Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8090
Peer-review started: March 31, 2021
First decision: April 28, 2021
Revised: May 6, 2021
Accepted: August 19, 2021
Article in press: August 19, 2021
Published online: September 26, 2021
Processing time: 168 Days and 19.7 Hours
Gastric stump cancer, also known as gastric remnant cancer (GRC), is one of the main complications of postgastrectomy syndrome, which usually occurs following Billroth II reconstruction. The predominant histological subtype of GRC is adenocarcinoma, whereas neuroendocrine carcinoma is relatively rare. In particular, there are few recently reported cases of mixed neuroendocrine carcinoma (MNEC) in the English literature. Here, we present an extremely rare case of MNEC of the gastric stump.
A 59-year-old patient presented to our department owing to chronic constipation. He had undergone subtotal gastric resection 35 years prior to admission because of benign peptic ulcer. After admission, the patient underwent several tests, and gastroendoscopy showed evidence of Billroth II gastrectomy and local thickening of the gastric stump mucosa at the gastrojejunostomy site, with bile reflux; pathological biopsy revealed adenocarcinoma. He was then diagnosed with GRC and underwent total gastrectomy, D2 Lymphadenectomy, and esophagojejunal Roux-en-Y reconstruction. Histopathological examination of the specimen identified MNEC comprising MNEC (60%), adenocarcinoma (30%), and squamous cell carcinoma (10%). Postoperative adjuvant chemotherapy was initiated on September 17, 2020. Taxol plus cisplatin was administered for only one cycle because of severe liver function damage, and the regimen was changed to etoposide plus cisplatin on October 10, 2020 for five cycles. The patient recovered, with no recurrence after 6 mo of follow-up.
Gastric MNECs (GMNECs) is a rare type of GRC. This study presented the unusual occurrence of GMNEC in the gastric stump. This case will contribute to improvements in our understanding of the carcinogenesis, biology, pathology, and behavior of GMNEC and GRC.
Core Tip: The predominant type of gastric remnant cancer is adenocarcinoma, whereas neuroendocrine carcinoma is particularly rare. Currently, there are few previous reports of gastric mixed neuroendocrine carcinomas (GMNECs). The information provided in this report will improve our understanding of the carcinogenesis, biology, and behavior of GMNEC and gastric remnant cancer.
- Citation: Zhu H, Zhang MY, Sun WL, Chen G. Mixed neuroendocrine carcinoma of the gastric stump: A case report. World J Clin Cases 2021; 9(27): 8090-8096
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8090.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8090
Gastric remnant cancer (GRC) is a well-known disease worldwide, first described by Balfour[1] in 1922. GRC was summarized as gastric carcinoma originating in the gastric stump after surgery, including partial or subtotal gastrectomy (SG), especially following Billroth II reconstruction[2,3]. GRC typically develops at least 5 years after gastric surgery for benign peptic ulcer disease or malignant gastric cancer[4]. The predominant histological subtype of GRC is adenocarcinoma[5], whereas there are few previous reports of adenoneuroendocrine carcinomas, especially gastric mixed neuroendocrine carcinoma (GMNEC). Here, we present a 59-year-old patient with GMNEC.
A 59-year-old patient presented to our department on August 14, 2020 because of chronic constipation.
The patient had suffered from chronic constipation for 3 mo without other symptoms.
The patient had undergone subtotal gastric resection 35 years prior to admission because of benign peptic ulcer bleeding. He had no history of other severe systemic diseases.
He had a 40-year history of smoking and drinking, and a family history of pancreatic cancer. He had no history of congenital or allergic diseases.
After admission, the patient's vital signs were normal, and there were no abnormalities observed on physical examination (e.g., palpation).
All laboratory test results, including routine bloodwork, biochemical tests, and tumor markers, were within normal limits.
