Published online Sep 26, 2021. doi: 10.12998/wjcc.v9.i27.8061
Peer-review started: May 18, 2021
First decision: June 15, 2021
Revised: June 29, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: September 26, 2021
Processing time: 121 Days and 5.3 Hours
In recent years, the incidence of cervical cancer has increased with increasing life pressures and changes in women's social roles, posing a serious threat to women's physical and mental health.
To explore the clinical effect of Endo combined with concurrent radiotherapy and chemotherapy in the treatment of advanced cervical squamous cell carcinoma.
A total of 120 patients admitted to the oncology department of our hospital were selected as the research subjects. They were equally divided into the test group and the control group (60 patients each) with a random number table. The test group was treated with Endo combined with concurrent radiotherapy and chemotherapy, and the control group was treated with concurrent radiotherapy and chemotherapy. We compared the serum thymidine kinase 1 (TK1), human epididymis protein 4 (HE4), vascular endothelial growth factor (VEGF), and squamous cell carcinoma-associated antigen (SCC-Ag) levels, the clinical effects and survival before and after radiotherapy and chemotherapy, the quality score, and the 3-year follow-up outcomes between the two groups.
After chemotherapy, the complete remission + partial remission rate was 85.00% in the test group and 68.33% in the control group; the difference was not statistically significant (P > 0.05). Before chemotherapy, the serum TK1, HE4, VEGF, and SCC-Ag levels of the two groups were not significantly different (P > 0.05). After chemotherapy, the levels of serum TK1 (1.27 ± 0.40 pmol/L), HE4 (81.4 ± 24.0 pmol/L), VEGF (235.1 ± 38.0 pg/mL), and SCC-Ag (1.76 ± 0.55 ng/mL) were lower than those in the control group [TK1 (1.58 ± 0.51 pmol/L), HE4 (98.0 ± 28.6) pmol/L, VEGF (284.2 ± 54.1 pg/mL), and SCC-Ag (2.34 ± 0.78 ng/mL)]. The difference was statistically significant (P < 0.05). Before chemotherapy, there were no significant differences in the physical, role, mood, cognition, social and symptom scale scores of the two groups (P > 0.05). After chemotherapy, the physical, role, mood, cognitive and social scores were higher in the test group than in the control group, and the difference was statistically significant (P < 0.05). The symptom scale scores of the test group were all lower than those of the control group, and the difference was statistically significant (P < 0.05). The 3-year progression-free survival (PFS) rate was 43.33% in the test group and 26.67% in the control group; the overall survival (OS) rate was 48.33% in the test group and 33.33% in the control group; the differences were not statistically significant (P > 0.05). The 3-year PFS time of the test group was 20.0 mo, which was longer than that of the control group (15.0 mo), and the difference was significant (P < 0.05). The OS time of the test group was 30.0 mo, which was longer than that of the control group (18.0 mo), and the difference was significant (P < 0.05).
Endo combined with concurrent radiotherapy and chemotherapy for the treatment of advanced cervical squamous cell carcinoma has a positive effect on reducing the level of tumor markers in patients, prolonging the PFS and OS times of patients, and improving the quality of life.
Core Tip: Through a set of retrospective studies, it was confirmed that the combination of Endo combined with radiotherapy and chemotherapy for the treatment of advanced cervical squamous cell carcinoma has a positive effect on reducing the level of tumor markers in patients and prolonging the survival time of patients.
- Citation: Zhao FJ, Su Q, Zhang W, Yang WC, Zhao L, Gao LY. Endu combined with concurrent chemotherapy and radiotherapy for stage IIB-IVA cervical squamous cell carcinoma patients. World J Clin Cases 2021; 9(27): 8061-8070
- URL: https://www.wjgnet.com/2307-8960/full/v9/i27/8061.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i27.8061
Cervical cancer is one of the most common malignant tumors in the female reproductive system, with a high incidence, second only to breast cancer. Generally, chronic cervical inflammation evolves into precancerous lesions. In recent years, the incidence of cervical cancer has increased with increasing life pressures and changes in women's social roles, posing a serious threat to women's physical and mental health. Most patients are in the middle and late stages at presentation[1-3]. Comprehensive clinical treatment is mainly adopted for middle-stage and advanced cervical cancer, and radiotherapy and chemotherapy are widely used in the clinic. Dana has no obvious effect in some patients, leads to a poor prognosis, and is prone to recurrence and distant metastasis. Anti angiogenic therapy is a kind of targeted therapy that can directly or indirectly inhibit angiogenic factors and their pathways and increase endogenous or exogenous angiogenic inhibitory factors. Some clinical experience has been gained in the combination of recombinant human vascular endostatin and chemotherapy to inhibit the degradation of extravascular matrix. The prospect of the combination of recombinant human vascular endostatin with radiotherapy or chemotherapy is optimistic. It is one of the hotspots in radiation oncology[4]. This study analyzed the effect of Endu combined with concurrent radiotherapy and chemotherapy in the treatment of advanced cervical squamous cell carcinoma, providing a basis for the clinical selection of treatments for advanced cervical cancer.
