Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7876
Peer-review started: April 12, 2021
First decision: June 23, 2021
Revised: July 4, 2021
Accepted: July 14, 2021
Article in press: July 14, 2021
Published online: September 16, 2021
Processing time: 151 Days and 0.2 Hours
The ATP6AP1 gene coding for the accessory protein Ac45 of the vacuolar-type adenosine triphosphatases (V-ATPase) is located on chromosome Xq28. Defects in certain subunits or accessory subunits of the V-ATPase can lead to congenital disorders of glycosylation (CDG). CDG is a group of metabolic disorders in which defective protein and lipid glycosylation processes affect multiple tissues and organs. Therefore, the clinical presentation of patients with ATP6AP1-CDG varies widely. In this report, we present a case of ATP6AP1-CDG in a Chinese infant, with clinical features and genotype.
An 8-mo-old boy was admitted to our hospital because unexplained hepatosplenomegaly and elevated transaminases that had been noted while he was being treated for a cough at a local hospital. A post-admission examination at our hospital revealed abnormalities in the infant’s liver, brain, and immune system. Trio-based whole exome gene analysis identified a hemizygous pathogenic mutation c.1036G>A (p.E346K) in exon 9 of the ATP6AP1 gene. This variant of the ATP6AP1 gene has not been reported in East Asian countries until now.
Based on the infant’s clinical manifestations and the results of genetic detection, he was clearly diagnosed with ATP6AP1-CDG. The clinical manifestations of children with CDG vary widely. Genetic testing analysis helps in the clinical diagnosis of children with CDG.
Core Tip: This article reports on an 8-mo-old male infant with hemizygous pathogenic mutation c.1036G>A (p.E346K) in the ATP6AP1 gene. ATP6AP1-congenital disorders of glycosylation (ATP6AP1-CDG) is a recently identified disease and rarely occurs in Asia. If a patient shows liver, neurological, and immune deficiencies, or other multisystem abnormalities, early genetic screening is advised for a definitive diagnosis. This study expands the disease spectrum of ATP6AP1-CDG and improves its understanding.
- Citation: Yang X, Lv ZL, Tang Q, Chen XQ, Huang L, Yang MX, Lan LC, Shan QW. Congenital disorder of glycosylation caused by mutation of ATP6AP1 gene (c.1036G>A) in a Chinese infant: A case report. World J Clin Cases 2021; 9(26): 7876-7885
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7876.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7876
Congenital disorders of glycosylation (CDG) are a group of monogenic genetic metabolic disorders that are caused by defects in protein and lipid glycosylation processes affecting multiple tissues and organs[1]. Vesicular- or vacuolar-type adenosine triphosphatases (V-ATPase) is a protein complex widely expressed in cell or organelle membranes. It helps to maintain the dynamic pH balance in the cellular or organelle internal environment by consuming ATP for H+ transport[2]. V-ATPase consists of two core structural domains (the soluble V1 domain and the intramem
The patient's parents reported that their baby was treated at a local hospital for a cough when he was about 8 mo old. During hospitalization, abnormal liver function and hepatosplenomegaly were detected.
The patient was diagnosed with bronchopneumonia and abnormal liver function at a local hospital. After 7 d of treatment with ceftazidime for anti-infection and liver protection with compound glycyrrhizin, the patient's cough stopped and the bronchopneumonia disappeared. However, the liver function indexes remained abnormal. The patient was then referred to our department for further treatment.
At 4 mo of age, the patient underwent an abdominal ultrasonography examination. The results showed that the mesenteric lymph nodes were enlarged, but no liver or spleen abnormalities were found at that time. When he was 7 mo and 13 d old, he had a head magnetic resonance imaging examination which showed delayed cerebral myelination. The patient subsequently had a period of brain rehabilitation treatments.