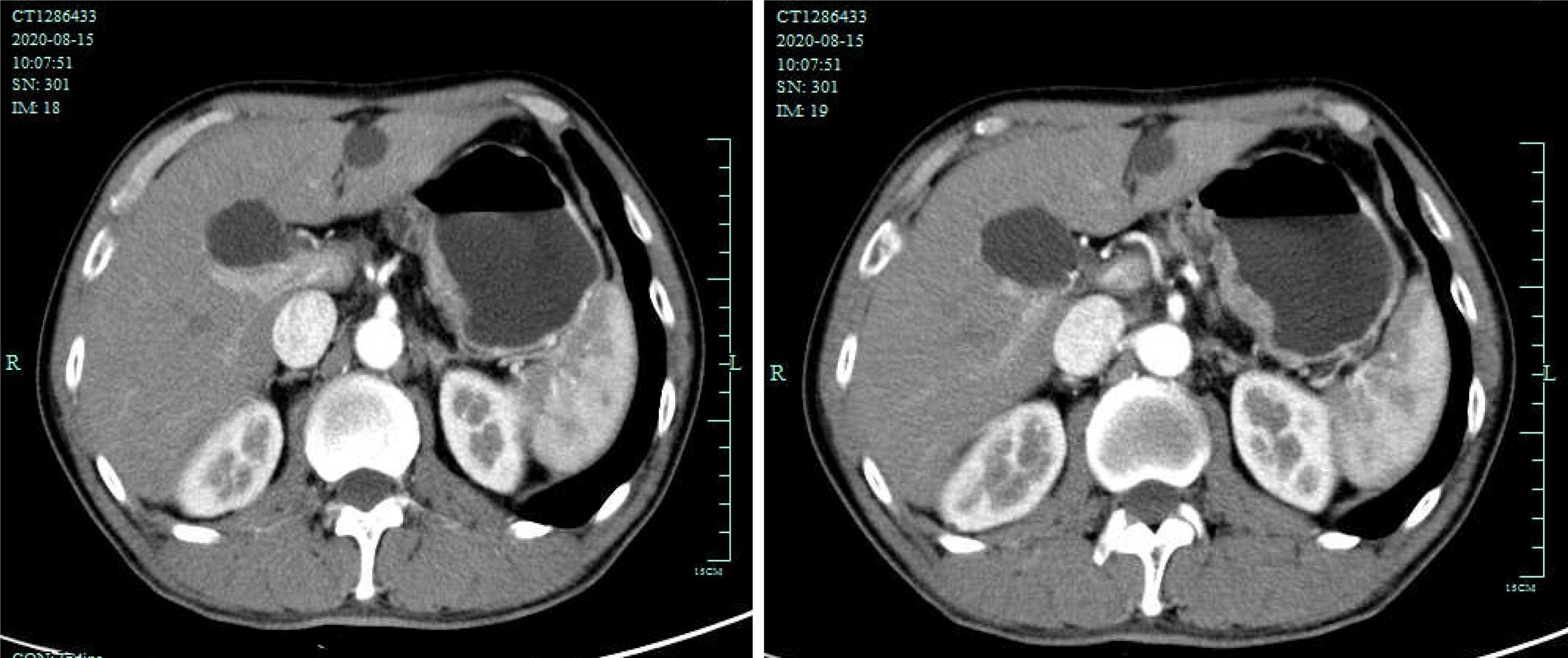
Abdominal computed tomography (CT) revealed thickening of the gastric wall at the lesser curvature (Figure 1). He then underwent gastroendoscopy, which showed evidence of Billroth II gastrectomy with Braun anastomosis and local thickening of the gastric stump mucosa at the gastrojejunostomy site with bile reflux. Additionally, pathological analysis of a biopsy sample revealed moderately differentiated adenocarcinoma.
According to the pathological analysis of the biopsy specimen, the final diagnosis was gastric stump cancer.
The patient underwent open total gastrectomy (TG), D2 Lymphadenectomy, and esophagojejunal Roux-en-Y reconstruction. The surgery required 220 min, with only 30 mL of blood loss, and no postoperative complications occurred. The patient was discharged from the hospital 12 d after surgery after the drainage catheters were removed.