A total of 120 patients treated in the oncology department of our hospital were randomly divided into two groups: the test group (n = 60) and the control group (n = 60). The patients were enrolled from July 2015 to June 2017. The inclusion criteria were as follows: (1) the diagnostic criteria of cervical cancer referred to those in the 2013 edition of the cervical cancer diagnosis and treatment guidelines of the Ministry of Health; (2) patients with a Karnofsky performance status (KPS) score ≥ 70 before treatment; (3) patients with stage IIB-IVA cervical squamous cell carcinoma diagnosed by pathology and/or cytology; (4) patients aged 19 to 70 years; (5) patients with expected survival time > 6 mo; and (6) this clinical trial must follow the Helsinki Declaration (1996 edition), the Drug Clinical trial Management Code issued by the Food and Drug Administration and related regulations. The exclusion criteria were as follows: (1) patients with recurrent cervical cancer after surgery; (2) patients with other malignant tumors; (3) patients with major organ dysfunction and severe heart disease, including congestive heart failure, uncontrollable arrhythmia, angina pectoris requiring long-term medication, valvular heart disease, myocardial infarction and intractable hypertension, pregnant or lactating women, those with prolonged infectious wounds, and those with a history of uncontrollable mental illness; and (4) patients lost to follow-up.
In the test group, the age range was from 42 to 70 years, with an average age of 57.6 ± 6.0 years. In the control group, the age range was 38 to 70 years old, and the average age was 55.9 ± 6.8 years. There was no significant difference in age between the two groups (P > 0.05).
Test group: Patients were treated with Endu combined with concurrent radiotherapy and chemotherapy, and the chemotherapy regimen was docetaxel 60 mg/m2 day 1 and cisplatin mg/m2 day 1-4, with 21-28 d in one cycle and at least 2 cycles. Endu (produced by Shandong XianshengMaidejin Biopharmaceutical Co., Ltd., S20050088) 7.5 mg/m2 per day was administered for 7 d, starting within one week before radiotherapy, intravenous pump or intravenous drip, with 21 d in one cycle for at least 2 cycles. For the radiotherapy regimen, the radiotherapy technique consisted of irradiation of primary lesions, subclinical lesions, positive lymph nodes and flow areas with 3D-conformal radiation therapy, intensity-modulated radiotherapy or image-guided radiation therapy. For the split dose, conventional segmentation with a prescription dose of 95% volume gross tumor volume T45-50Gy/23-25 times, planning target volume N 45-50Gy/23-25 times, and planning clinical tumor volume N46-50Gy/23-25 times was used. This was combined with Endu 15 mg once a day 5 times a week. Control group: patients were treated with only radiotherapy and chemotherapy at the same time, and the method was the same as that of the test group.
Short-term efficacy was evaluated in terms of complete remission (CR), partial remission (PR), stable disease and progressive disease according to the Response Evaluation Criteria in Solid Tumors.
The levels of serum thymidine kinase 1 (TK1), human epididym is protein 4 (HE4), vascular endothelial growth factor (VEGF) and squamous cell carcinoma-associated antigen (SCC-Ag) were compared between the two groups before and after chemotherapy.
The European Organisation for Research and Treatment of Cancer quality of life scale was used to evaluate the quality of life from five dimensions: body, role, emotion, cognition and sociality. The higher the score was, the higher the quality of life of the patients. This assessment also included a symptom scale (a total of 7 clinical symptoms); the higher the symptom score was, the lower the quality of life of the patients.