The patient is the parents’ first child. His mother had no history of miscarriage. At the full term of the pregnancy, the mother had a cesarean section because prenatal ultrasound examination showed "oligohydramnios". The patient was hospitalized for neonatal jaundice. He was cured and discharged a few weeks later. The mother is a hepatitis B carrier. There was no family history of hereditary diseases.
Upon admission, the patient’s height was 71.5 cm (66th percentile) and his weight was 7.7 kg (15th percentile). No cutis laxa (CL), rash, hemorrhagic spots, petechiae, or jaundice were observed on the child’s skin. The abdomen was soft. The liver was 4 cm below the right rib with a hard texture, and the spleen was 2.5 cm below the rib with a medium texture. The extremities’ muscle strength was normal, and no abnormal neurological pathological reflexes were observed. The patient was lagging his peers in growth and development. He turned over at 4.5 mo, held his head upright and stable at 5 mo, and was still unable to sit at 8 mo.
Because of the patient’s young age and the predominant manifestation of elevated transaminases and liver damage, infection, congenital genetic metabolic disease, autoimmune liver disease, abnormal development of intrahepatic bile duct, and other factors could not be ruled out. Laboratory examinations showed that the hepatitis B and C viruses were negative, EB virus was negative, autoimmune liver disease antibody spectrum was normal, and T lymphocyte subsets were normal. The results of other laboratory examinations are shown in Table 1.
| Panel | Index/reference range | Result |