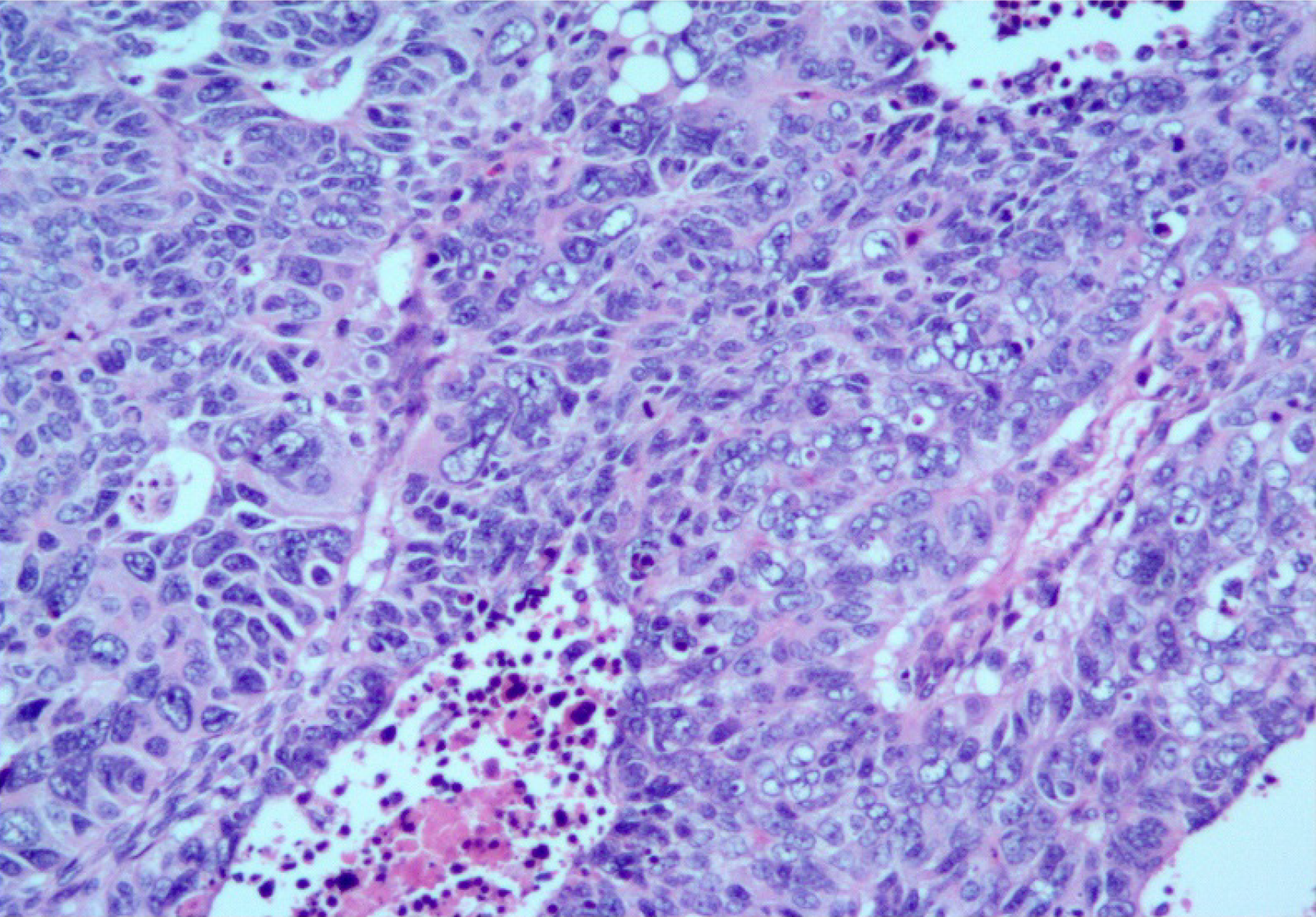
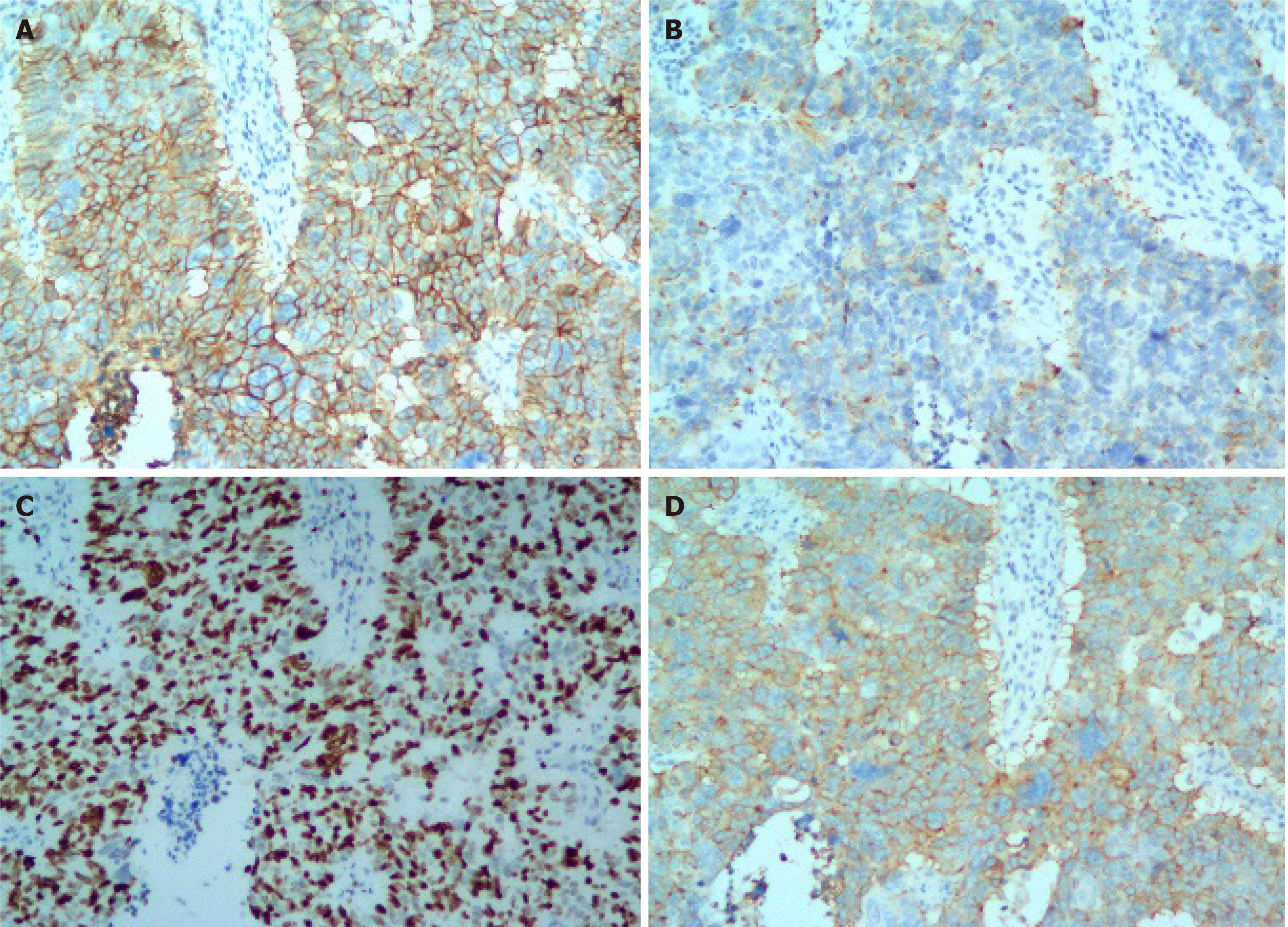
Histopathological examination of the specimen showed that it was an MNEC comprising neuroendocrine carcinoma (60%), adenocarcinoma (30%), and squamous cell carcinoma (10%). The tumor was poorly differentiated, with necrosis, (Figure 2) and had infiltrated the subserosal level without perineural invasion. Tumor emboli were present in the lymphatic vessels, none of the lymph nodes showed metastases (0/16), and the surgical margins were clear. Accordingly, the tumor-node-metastasis (TNM) classification was T3N0M0 (IIA). The immunohistochemical results were as follows: MLH1 (+), MSH2 (+), MSH6 (+), PMS2 (+), CEA (+), CA 19-9 (+), E-cadherin (+), Ki67 (80%), P16 (+), CDX2 (−), Her2 (−), P53 (−), EGFR (−), P16 (+), LcA (−), CK (+), TTF-1 (−), CgA (neuroendocrine carcinoma +), Syn (neuroendocrine carcinoma +), CD56 (neuroendocrine carcinoma +), CK7 (adenocarcinoma +), CK20 (adenocarcinoma +), villin (adenocarcinoma +), P40 (squamous cell carcinoma +), CK5/6 (squamous cell carcinoma +), and P63 (squamous cell carcinoma +). Together, these results confirmed the diagnosis of GMNEC (Figure 3).
Postoperative adjuvant chemotherapy was initiated on September 17, 2020. Taxol plus cisplatin was administered for only one cycle because of severe liver function damage, and the regimen was changed to etoposide plus cisplatin on October 10, 2020, for five cycles. The patient experienced mild side effects, such as vomiting, during chemotherapy, but no dose reduction was necessary. He has been followed-up for 6 mo without recurrence.