The Common Toxicity Criteria (CTC3.0) includes nausea, vomiting, loss of appetite, diarrhea, neutropenia, leukopenia and thrombocytopenia. Each toxicity and side effect index was divided into grade 1, grade 2, grade 3, grade 4 and grade 5. The higher the grade was, the more serious the patient's condition.
The fasting venous blood of the patients was centrifuged for 30 min with a radius of 15 cm. The levels of TK1, HE4 and VEGF were determined by enzyme-linked immunosorbent assay. The concentration of SCC-Ag was determined by microparticle enzyme immunoassay. The kits were provided by Shanghai Kanglang Biotechnology Co., Ltd.
The patients were followed up by outpatient follow-up and telephone follow-up. In the first year of treatment, the patients went to the hospital for a follow-up examination at least once every 3 mo. The patients' progression-free survival (PFS) and overall survival (OS) times were mainly recorded.
SPSS21.0 was adopted for data processing. The measurement indexes in this study, such as age, body mass index (BMI) and KPS score, all followed an approximate normal distribution or a normal distribution, so they are expressed as mean ± SD, and the data were compared by t-test. The count data were compared by the χ2 test. The Kaplan-Meier method was used for the survival analysis, and survival times were compared by the log-rank test.
There were no significant differences in age, BMI, KPS score, International Federation of Gynecology and Obstetrics stage or degree of differentiation between the test group and the control group (P > 0.05, Table 1).
| Group | n | Age (yr) | BMI (kg/m2) | KPS score (scores) | FIGO stage (%) | Differentiation (%) | ||||
| Stage IIB | Stage III | Stage IVA | Well differentiated | Moderately differentiated | Poorly differentiated | |||||
| Test group | 60 | 57.6 ± 6.0 | 23.2 ± 2.4 | 78.4 ± 3.0 | 13 (21.67) | 26 (43.33) | 21 (35.00) | 18 (30.00) | 27 (45.00) | 15 (25.00) |
| Control group | 60 | 55.9 ± 6.8 | 22.8 ± 2.7 | 77.8 ± 2.8 | 17 (28.33) | 21 (35.00) | 22(36.67) | 23 (38.33) | 20 (33.33) | 17 (28.33) |
| t/χ2 | 1.452 | 0.858 | 1.133 | 1.825 | 1.565 | |||||
| P value | 0.149 | 0.393 | 0.260 | 0.401 | 0.457 | |||||
After chemotherapy, the CR + PR rate was 85.00% in the test group and 68.33% in the control group. The difference was not statistically significant (P > 0.05, Table 2).
| Group | n | CR | PR | SD | PD | CR + PR |
| Test group | 60 | 27 | 24 | 9 | 0 | 51 (85.00) |
| Control group | 60 | 21 | 20 | 17 | 2 | 41 (68.33) |
| χ2 | 4.658 | |||||
| P value | 0.031 |
Before chemotherapy, there were no significant differences in the levels of serum TK1, HE4, VEGF and SCC-Ag between the two groups. However, after chemotherapy, the levels of serum TK1 (1.27 ± 0.40 pmol/L), HE4 (81.4 ± 24.0 pmol/L), VEGF (235.1 ± 38.0 pg/mL), and SCC-Ag (1.76 ± 0.55 ng/mL) were lower than those in the control group [TK1 (1.58 ± 0.51 pmol/L), HE4 (98.0 ± 28.6 pmol/L), VEGF (284.2 ± 54.1 pg/mL), and SCC-Ag (2.34 ± 0.78 ng/mL)]. The difference was statistically significant (P < 0.05, Table 3).
| Group | n | TK1 (pmol/L) | HE4 (pmol/L) | VEGF (pg/mL) | SCC-Ag (ng/mL) | ||||
| Before chemotherapy | After chemotherapy | Before chemotherapy | After chemotherapy | Before chemotherapy | After chemotherapy | Before chemotherapy | After chemotherapy | ||
| Test group | 60 | 3.64 ± 0.95 | 1.27 ± 0.40 | 138.5 ± 29.5 | 81.4 ± 24.0 | 549.6 ± 98.3 | 235.1 ± 38.0 | 6.19 ± 2.04 | 1.76 ± 0.55 |
| Control group | 60 | 3.40 ± 1.03 | 1.58 ± 0.51 | 132.0 ± 32.7 | 98.0 ± 28.6 | 541.0 ± 88.6 | 284.2 ± 54.1 | 5.95 ± 2.23 | 2.34 ± 0.78 |
| t | 1.327 | -3.705 | 1.143 | -3.444 | 0.503 | -5.753 | 0.615 | -4.707 | |
| P value | 0.187 | 0.000 | 0.255 | 0.001 | 0.616 | 0.000 | 0.540 | 0.000 | |
There were no significant differences in the degree of nausea, vomiting, loss of appetite, diarrhea, granulocytopenia, leukopenia or thrombocytopenia between the test group and the control group (P > 0.05, Table 4).
