Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8104
Peer-review started: May 7, 2021
First decision: June 6, 2021
Revised: June 19, 2021
Accepted: July 27, 2021
Article in press: July 27, 2021
Published online: September 26, 2021
Processing time: 131 Days and 16.9 Hours
Thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy characterized by the pentad of hemolytic anemia, fever, thrombocytopenia, renal failure, and neurological dysfunction. The formation of microthrombi in the arterioles and capillaries of various organs is one of the main pathophysiological mechanisms. Clinical manifestations of cardiac involvement in TTP patients are variable. Acute myocardial infarction has been reported as a complication with TTP as the secondary thrombotic event. Its emergence as the initial thrombotic event is extremely rare.
A 49-year-old previously healthy man was admitted for fever, typical angina chest pain 3 d prior to presentation, and newly onset left lower limb pain. The electrocardiogram illustrated ST-elevation acute myocardial infarction of the antero-lateral wall of the left ventricle. Transthoracic echocardiography depicted two large thrombi at the apex of the left ventricle and moderately reduced ejection fraction (40%). Venous Doppler ultrasound showed occlusion of the left popliteal artery. Laboratory tests showed severe thrombocytopenia, mild hemolytic anemia, elevated D-dimers, and high troponin and creatine kinase-MB. Abdo
Cardiac involvement in TTP patients is common, challenging and more often fatal, especially when other thrombotic complications coexist.
Core Tip: Physicians should be vigilant regarding the possible diagnosis of thrombotic thrombocytopenic purpura when identifying multiple thrombotic sites in the context of an acute myocardial infarction associated with thrombocytopenia. At the moment of diagnosis, plasma exchange with fresh frozen plasma replacement and immunosuppression with corticosteroids should be considered. The prognosis is severe.
- Citation: Șalaru DL, Adam CA, Marcu DTM, Șimon IV, Macovei L, Ambrosie L, Chirita E, Sascau RA, Statescu C. Acute myocardial infarction and extensive systemic thrombosis in thrombotic thrombocytopenic purpura: A case report and review of literature. World J Clin Cases 2021; 9(27): 8104-8113
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8104.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8104
Thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy characterized by the pentad of hemolytic anemia, fever, thrombocytopenia, renal failure, and neurological dysfunction. TTP affects any organ in the body as a consequence of the thromboses in terminal arterioles and capillaries. The formation of microthrombi in the arterioles and capillaries of various organs is one of the main pathophysiological mechanisms. In adults, the condition is most often immune-mediated TTP (iTTP), can be lethal without prompt diagnosis and treatment, and occurs more often in women. Congenital TTP is often detected in childhood or during pregnancy[1].
The center of TTP pathophysiology is a defect of a disintegrin and metalloproteinase with a thrombospondin type 1 motif member 13 (ADAMTS13). It can can be inherited or most commonly appears as a consequence of the development of an autoantibody (deficiency can be induced even by a small amount of anti-ADAMTS13 autoanti
ADAMTS13 is an enzyme synthesized in hepatic stellate cells whose function is to regulate von Willebrand factor (VWF) multimers. VWF, secreted by platelets and endothelial cells, is in a globular state. Proteolytic activity of ADAMTS13 on VWF is dependent on the conformational change of both proteins[3,4]. The proteolysis process reduces VWF multimer size and its hemostatic function, which leads to platelet adhesion to the endothelium, platelet activation, and consequently the formation of a platelet plug[5]. When severe ADAMTS13 deficiency (< 10%) is present, ultra large VWF multimers can accumulate, which leads to unregulated platelet adhesion and aggregation, resulting in TTP with disseminated microthrombi. An ADAMTS13 activity lower than 3% correlates with earlier age of disease onset, need for prophylactic plasma infusions, and an annual event rate > 1. To induce the clinical syndrome, a severe ADAMTS13 deficiency alone is not sufficient, and activation of the comple
The annual incidence is 1.5 up to 6 cases per million per year in adults, and approximately 90% of the cases develop in adulthood (immune-mediated). African Ame
The clinical presentation is characterized by the pentad of hemolytic anemia, fever, thrombocytopenia, renal failure, and neurological dysfunction. Less than 10% of patients with acute TTP present with the pentad described previously[17]. The pre
Clinical manifestations of cardiac involvement in TTP patients are variable and can be seen at any age. Patients can develop acute myocardial infarction (AMI) as a secon
The diagnosis is confirmed by a severe deficiency (< 10%) of ADAMTS13 activity. Undetectable haptoglobin concentration, an elevated reticulocyte count or total bilirubin (especially unconjugated), and an elevated lactate dehydrogenase level, a marker for both red cell destruction and organ ischemia, can be found in the biological profile of patients with TTP. Coombs’ testing is frequently negative, and coagulation parameters are not severely affected[1,22].