| Liver function | ALT (9-60 U/L) | 213 |
| AST (15-45 U/L) | 242 | |
| TBIL (3.40-20.50 μmol/L) | 1.60 | |
| IBIL (3.10-14.30 μmol/L) | 0.60 | |
| TBA (0.00-10.00 μmol/L) | 21.46 | |
| TP (65.0-85.0 g/L) | 62.2 | |
| GLO (20.0-40.0 g/L) | 11.3 | |
| ALB (40.0-55.0 g/L) | 50.9 | |
| GGT (0.0-50.0 U/L) | 64.0 | |
| ALP (40-500 U/L) | 557 | |
| Liver genetic metabolic index | 24h-Cu (0.24-0.48 μmol/24 h) | 0.03 |
| CER (180-450 mg/L) | 133 | |
| LACT (0.63-2.44 mmol/L) | 2.79 | |
| Blood ammonia (18-72 μg/dL) | 181 | |
| Immunoglobulin | IgG (8.0-18.0 g/L) | 4.20 |
| IgA (0.90-4.50 g/L) | 1.30 | |
| IgM (0.84-1.32 g/L) | 0.54 | |
| Routine blood test | PLT (125-350 × 109/L) | 138 |
| Blood lipids | HDL-C (1.03-2.07 mmoL/L) | 0.09 |
| LDL-C (1.0-4.4 mmol/L) | 1.74 | |
| Cardiac enzymes | CK (10-174 U/L) | 94 |
| CK-MB (0.0-25.0 U/L) | 56.0 | |
| LD (109-245 U/L) | 331 |
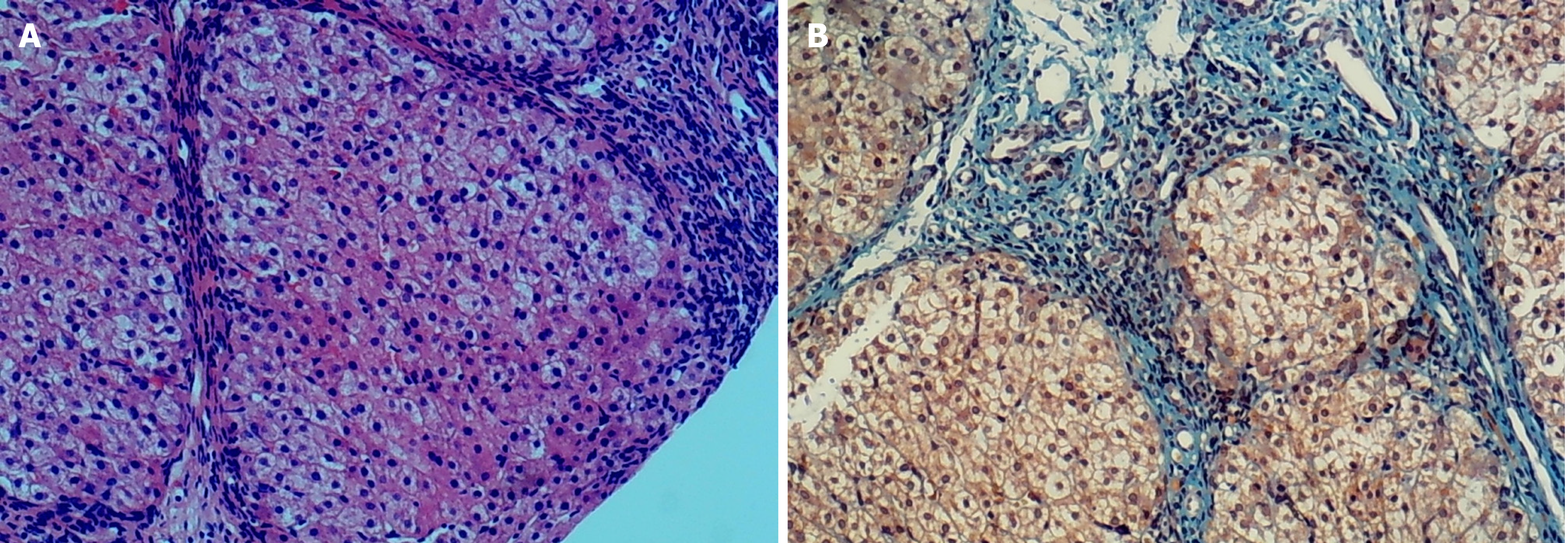
After a liver aspiration biopsy, hematoxylin and eosin staining showed disorganized hepatic lobules with a nodular arrangement of hepatocytes, mild edema, dotted necrosis, fragmented necrosis, and bridging necrosis. The confluent area was significantly widened. There was a large amount of fibrous tissue proliferation and a division of hepatic lobules to form pseudo bullets; with more lymphocytes and individual plasma cell infiltration in the confluent area (Figure 1A). Collagen fiber staining (Masson) showed massive collagen fibrous hyperplasia and a division of hepatic lobules to form pseudo bullets (Figure 1B).
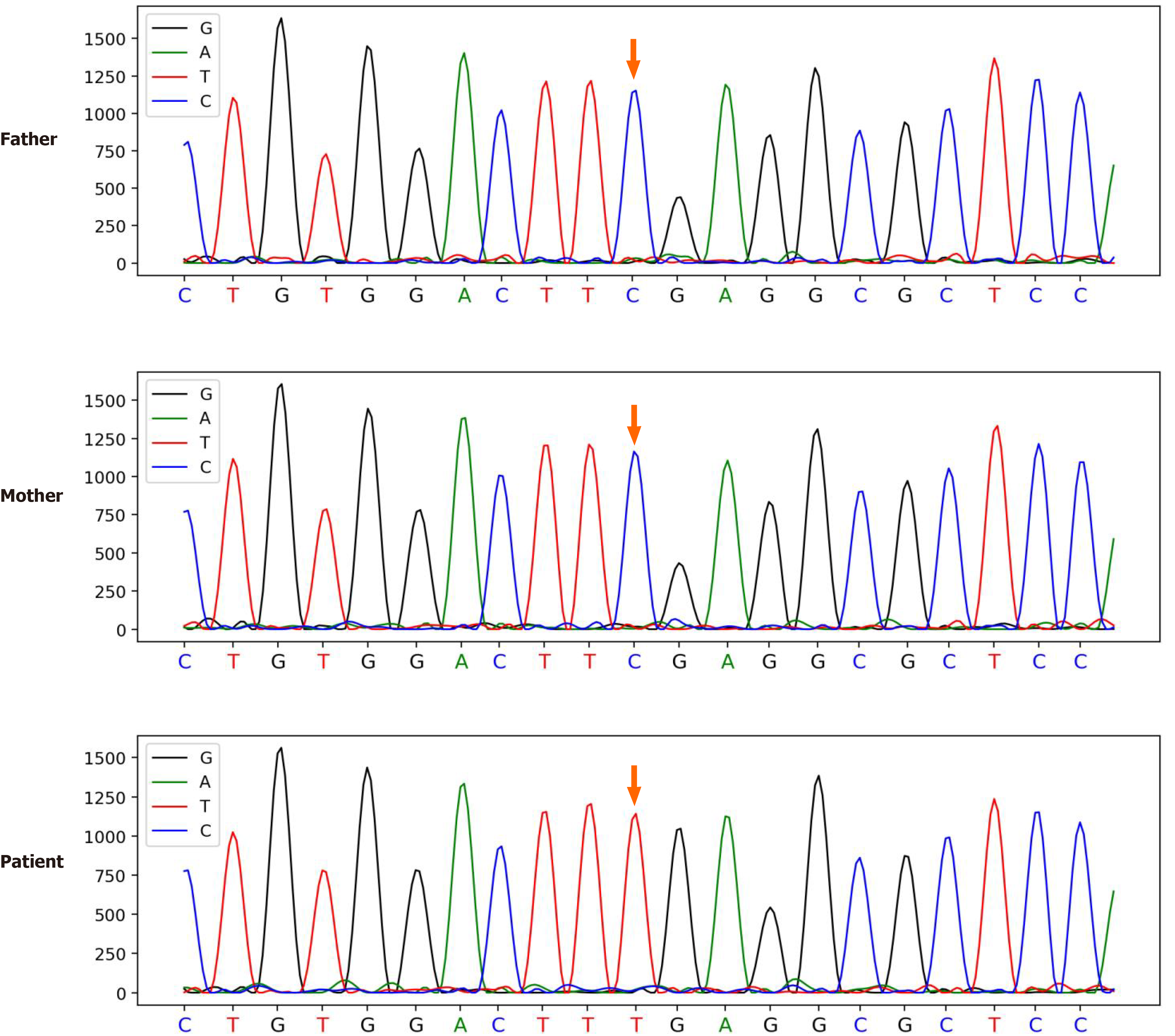