| Toxic side effects | Test group (n = 60) | Control group (n = 60) | χ2 | P value | ||
| Grade 1 | Grade ≥ 2 | Grade 1 | Grade ≥ 2 | |||
| Nausea | 13 (21.67) | 47 (78.33) | 18 (30.00) | 42 (70.00) | 1.087 | 0.297 |
| Vomiting | 19 (31.67) | 41 (68.33) | 24 (40.00) | 36 (60.00) | 0.906 | 0.341 |
| Loss of appetite | 24 (40.00) | 36 (60.00) | 20 (33.33) | 40 (66.67) | 0.574 | 0.449 |
| Diarrhea | 34 (56.67) | 26 (43.33) | 39 (65.00) | 21 (35.00) | 0.874 | 0.35 |
| Neutropenia | 16 (26.67) | 44 (73.33) | 22 (36.67) | 38 (63.33) | 1.386 | 0.239 |
| Leukopenia | 11 (18.33) | 49 (81.67) | 17 (28.33) | 43 (71.67) | 1.677 | 0.195 |
| Thrombocytopenia | 14 (23.33) | 46 (76.67) | 23 (38.33) | 37 (61.67) | 3.165 | 0.075 |
Before chemotherapy, there were no significant differences in the scores of the body, role, emotion, cognition, social and symptom scales between the two groups (P > 0.05). However, after chemotherapy, the body (72.0 ± 7.4 vs 68.8 ± 8.3), role (70.5 ± 5.5 vs 66.7 ± 6.5), emotion (68.8 ± 6.6 vs 66.5 ± 6.3), cognition (65.0 ± 7.1 vs 63.0 ± 7.6) and social interaction (72.1 ± 7.5 vs 69.8 ± 7.1) scales were significantly higher in the test group than in the control group (P < 0.05). In addition, the symptom scale scores in the test group (158.4 ± 15.5) were significantly lower than those in the control group (173.0 ± 18.3) (P < 0.05, Table 5).
| Project | Before chemotherapy | t | P value | After chemotherapy | t | P value | ||
| Test group (n = 60) | Control group (n = 60) | Test group (n = 60) | Control group (n = 60) | |||||
| Physical function | 55.3 ± 6.2 | 53.8 ± 5.8 | 1.369 | 0.174 | 72.0 ± 7.4 | 68.8 ± 8.3 | 2.229 | 0.028 |
| Role function | 51.7 ± 5.5 | 53.5 ± 6.0 | -1.713 | 0.089 | 70.5 ± 5.5 | 66.7 ± 6.5 | 3.457 | 0.001 |
| Emotional function | 48.8 ± 6.6 | 50.2 ± 6.2 | -1.198 | 0.233 | 68.8 ± 6.6 | 66.5 ± 6.3 | 1.953 | 0.053 |
| Cognitive function | 58.2 ± 7.1 | 56.4 ± 6.6 | 1.438 | 0.153 | 65.0 ± 7.1 | 63.0 ± 7.6 | 1.490 | 0.139 |
| Social function | 65.8 ± 7.5 | 64.3 ± 7.2 | 1.118 | 0.266 | 72.1 ± 7.5 | 69.8 ± 7.1 | 1.725 | 0.087 |
| Symptom scale score | 229.1 ± 24.0 | 225.9 ± 21.4 | 0.771 | 0.442 | 158.4 ± 15.5 | 173.0 ± 18.3 | -4.716 | 0.000 |
The 3-year PFS and OS rates of the test group were 43.33% and 48.33%, and those of the control group were 26.67% and 33.33%, respectively; there were no significant differences between the two groups (P > 0.05, Table 6).