Treatment should be immediately initiated in patients with suspected TTP, being known the proportional relation with the poor prognosis suggested by high mortality and morbidity rates. Plasma exchange with fresh frozen plasma replacement and immunosuppression with corticosteroids represent the first-line therapy[23]. Immunosuppression targeting ADAMTS13 autoantibodies with the humanized anti-CD20 monoclonal antibody rituximab is frequently added to the initial therapy, as well as splenectomy can be considered. Novel agents, such as recombinant ADAMTS13, show promise for the treatment of TTP. Long-term follow-up after the acute episode is critical to monitor for relapse and to diagnose and manage chronic sequelae of this disease[1,24].
A 49-year-old previously healthy man was admitted for fever, typical angina chest pain 3 d prior to presentation, and newly onset left lower limb pain.
No history declared.
No relevant medical history.
This patient declared no smoking or alcohol history or any other noteworthy family medical history.
The heart sounds were tachycardic without any pathological murmurs. The left leg was cold and cyanotic, with no palpable pulse of the dorsalis pedis and posterior tibial arteries but with preserved sensibility and active movements.
Troponin I and creatine kinase-MB levels were elevated. Severe thrombocytopenia, anemia and elevated D-dimers were also present. The blood urea nitrogen level was elevated. Summary of laboratory data are shown in Table 1. The laboratory data included serological testing for markers of hepatitis B infection, human immunodeficiency virus status as well as hepatitis C virus. The results were negative, and thus we excluded a viral causative agent of TTP. We also performed specific tests for some of the most common bacteria cited in the literature such as Brucella melitensis, Campylobacter jejuni, Chlamydia pneumoniae, Legionella pneumophila, Mycobacterium tuberculosis, Mycoplasma pneumoniae and Salmonella typhi, all results being negative. Unfortunately, testing for Arcanobacterium pyogenes could not be performed. The electrocardiogram showed sinus tachycardia with marked ST-elevation and Q waves in DI and aVL and r waves in V1-V3.