GMNECs are a rare type of malignant tumor in the digestive system[6]. Mak et al[7] reported that the occurrence rate of GRC ranged from 0.9%–12.9%, and the pooled prevalence of GRC was 2.6%. GMNEC is a particularly rare type that exhibits aggressive behavior and a poor prognosis, and it accounts for 0.1%–0.6% of all gastric carcinomas[8]. Following a PubMed literature search, we identified no cases with similar histopathological findings before July 15, 2014[5]. Although GRC develops at least 5 years after primary gastrectomy, it is typically considered a separate clinical entity[1].
The carcinogenesis of GRC is closely related to chronic duodenogastric reflux of bile or pancreatic juice, sometimes it also related to hypochlorhydria second to denervation after vagotomy[9-11]. In the present case, the Braun anastomosis site was only approximately 5 centimeters away from the gastrojejunostomy site, which explains why a large amount of bile and pancreatic juice refluxed. The carcinogenesis of GMNECs is likely closely related to this reflux.
The Japanese histological classification of gastric carcinoma defines neuroendocrine carcinoma as a specific type. In 1993, Rindi et al[12] raised a classification system for gastric neuroendocrine carcinoma in which cancers were divided into three types according to their underlying etiology, pathophysiology, and presentation, as follows: (1) Associated with chronic atrophic gastritis and hypergastrinemia; (2) Associated with multiple endocrine neoplasia type 1, Zollinger–Ellison syndrome, and hypergastrinemia; and (3) Gastrin-independent and sporadic in occurrence, which is considered to be the most invasive gastric neuroendocrine tumor. In our study, there was no optimal strategy for tumor classification because of its rarity and heterogenous components according to histological examination and immunohistochemical staining.
For patients with gastric neuroendocrine carcinoma, radical gastrectomy with regional lymph node dissection should be considered a priority. Cazzo and de Saito[5] reported that there is no optimal strategy for the management of GMNECs because of their rarity and complicated pathological characteristics. Therefore, the most aggressive component should be taken into account when treatment options are considered. TG is a widely accepted approach for treating GRC. Ma et al[13] reported that SG was a suitable alternative surgical procedure for GRC located at the anastomotic site after distal gastrectomy for benign diseases. Of 45 patients, 21 patients underwent SG with lymph node dissection. A similar rate of postoperative complications was observed between the two groups, similar to the findings reported by Hosokawa et al[14] and Irino et al[15]. The short-term outcomes and long-term prognosis of SG are comparable to those of TG. In the present study, we performed TG with lymph node dissection (similar to radical gastrectomy) because the patient strongly desired that his entire stomach be removed.
After surgery, adjuvant chemotherapy should also be provided[11,16,17]. However, there is no standardized chemotherapy for GMNECs because of the rarity of these tumors. For extra-pulmonary high-grade NECs, EP regimen, which combined with etoposide and cisplatin, is always suggested as first-line treatment[18]. In the present study, we chose taxol plus cisplatin (TP regimen) as the first-line chemotherapy, but because of severe liver function damage after the first cycle, we decided to treat this patient with the EP regimen as an alternative first-line therapy. The patient experienced mild side effects during treatment, with no cancer recurrence. Although the survival rate of patients with resectable small cell lung cancer has been improved by radiotherapy, the role of radiotherapy in the treatment of GMNECs remains controversial[11].
The prognosis or GMNECs is not well-defined[5,19]. Compared with benign primary gastric diseases, malignant disease is much more aggressive, according to the TNM classification, which can reflect the outcomes of GRC. A lower TNM stage is associated with a higher 5-year survival rate[20]. However, Mak et al[7] reported that the 5-year survival rate of GRC patients with primary gastric carcinoma was higher than that for patients with benign diseases. The present data indicate that high-grade GMNECs appear to lead to a better prognosis than pure neuroendocrine carcinomas, whereas the overall prognosis of intermediate-grade MNECs is poorly understood[5,6,21]. Furthermore, low-grade GMNECs, especially presenting as the linitis plastica form, are associated with a poor prognosis as most patients die within 10 mo after surgery[5]. Veits et al[21] reported that the prognosis of GMNECs is very poor (overall survival: < 6 mo). Ma et al[11] reported a similar case of GMNEC in 2018. The tumor classification was T3N0M0 (stage IIIA), and the patient died 31 mo after surgery because of disease progression. Our patient with GMNEC is still alive, without recurrence.
There are limitations to our study. This was a single case of a rare type of carcinoma, and with the short follow-up of 6 mo, further studies are needed to better understand the carcinogenesis, behavior, and treatment of this disease.