| Group | n | Progression-free survival | Overall survival |
| Test group | 60 | 26 (43.33) | 29 (48.33) |
| Control group | 60 | 16 (26.67) | 20 (33.33) |
| χ2 | 3.663 | 2.794 | |
| P value | 0.056 | 0.095 |
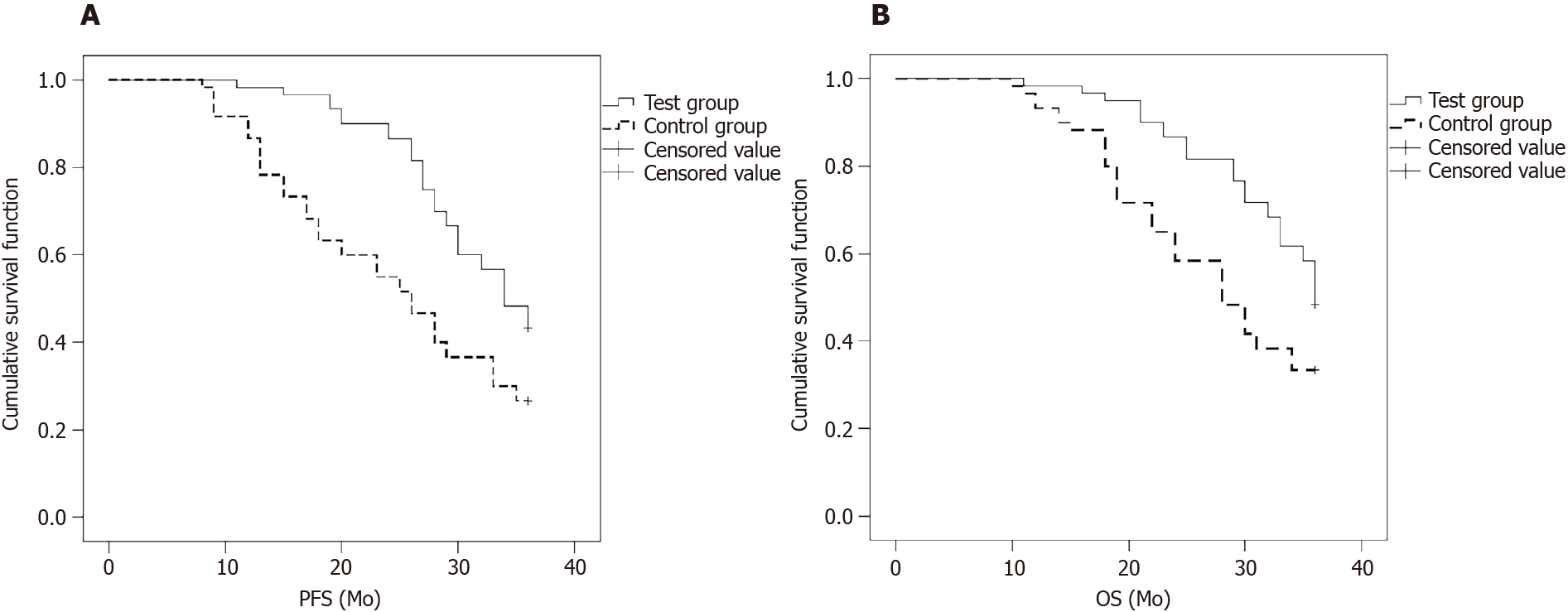
The 3-year PFS time was 20.0 mo in the test group and 15.0 mo in the control group, and the difference was significant (P < 0.05, Figure 1A).
The OS time was 30.0 mo in the test group and 18.0 mo in the control group, and the difference was significant (P < 0.05, Figure 1B).
In recent years, a number of clinical studies in China and elsewhere have confirmed that radiotherapy combined with chemotherapy is beneficial for the control of local and distant metastasis. The National Cancer Institute of the United States has listed cisplatin-based concurrent radiotherapy and chemotherapy as the standard treatment for locally advanced cervical cancer and early high-risk cervical cancer[5-9]. For local middle-stage and advanced tumors, due to the large tumor load, poor differentiation, wide range of local invasion, and high proportion of hypoxic cells, the 5-yearPFS rate is approximately 67%. In addition, 33% of patients experience local recurrence and/or distant metastasis within two years[10-13]. One of the key factors leading to local tumor recurrence after chemotherapy and radiotherapy is the increased expression of VEGF induced by radiotherapy, which results in increased local neovascularization, radiation resistance and possible distant effects. In addition, the inhibition of angiogenesis and vascular injury affects the above resistance factors and improves the efficacy of simultaneous chemotherapy and radiotherapy[14].