| Test name | Value | Analytical measurement range |
| Alanine aminotransferase | 39 U/L | 0-31 U/L |
| Aspartate aminotransferase | 57 U/L | 0-31 U/L |
| Gamma glutamyl transferase | 16 U/L | 7-32 U/L |
| Glucose | 141 mg/dL | 75-115 mg/dL |
| Alkaline phosphatase | 181 U/L | 98-279 U/L |
| Amylase | 31 U/L | 28-100 U/L |
| Total bilirubin | 1.4 mg/dL | 0.05-0.02 mg/dL |
| Creatine kinase-MB | 29 U/L | < 25 U/L |
| Lactate dehydrogenase | 1081 U/L | 250-460 U/L |
| Troponin I | 540 ng/L | < 25 ng/L |
| Creatinine | 1.4 mg/dL | 0.5-0.9 mg/dL |
| Urea | 100 mg/dL | 10 -50 mg/dL |
| Uric acid | 6.31 mg/dL | 2.4-5.7 mg/dL |
| Na | 130 mmol/L | 135-148 mmol/L |
| K | 3.8 mmol/L | 3.5-5.1 mmol/L |
| Cholesterol | 77 mg/dL | 0-200 mg/dL |
| Low-density lipoprotein cholesterol | 43 mg/dL | 0-160 mg/dL |
| High-density lipoprotein cholesterol | 17 mg/dL | 42-98 mg/dL |
| Triglyceride | 86 mg/dL | 35 - 150 mg/dL |
| Thyroid-stimulating hormone | 2.05 uUI/mL | 0.25-5 uUI/mL |
| D-dimer | > 2000 ng/mL | < 200 ng/mL |
| C reactive protein | 333 mg/L | 0-5 mg/L |
| Erythrocyte sedimentation rate | 121 mm/h | 2-25 mm/h |
| White blood cells | 5910 /mm3 | 4000-10500/mm3 |
| International normalized ratio | 1.15 | 0.76-1.24 |
| Thrombocytes | 9000/mm3 | 150000-450000/mm3 |
| Red blood cells | 4.1 mil/mm3 | 4.2-5.4 mil/mm3 |
| Reticulocytes | 2% | 0.5%-1.5% |
| Hemoglobin | 11 g/dL | 12.5-16 g/dL |
| Mean corpuscular volume | 93 fL | 78-100 fL |
| Mean corpuscular hemoglobin concentration | 34 g/dL | 32-36 g/dL |
| Packed-cell volume | 38% | 37%-47% |
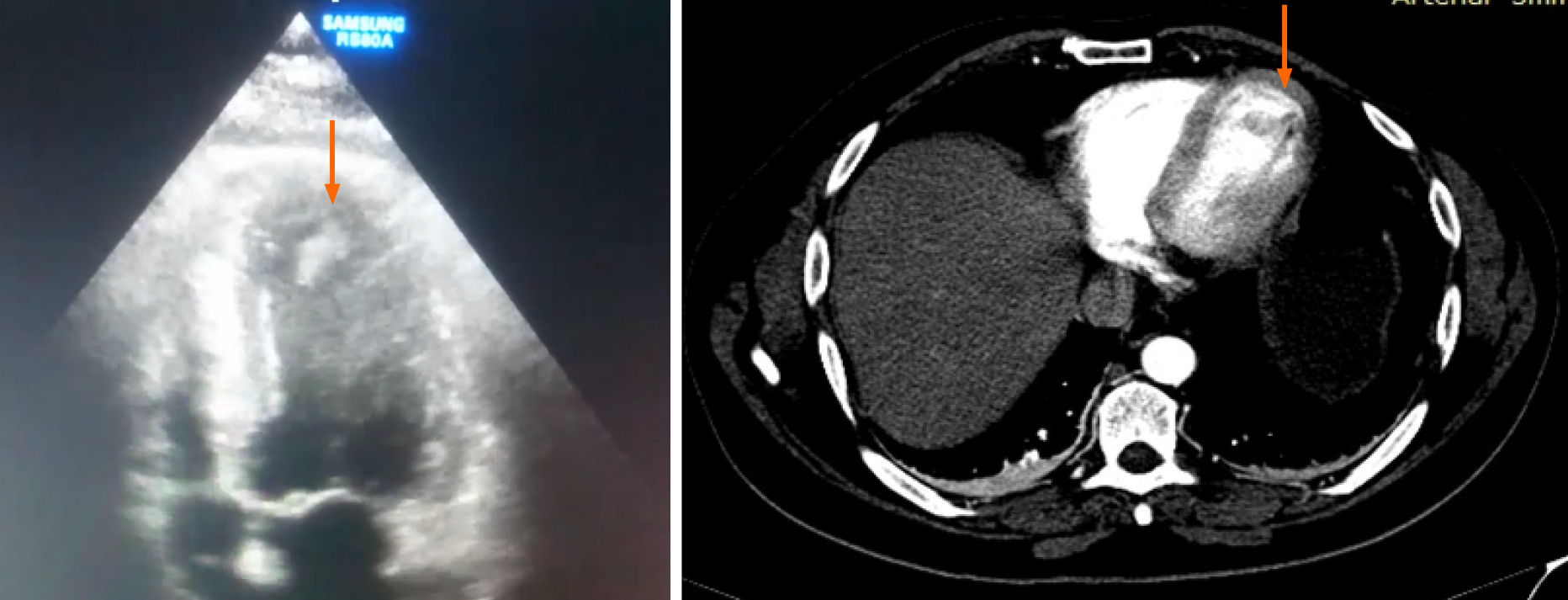
Transthoracic echocardiography depicted a mildly hypertrophied left ventricle (LV), with akinesia of the apical third of the interventricular septum, the apex, and the antero-lateral wall. The LV function was moderately depressed, with an ejection fraction of 40%. The apex was occupied by two thrombi, one adherent, 15/18 mm, and the other one very mobile, tongue-shaped, 18/9 mm (Figure 1).
Venous Doppler ultrasound showed occlusion of the left popliteal artery.