GMNECs are a rare type of GRC. Our study presented the particularly unusual occurrence of GMNEC in the remnant stomach following gastrectomy surgery with Billroth II reconstruction. This case will contribute to improvements in our understandings of the carcinogenesis, biology, behavior,pathology, and treatment of GMNEC and GRC.
We thank all of our colleagues at the Department of Gastrointestinal Surgery and the Department of Pathology, the Affiliated People's Hospital of Ningbo University.
| 1. | Balfour DC. Factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg. 1922;76:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 3. | Kondo K. Duodenogastric reflux and gastric stump carcinoma. Gastric Cancer. 2002;5:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Kung CY, Fang WL, Wang RF, Liu CA, Li AFY, Wu CW, Shyr YM, Chou SC, Huang KH. Prognosis and clinicopathologic features in patients with gastric stump cancer after curative surgery. Curr Oncol. 2020;27:e259-e264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Cazzo E, de Saito HP. Mixed adenoneuroendocrine carcinoma of the gastric stump following Billroth II gastrectomy: case report and review of the literature. Sao Paulo Med J. 2016;134:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel). 2012;4:11-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Mak TK, Guan B, Peng J, Chong TH, Wang C, Huang S, Yang J. Prevalence and characteristics of gastric remnant cancer: A systematic review and meta-analysis. Asian J Surg. 2021;44:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Kang SH, Kim KH, Seo SH, An MS, Ha TK, Park HK, Bae KB, Choi CS, Oh SH, Choi YK. Neuroendocrine carcinoma of the stomach: A case report. World J Gastrointest Surg. 2014;6:77-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hu X, Tian DY, Cao L, Yu Y. Progression and prognosis of gastric stump cancer. J Surg Oncol. 2009;100:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Tanigawa N, Nomura E, Niki M, Shinohara H, Nishiguchi K, Okuzawa M, Toyoda M, Morita S. Clinical study to identify specific characteristics of cancer newly developed in the remnant stomach. Gastric Cancer. 2002;5:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Ma FH, Xue LY, Chen YT, Xie YB, Zhong YX, Xu Q, Tian YT. Neuroendocrine carcinoma of the gastric stump: A case report and literature review. World J Gastroenterol. 2018;24:543-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 379] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Ma F, Li Y, Li W, Kang W, Liu H, Ma S, Wang B, Xie Y, Zhong Y, Chen Y, Xue L, Tian Y. Is subtotal gastrectomy feasible for the treatment of gastric stump cancer located at the anastomotic site after distal gastrectomy for benign lesions? World J Surg Oncol. 2020;18:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Hosokawa Y, Konishi M, Sahara Y, Kinoshita T, Takahashi S, Gotohda N, Kato Y. Limited subtotal gastrectomy for early remnant gastric cancer. Gastric Cancer. 2014;17:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Irino T, Hiki N, Nunobe S, Ohashi M, Tanimura S, Sano T, Yamaguchi T. Subtotal gastrectomy with limited lymph node dissection is a feasible treatment option for patients with early gastric stump cancer. J Gastrointest Surg. 2014;18:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Zhang M, Zhao P, Shi X, Zhao A, Zhang L, Zhou L. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine neoplasms in a Chinese population: a large, retrospective single-centre study. BMC Endocr Disord. 2017;17:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Xie JW, Sun YQ, Feng CY, Zheng CH, Li P, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Yang YH, Huang CM. Evaluation of clinicopathological factors related to the prognosis of gastric neuroendocrine carcinoma. Eur J Surg Oncol. 2016;42:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Okita NT, Kato K, Takahari D, Hirashima Y, Nakajima TE, Matsubara J, Hamaguchi T, Yamada Y, Shimada Y, Taniguchi H, Shirao K. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer. 2011;14:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Lee EJ, Park SM, Maeng L, Lee A, Kim KM. Composite glandular-endocrine cell carcinomas of the stomach: clinicopathologic and methylation study. APMIS. 2005;113:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K; Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 764] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 21. | Veits L, Lang-Schwarz C, Volkholz H, Falkeis C, Vieth M, Schulz H. Mixed adenoneuroendocrine carcinoma (MANEC) of the esophagogastric junction predominantly consisting of poorly differentiated neuroendocrine carcinoma. Endoscopy. 2013;45 Suppl 2:E16-E17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Syahputra DA S-Editor: Liu M L-Editor: A P-Editor: Li JH