Endostatin can specifically act on endothelial cells during neovascularization. It can also have an antiangiogenic effect by regulating the expression of VEGF and the activity of proteolytic enzymes on the surface of tumor cells, which indirectly leads to tumor dormancy or retraction[15,16]. The advantage of antiangiogenic therapy is that it can be targeted toward pathological blood vessels and tumor blood vessels, and resistance to antiangiogenic therapy does not easily develop, mainly because the vascular endothelial genome is relatively stable. Some scholars have found that targeted therapeutic drugs are beneficial for the control of tumor metastasis, have the characteristics of low toxicity and safety, and can be used to treat a broad spectrum of malignant tumors[17]. Endu is a representative antiangiogenic cancer drug. A number of previous experimental studies have shown that Endu can specifically inhibit vascular endothelial cell proliferation and tumor growth. Phase I and II clinical studies have found that Endu monotherapy has certain antitumor effects. The combination of Endu with chemotherapy does not increase adverse reactions and is safe[18]. Some scholars found that the response of tumors to radiation depends not only on the cell type but also on the radiosensitivity of tumor microvessels. Animal experiments have shown that endostatin can improve the efficacy of radiotherapy and chemotherapy[19]. Some scholars believe that antiangiogenic therapy can improve the disordered vascular network and normalize its structure and function in tumors to improve local blood circulation, reduce tumor interstitial pressure, increase the local partial pressure of oxygen, and enhance the sensitivity of tumor cells to radiotherapy[20]. Antiangiogenic therapy can directly or indirectly inhibit angiogenic factors and their pathways, increase endogenous or exogenous angiogenesis inhibitors, and inhibit the degradation of the extravascular matrix. Studies in recent years have found that the rational use of antiangiogenic drugs can repair abnormal tumor vascular systems before vascular regression, promote the normalization of tumor blood vessels, and increase the effectiveness of oxygen and drug transport to tumor cells. As a result, antiangiogenic therapy can improve the sensitivity of radiotherapy and chemotherapy. Some scholars have used Endu combined with radiotherapy and chemotherapy in the treatment of inoperable patients with cervical cancer without metastasis. In one study, Endu combined with radiotherapy and chemotherapy improved the CR rate and 1-and 3-year OS rates of patients[21].
There were no differences in the CR and PR rates between the two groups, which was related to the small number of patients enrolled in the groups. The serum levels of TK1, HE4, VEGF and SCC-Ag in the test group were lower than those in the control group after chemotherapy, suggesting that Endu combined with radiotherapy and chemotherapy can effectively inhibit tumor angiogenesis and reduce the concentration of tumor markers in patients with advanced cervical cancer. There was no difference in the occurrence of adverse reactions between the two groups, suggesting that Endu combined with simultaneous radiotherapy and chemotherapy does not increase toxicity or side effects in patients with advanced cervical cancer. After chemotherapy, the body, role, emotion, cognition and social interaction scores in the test group were higher than those in the control group. However, the symptom scale scores in the test group were lower than those in the control group. These results suggest that Endu combined with radiotherapy and chemotherapy is more effective in improving the quality of life of patients with advanced cervical cancer than the control treatment. During the 3-year follow-up, it was found that the PFS and OS times of the test group were better than those of the control group. This result suggests that Endu combined with simultaneous radiotherapy and chemotherapy can prolong the survival time of patients with advanced cervical cancer. Endu combined with concurrent radiotherapy and chemotherapy was used in the treatment of locally advanced cervical cancer without increasing the number of adverse reactions in response to chemotherapy. In addition, the treatment strategy retained the advantages of simultaneous radiotherapy and chemotherapy, and given the inhibition of tumor angiogenesis by endostatin, Endu has important clinical application prospects in terms of exerting synergistic effects with radiotherapy and chemotherapy. However, the treatment dose of Endu to use in combination with radiotherapy and chemotherapy, the mode with which it is combined with radiotherapy and chemotherapy, and the course of treatment remain to be optimized and standardized, and further research is needed.