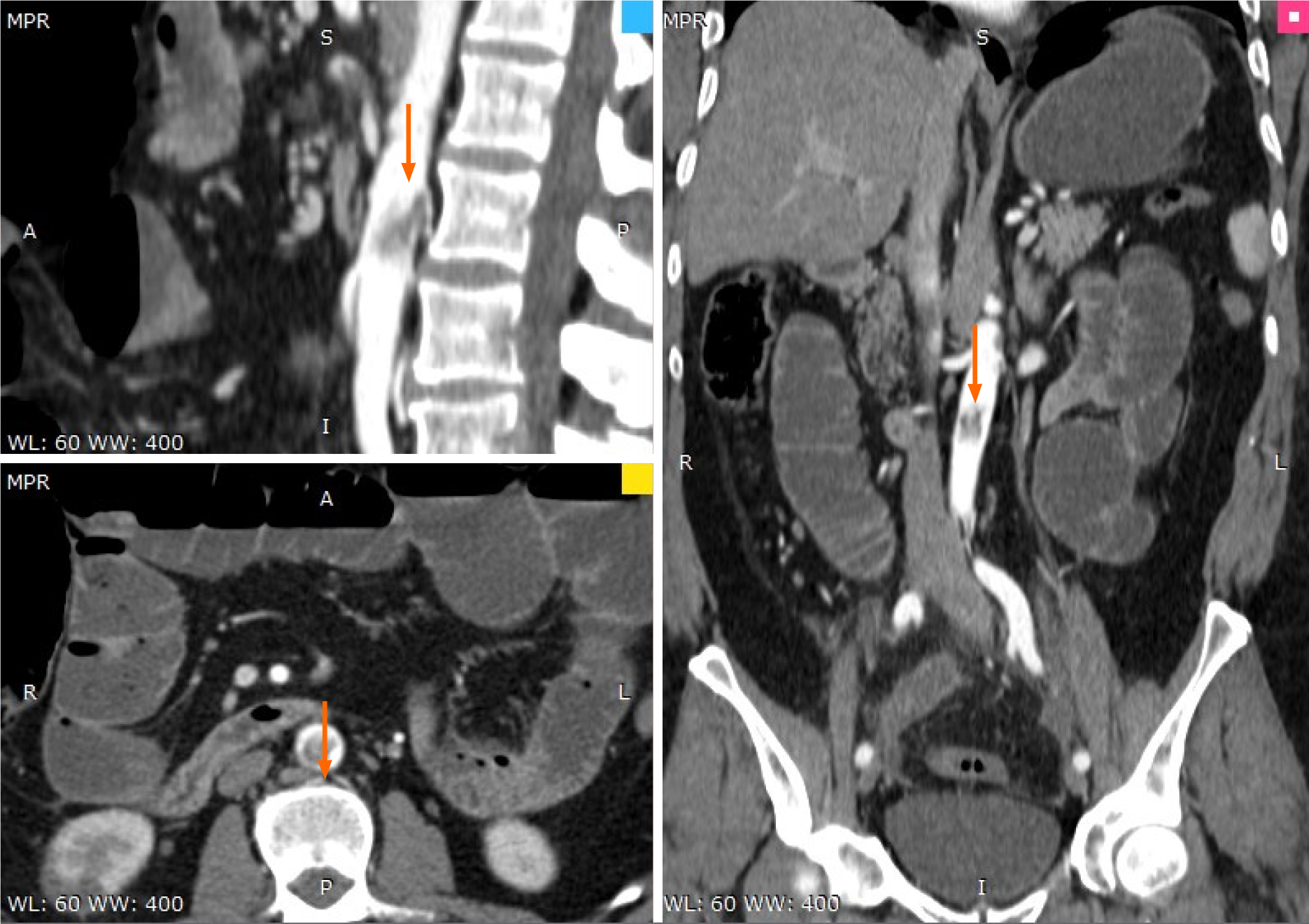
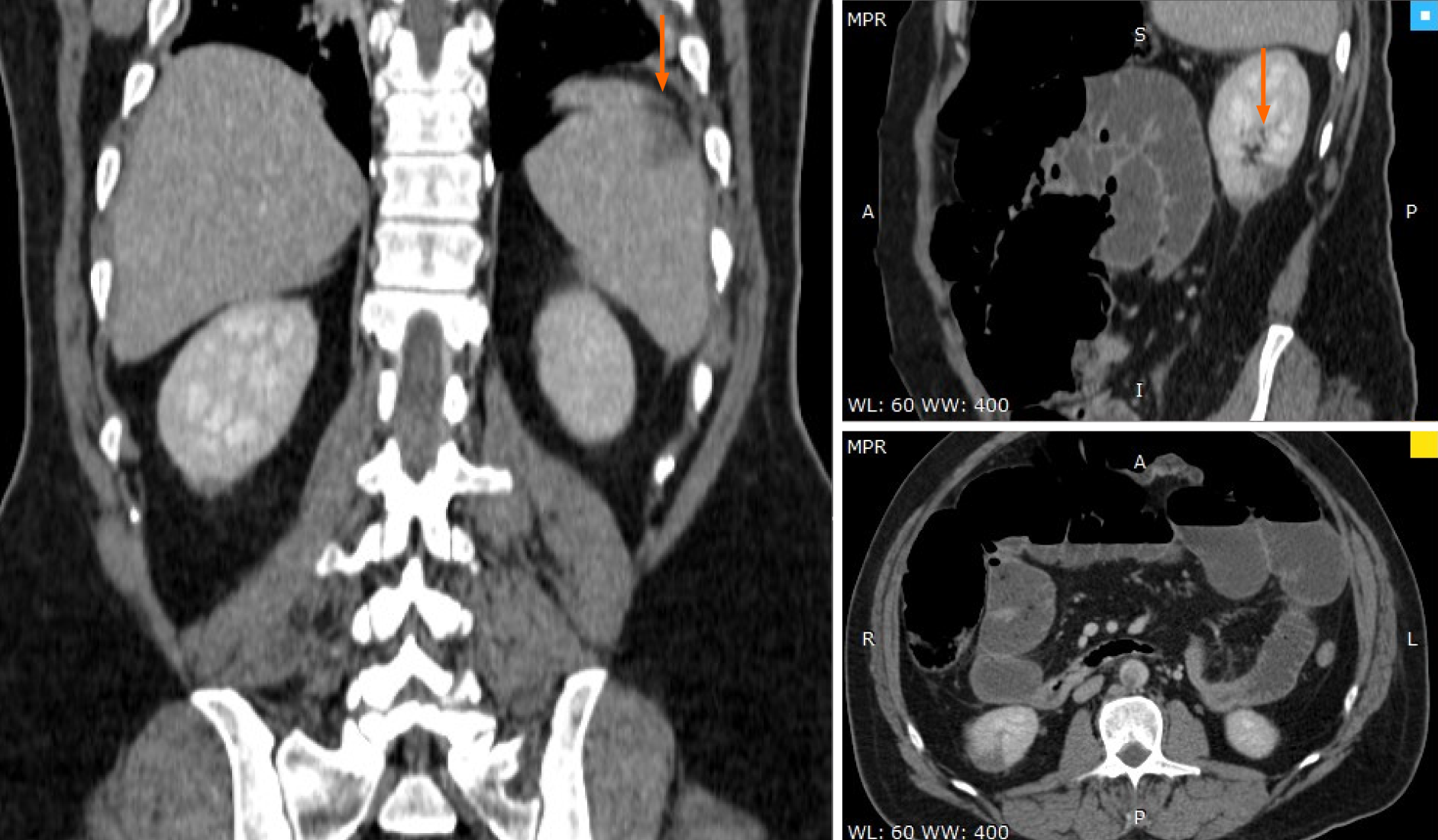
Abdominal computer scan revealed several other thrombotic sites: subocclusion of the superior mesenteric artery (approximately 40 mm), thrombosis (70% stenosis) of the posterior aortic wall, with several irregularities, spleen infarction (superior pole), renal infarction (inferior pole) as well as distended bowel with air-fluid levels and widespread edema, and areas of ileum necrosis (Figures 2-4).
AMI complicated by left ventricular thrombi, acute limb ischemia, subocclusion of the superior mesenteric artery, spleen and renal infarction, ileum necrosis, and TTP.
