic pyloric stenosis]. Radiologia. 2016;58:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Takeyama J, Sato T, Tanaka H, Nio M. Adenomyoma of the stomach mimicking infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2007;42:E11-E12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Bush WH Jr, Hall DG, Ward BH. Adenomyosis of the gastric antrum in children. Radiology. 1974;111:179-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Stewart TW Jr, Mills LR. Adenomyoma of the stomach. South Med J. 1984;77:1337-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Eisenberger CF, Kropp A, Langwieler TE, Gocht A, Izbicki JR, Knoefel WT. Heterotopic pancreatitis: gastric outlet obstruction due to an intramural pseudocyst and hamartoma. Z Gastroenterol. 2002;40:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Lasser A, Koufman WB. Adenomyoma of the stomach. Am J Dig Dis. 1977;22:965-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Janota I, Smith PG. Adenomyoma in the pylorus. Gut. 1966;7:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Chu K-. Endosonographic appearance of gastric adenomyoma. Endoscopy. 2002;34:682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Anand S, Dhua AK, Bhatnagar V, Agarwala S, Kandasamy D, Kakkar A. Gastric Adenomyosis: A Rare Cause of Pyloric Mass in Children. J Indian Assoc Pediatr Surg. 2020;25:172-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Nabi J, Authoy FN, Akhter SM. Atypical presentation of myoepithelial hamartoma in the antrum of the stomach, mimicking a gastrointestinal stromal tumor: a case report. J Med Case Rep. 2012;6:382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Song DE, Kwon Y, Kim KR, Oh ST, Kim JS. Adenocarcinoma arising in gastric heterotopic pancreas: a case report. J Korean Med Sci. 2004;19:145-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Keshgegian AA, Enterline HT. Gardner's syndrome with duodenal adenomas, gastric adenomyoma and thyroid papillary--follicular adenocarcinoma. Dis Colon Rectum. 1978;21:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Portale TR, Mosca F, Vicari S, Pulvirenti G, Fichera S, Salomone E, Puleo S. Myoepithelial hamartoma of the stomach simulating a gastric carcinoma. A case report. Tumori. 2007;93:220-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Chapple CR, Muller S, Newman J. Gastric adenocarcinoma associated with adenomyoma of the stomach. Postgrad Med J. 1988;64:801-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Böck P, Abdel-Moneim M, Egerbacher M. Development of pancreas. Microsc Res Tech. 1997;37:374-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Ling CH, Situ ZX. [Gastric adenomyoma: with report of 9 cases]. Zhonghua Wai Ke Za Zhi. 1985;23:424-425, 446. [PubMed] |
| 33. | Wang ZC, Huang XR, Xiao H, Lai RQ. Adenocarcinoma arising in gastric heterotopic pancreas type Ⅲ. Zhonghua Zhenduan Binglixue Zazhi. 2010;. |
| 34. | Inoue S, Masuda H. [A case of adenomyoma of the gallbladder with gastric polyps (author's transl)]. Nihon Shokakibyo Gakkai Zasshi. 1978;75:366-373. [PubMed] |
| 35. | Campbell RJ. Gastric adenomyosis; report of a case. Br J Radiol. 1949;22:284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Faigel DO, Gopal D, Weeks DA, Corless C. Cap-assisted endoscopic submucosal resection of a pancreatic rest. Gastrointest Endosc. 2001;54:782-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Floros D, Dosios T, Gourtsoyiannis N, Vyssoulis C. Gastric duplication associated with adenomyoma. J Surg Oncol. 1982;19:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Kagawa S, Fujiwara T, Nishizaki M, Naomoto Y, Hiroshi I, Tanaka N. Adenomyoma of the stomach presenting as localized peritonitis. Dig Dis Sci. 2007;52:3184-3187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Kamrani K, Cutler J, Austin C, Hudacko R, Bhattacharyya N. Adenomyoma causing gastric outlet obstruction. J Pediatr Surg Case Rep. 2019;42:51-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Reardon PR, Schwartz MR, Fagan SP, Reardon MJ, Brunicardi FC. Completely laparoscopic resection of a rare pyloric tumor with laparoscopically sutured gastroduodenostomy. J Laparoendosc Adv Surg Tech A. 1999;9:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Zarling EJ. Gastric adenomyoma with coincidental pancreatic rest: a case report. Gastrointest Endosc. 1981;27:175-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Vandelli A, Cariani G, Bonora G, Padovani F, Saragoni L, Dell'Amore D. Adenomyoma of the stomach. Report of a case and review of the literature. Surg Endosc. 1993;7:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe T S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Li X