In summary, Endu combined with radiotherapy and chemotherapy for the treatment of advanced cervical squamous cell carcinoma reduces the level of tumor markers, prolongs the PFS and OS times, and improves quality of life.
Cervical cancer is one of the most common malignant tumors in the female reproductive system, with a high incidence, second only to breast cancer. Generally, chronic cervical inflammation evolves into precancerous lesions. In recent years, the incidence of cervical cancer has increased with increasing life pressures and changes in women's social roles, posing a serious threat to women's physical and mental health. Most patients are in the middle and late stages at presentation.
This study provides guidance for clinical treatment of advanced cervical squamous cell carcinoma.
This study aimed to explore the clinical effect of Endo combined with concurrent radiotherapy and chemotherapy in the treatment of advanced cervical squamous cell carcinoma.
A total of 120 patients admitted to the oncology department of our hospital were selected as the research subjects. They were equally divided into the test group and the control group (60 patients each) with a random number table. The test group was treated with Endo combined with concurrent radiotherapy and chemotherapy, and the control group was treated with concurrent radiotherapy and chemotherapy. The serum thymidine kinase 1 (TK1), human epididymis protein 4 (HE4), vascular endothelial growth factor (VEGF), and squamous cell carcinoma-associated antigen (SCC-Ag) levels, the clinical effects and survival before and after radiotherapy and chemotherapy, the quality score, and the 3-year follow-up outcomes between the two groups were compared.
After chemotherapy, the complete remission + partial remission rate was 85.00% in the test group and 68.33% in the control group; the difference was not statistically significant. Before chemotherapy, the serum TK1, HE4, VEGF, and SCC-Ag levels of the two groups were not significantly different. After chemotherapy, the levels of serum TK1, HE4, VEGF, and SCC-Ag were lower than those in the control group. The difference was statistically significant.
Endo combined with concurrent radiotherapy and chemotherapy for the treatment of advanced cervical squamous cell carcinoma has a positive effect on reducing the level of tumor markers in patients, prolonging the progression-free survival and overall survival times of patients, and improving the quality of life.
Endo combined with concurrent radiotherapy and chemotherapy has a positive effect on patients with advanced cervical squamous cell carcinoma, and has certain practical significance for clinical treatment.
| 1. | Mancilla-Jimenez R, Stanley RJ, Blath RA. Papillary renal cell carcinoma: a clinical, radiologic, and pathologic study of 34 cases. Cancer. 1976;38:2469-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Liu YY, Guo RX, Li BJ, Wu Y, Bai J, Li LX, Wang CF. [Analysis of clinical features of cervical precancerous lesions in postmenopausal women]. Zhonghua Fu Chan Ke Za Zhi. 2021;56:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Gredmark T, Kvint S, Havel G, Mattsson LA. Adipose tissue distribution in postmenopausal women with adenomatous hyperplasia of the endometrium. Gynecol Oncol. 1999;72:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Boldrini L, Piras A, Chiloiro G, Autorino R, Cellini F, Cusumano D, Fionda B, D'Aviero A, Campitelli M, Marazzi F, Balducci M, Valentini V, Gambacorta MA. Low Tesla magnetic resonance guided radiotherapy for locally advanced cervical cancer: first clinical experience. Tumori. 2020;106:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Vasilev SA, Schlaerth JB. Scalene lymph node sampling in cervical carcinoma: a reappraisal. Gynecol Oncol. 1990;37:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Trojanowski T, Peszyński J, Turowski K, Kamiński S, Gościński I, Reinfus M, Krzyszkowski T, Pyrich M, Bielawski A, Leszczyk C. Postoperative radiotherapy and radiotherapy combined with CCNU chemotherapy for treatment of brain gliomas. J Neurooncol. 1988;6:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Guo Q, Sun Y, Kong E, Rao L, Chen J, Wu Q, Zhang T, Liu N, Li M, Sun L. Apatinib combined with chemotherapy or concurrent chemo-brachytherapy in patients with recurrent or advanced cervical cancer: A phase 2, randomized controlled, prospective study. Medicine (Baltimore). 