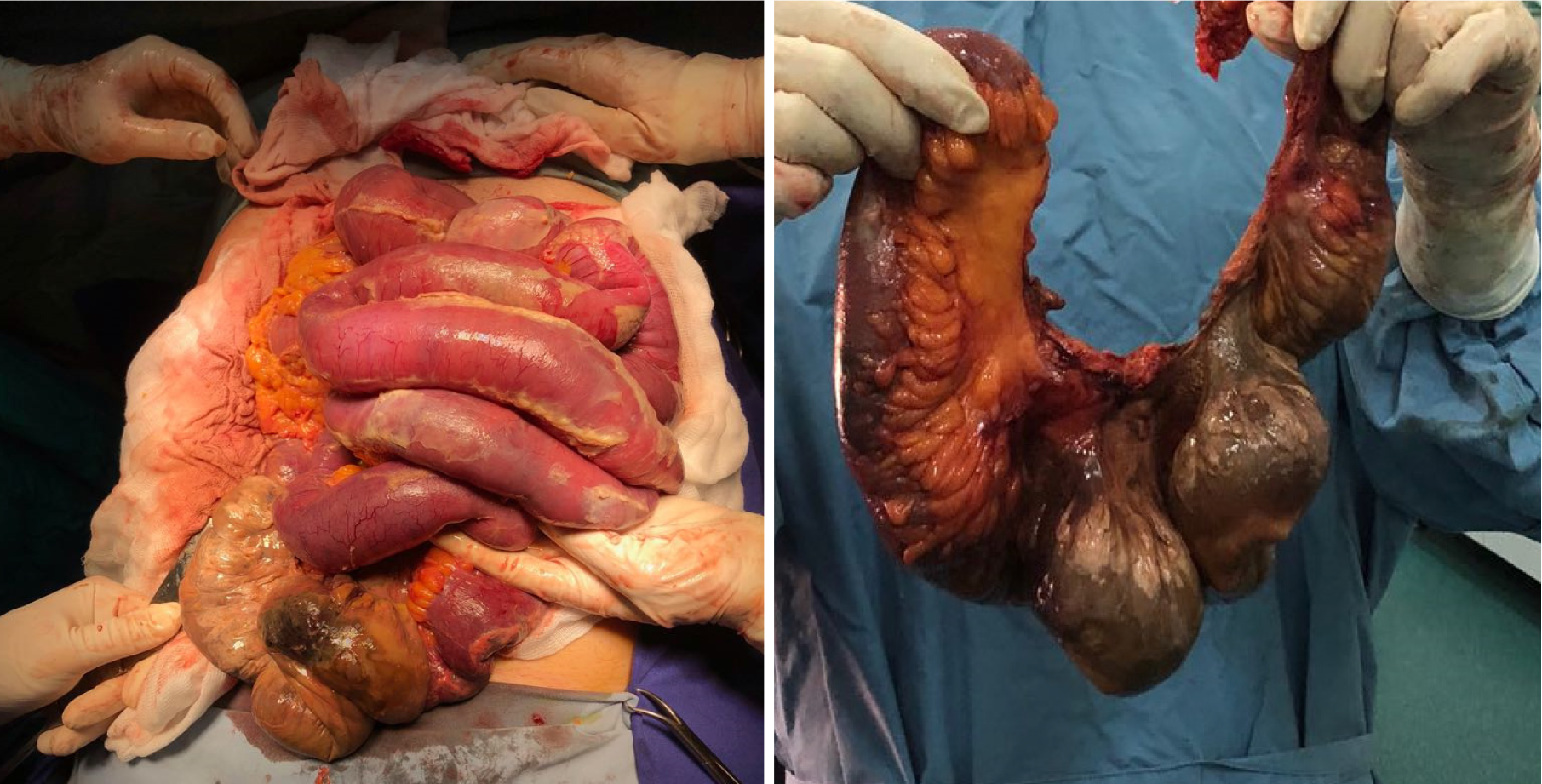
Corticosteroids were started promptly, and the patient was addressed for surgery as he developed acute abdominal pain. After an initial stabilization of the hematological profile, the patient was operated for necrotic bowel resection (enterectomy, end ileostomy, drainage, Figure 5) as well as a popliteal artery embolectomy.
The patient died soon after the surgical intervention due to multiple organ failure. None of the diagnostic workup tests proved any specific form of hereditary coagu
TTP commonly affects solid organs and is characterized by the formation of platelet or fibrin thrombi within small blood vessels, which cause laboratory abnormalities and can lead to multiple organ dysfunction. Cardiac ischemic events increase the risk of mortality in patients with TTP as the mortality rate for AMI with TTP has been reported to be 38%-50%. Due to severe thrombocytopenia and renal failure coexistent at the time of diagnosis, a cardiac catheterization sometimes cannot be performed. TTP that presented with AMI as the initial clinical presentation has been extremely rare[1].
In our case, AMI was diagnosed by typical electrocardiogram and echocardiography changes, increased serum cardiac troponin I levels, and clinical symptomatology. Chest discomfort may not be as alarming as other clinical manifestations of acute TTP (such as overt bleeding and stroke), but we must take into consideration that these patients are sometimes unable to give a complete history due to neurological manifestations of TTP such as disorientation or aphasia.