2020;99:e19372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Tinelli R, Uccella S, Nappi L, D'Amato G, Cicinelli E, Angioni S. Obturator nerve injury in a chemo and radio-resistant patient with a locally-advanced cervical cancer after two previous uterine artery embolizations for severe vaginal bleeding: Case report and review of literature. Eur J Obstet Gynecol Reprod Biol. 2020;252:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Scambia G, Ferrandina G, Distefano M, Fagotti A, Manfredi R, Zannoni GF, Mancuso S. Is there a place for a less extensive radical surgery in locally advanced cervical cancer patients? Gynecol Oncol. 2001;83:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Liu T, Kong W, Liu Y, Song D. Efficacy and prognostic factors of concurrent chemoradiotherapy in patients with stage Ib3 and IIa2 cervical cancer. Ginekol Pol. 2020;91:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Shim HJ, Kim HJ, Hwang JE, Bae WK, Chung IJ, Lee DH, Mi YT, Lee JK, Lim SC, Chung JW, Cho SH. Long term complications and prognostic factors in locally advanced nasopharyngeal carcinoma treated with docetaxel, cisplatin, 5-fluorouracil induction chemotherapy followed by concurrent chemoradiotherapy: A retrospective cohort study. Medicine (Baltimore). 2020;99:e23173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Liu B, Sun Z, Ma WL, Ren J, Zhang GW, Wei MQ, Hou WH, Hou BX, Wei LC, Huan Y, Zheng MW. DCE-MRI Quantitative Parameters as Predictors of Treatment Response in Patients With Locally Advanced Cervical Squamous Cell Carcinoma Underwent CCRT. Front Oncol. 2020;10:585738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Kallehauge J, Nielsen T, Haack S, Peters DA, Mohamed S, Fokdal L, Lindegaard JC, Hansen DC, Rasmussen F, Tanderup K, Pedersen EM. Voxelwise comparison of perfusion parameters estimated using dynamic contrast enhanced (DCE) computed tomography and DCE-magnetic resonance imaging in locally advanced cervical cancer. Acta Oncol. 2013;52:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Somasundaram A, Socinski MA, Villaruz LC. Immune Checkpoint Blockade in Oncogene-Driven Non-Small-Cell Lung Cancer. Drugs. 2020;80:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Brazelle WD, Shi W, Siemann DW. VEGF-associated tyrosine kinase inhibition increases the tumor response to single and fractionated dose radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Printzell L, Reseland JE, Edin NFJ, Ellingsen JE. Effects of ionizing irradiation and interface backscatter on human mesenchymal stem cells cultured on titanium surfaces. Eur J Oral Sci. 2019;127:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Crooke ST, Seth PP, Vickers TA, Liang XH. The Interaction of Phosphorothioate-Containing RNA Targeted Drugs with Proteins Is a Critical Determinant of the Therapeutic Effects of These Agents. J Am Chem Soc. 2020;142:14754-14771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 18. | Han KY, Chang JH, Azar DT. MMP14-Containing Exosomes Cleave VEGFR1 and Promote VEGFA-Induced Migration and Proliferation of Vascular Endothelial Cells. Invest Ophthalmol Vis Sci. 2019;60:2321-2329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Lang HB, Xie RX, Huang ML, Fang LY, Tang YB, Zhang F. The Effect and Mechanism of TRPC1, 3, and 6 on the Proliferation, Migration, and Lumen Formation of Retinal Vascular Endothelial Cells Induced by High Glucose. Ophthalmic Res. 2020;63:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Amemiya T, Hata N, Mizoguchi M, Yokokawa R, Kawamura Y, Hatae R, Sangatsuda Y, Kuga D, Fujioka Y, Takigawa K, Akagi Y, Yoshimoto K, Iihara K, Miura T. Mesenchymal glioblastoma-induced mature de-novo vessel formation of vascular endothelial cells in a microfluidic device. Mol Biol Rep. 2021;48:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Kanao H, Aoki Y, Omi M, Nomura H, Tanigawa T, Okamoto S, Chang EJ, Kurita T, Netsu S, Matoda M, Omatsu K, Matsuo K. Laparoscopic pelvic exenteration and laterally extended endopelvic resection for postradiation recurrent cervical carcinoma: Technical feasibility and short-term oncologic outcome. Gynecol Oncol. 2021;161:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goldstein BH S-Editor: Wang JL L-Editor: A P-Editor: Guo X