We took into consideration the presence of disseminated intravascular coagulation (DIC) due to cardiac dysfunction with intracardiac thrombi. The frequency of DIC is higher than that of TTP. Both DIC and TTP cause microvascular thrombosis associated with thrombocytopenia, bleeding tendency, and organ failure[25,26]. Although DIC and TTP are similar in appearance, fibrinolysis and platelet systems are activated in DIC, and only platelets are markedly activated in patients with TTP. On review of the literature regarding DIC associated with heart failure, an important percentage of those patients had a history of myocardial infarction with LV thrombus[23,27,28]. Most of the DIC-related thrombi tend to be small, short lived, and highly susceptible to fibrinolysis[28]. Solomon et al[29] proposed that disruption of the normal flow pattern in the LV cavity over a large (more than 1 cm in diameter) intraventricular thrombus results in damage to red blood cells, monocytes, and platelets, with activation of coagulation and widespread dispersion of activated coagulation factors[29-31].
We also considered the differential diagnosis with thrombophilia, but the specific serological tests were negative. We ruled out infectious and rheumatological causes, and thus we concluded towards iTTP.
The management of patients with TTP requires a multidisciplinary approach espe
| 1. | Takimoto T, Nakao M, Nakajo T, Chinen Y, Kuroda J, Taniwaki M. Acute myocardial infarction as the initial thrombotic event of thrombotic thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2016;27:948-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Mariotte E, Azoulay E, Galicier L, Rondeau E, Zouiti F, Boisseau P, Poullin P, de Maistre E, Provôt F, Delmas Y, Perez P, Benhamou Y, Stepanian A, Coppo P, Veyradier A; French Reference Center for Thrombotic Microangiopathies. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3:e237-e245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 3. | Scully M, Yarranton H, Liesner R, Cavenagh J, Hunt B, Benjamin S, Bevan D, Mackie I, Machin S. Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol. 2008;142:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Reese JA, Muthurajah DS, Kremer Hovinga JA, Vesely SK, Terrell DR, George JN. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 2013;60:1676-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Miesbach W, Menne J, Bommer M, Schönermarck U, Feldkamp T, Nitschke M, Westhoff TH, Seibert FS, Woitas R, Sousa R, Wolf M, Walzer S, Schwander B. Incidence of acquired thrombotic thrombocytopenic purpura in Germany: a hospital level study. Orphanet J Rare Dis. 2019;14:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Turner N, Sartain S, Moake J. Ultralarge von Willebrand factor-induced platelet clumping and activation of the alternative complement pathway in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndromes. Hematol Oncol Clin North Am. 2015;29:509-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Joly BS, Stepanian A, Leblanc T, Hajage D, Chambost H, Harambat J, Fouyssac F, Guigonis V, Leverger G, Ulinski T, Kwon T, Loirat C, Coppo P, Veyradier A; French Reference Center for Thrombotic Microangiopathies. Child-onset and adolescent-onset acquired thrombotic thrombocytopenic purpura with severe ADAMTS13 deficiency: a cohort study of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3:e537-e546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Roose E, Schelpe AS, Tellier E, Sinkovits G, Joly BS, Dekimpe C, Kaplanski G, Le Besnerais M, Mancini I, Falter T, Von Auer C, Feys HB, Reti M, Rossmann H, Vandenbulcke A, Pareyn I, Voorberg J, Greinacher A, Benhamou Y, Deckmyn H, Fijnheer R, Prohászka Z, Peyvandi F, Lämmle B, Coppo P, De Meyer SF, Veyradier A, Vanhoorelbeke K. Open ADAMTS13, induced by antibodies, is a biomarker for subclinical immune-mediated thrombotic thrombocytopenic purpura. Blood. 2020;136:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Réti M, Farkas P, Csuka D, Rázsó K, Schlammadinger Á, Udvardy ML, Madách K, Domján G, Bereczki C, Reusz GS, Szabó AJ, Prohászka Z. Complement activation in thrombotic thrombocytopenic purpura. J Thromb Haemost. 2012;10:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Ferrari S, Mudde GC, Rieger M, Veyradier A, Kremer Hovinga JA, Scheiflinger F. IgG subclass distribution of anti-ADAMTS13 antibodies in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2009;7:1703-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Martino S, Jamme M, Deligny C, Busson M, Loiseau P, Azoulay E, Galicier L, Pène F, Provôt F, Dossier A, Saheb S, Veyradier A, Coppo P; French Reference Center for Thrombotic Microangiopathies. Thrombotic Thrombocytopenic Purpura in Black People: Impact of Ethnicity on Survival and Genetic Risk Factors. PLoS One. 2016;11:e0156679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Kosugi N, Tsurutani Y, Isonishi A, Hori Y, Matsumoto M, Fujimura Y. Influenza A infection triggers thrombotic thrombocytopenic purpura by producing the anti-ADAMTS13 IgG inhibitor. Intern Med. 2010;49:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Franchini M. Thrombotic thrombocytopenic purpura: proposal of a new pathogenic mechanism involving Helicobacter pylori infection. Med Hypotheses. 2005;65:1128-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Talebi T, Fernandez-Castro G, Montero AJ, Stefanovic A, Lian E. A case of severe thrombotic thrombocytopenic purpura with concomitant Legionella pneumonia: review of pathogenesis and treatment. Am J Ther. 2011;18:e180-e185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Yagita M, Uemura M, Nakamura T, Kunitomi A, Matsumoto M, Fujimura Y. Development of ADAMTS13 inhibitor in a patient with hepatitis C virus-related liver cirrhosis causes thrombotic thrombocytopenic purpura. J Hepatol. 2005;42:420-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Gunther K, Garizio D, Nesara P. ADAMTS13 activity and the presence of acquired inhibitors in human immunodeficiency virus-related thrombotic thrombocytopenic purpura. Transfusion. 2007;47:1710-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Gandhi K, Aronow WS, Desai H, Amin H, Sharma M, Lai HM, Singh P. Cardiovascular manifestations in patients with thrombotic thrombocytopenic purpura: a single-center experience. Clin Cardiol. 2010;33:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | McCarthy LJ, Danielson CF, Skipworth EM, Peters SL, Miraglia CC, Antony AC. Myocardial infarction/injury is relatively common at presentation of acute thrombotic thrombocytopenic purpura: the Indiana University experience. Ther Apher. 2002;6:2-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | McCarthy CP, Steg G, Bhatt DL. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: a clinical conundrum. Eur Heart J. 2017;38:3488-3492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (61)] |
| 20. | Sonneveld MA, Kavousi M, Ikram MA, Hofman A, Rueda Ochoa OL, Turecek PL, Franco OH, Leebeek FW, de Maat MP. Low ADAMTS-13 activity and the risk of coronary heart disease - a prospective cohort study: the Rotterdam Study. J Thromb Haemost. 2016;14:2114-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Bongers TN, de Bruijne EL, Dippel DW, de Jong AJ, Deckers JW, Poldermans D, de Maat MP, Leebeek FW. Lower levels of ADAMTS13 are associated with cardiovascular disease in young patients. Atherosclerosis. 2009;207:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Wiernek SL, Jiang B, Gustafson GM, Dai X. Cardiac implications of thrombotic thrombocytopenic purpura. World J Cardiol. 2018;10:254-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Cataland SR, Jin M, Ferketich AK, Kennedy MS, Kraut EH, George JN, Wu HM. An evaluation of cyclosporin and corticosteroids individually as adjuncts to plasma exchange in the treatment of thrombotic thrombocytopenic purpura. Br J Haematol. 2007;136:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Cataland SR, Kourlas PJ, Yang S, Geyer S, Witkoff L, Wu H, Masias C, George JN, Wu HM. Cyclosporine or steroids as an adjunct to plasma exchange in the treatment of immune-mediated thrombotic thrombocytopenic purpura. Blood Adv. 2017;1:2075-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 25. | Belov D, Lyubarova R, Fein S, Torosoff M. Disseminated intravascular coagulation with congestive heart failure and left ventricular thrombus: a case report with literature review of 7 cases. Am J Case Rep. 2015;16:53-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Chen KT, Lin HJ, Lee WJ. Severe disseminated intravascular coagulation caused by congestive heart failure and left ventricular thrombus. Eur J Emerg Med. 2007;14:87-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Heckman TA, Rosove MH. Massive left ventricular mural thrombosis with consumption coagulopathy in congestive heart failure. West J Med. 1980;133:442-444. [PubMed] |
| 28. | Wada H, Matsumoto T, Yamashita Y, Hatada T. Disseminated intravascular coagulation: testing and diagnosis. Clin Chim Acta. 2014;436:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Solomon SA, Cotton DW, Preston FE, Ramsay LE. Severe disseminated intravascular coagulation associated with massive ventricular mural thrombus following acute myocardial infarction. Postgrad Med J. 1988;64:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Wada H, Matsumoto T, Hatada T. Diagnostic criteria and laboratory tests for disseminated intravascular coagulation. Expert Rev Hematol. 2012;5:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Dahal S, Ghimire DKC, Sapkota S, Dahal S, Kafle P, Bhandari M. Myocardial Infarction as an Early Presentation in Thrombotic Thrombocytopenic Purpura: A Rare Case Series. J Investig Med High Impact Case Rep. 2018;6:2324709618773789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan M, Yang X S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wu RR