To find the cause of the disease, we took peripheral blood samples from the patient and his parents for trio-based whole exome gene analysis. This revealed the presence of the c.1036G>A (p.E346K) mutation in the patient's ATP6AP1 gene. Both his parents were confirmed to be free of mutations at this locus (Figure 2). The pathogenicity of CDG caused by this site mutation has been reported in the PubMed database[4]. The patient's clinical presentation combined with the genetic sequencing conclusion resulted in a clear ATP6AP1-CDG diagnosis.
Referring to the literature, there was no effective treatment for the disease. During hospitalization, the patient was given reduced glutathione to protect the liver. He was also given ornithine and aspartate to lower the blood ammonia symptomatically. After treatment, transaminases levels were lower than the those at the time of admission (alanine aminotransferase 120 U/L, aspartate aminotransferase 140 U/L) and the child was generally doing well, so the parents requested his discharge.
During the current both outpatient and telephone follow-up, the parents reported that the child showed signs of muscle weakness and had slight difficulty going upstairs. His immunoglobulin levels were consistently low. He was subsequently treated with gamma-globulin at a local hospital several times because of hypogammaglobulinemia. The child is now two years and one month old. Some of the laboratory tests and medications used in the clinic follow-up are summarized in Table 2. The long-term prognosis requires continued follow-up and observation.
| Age | ALT | AST | IgG | IgA | IgM | Blood ammonia | Medication |
| 8 mo | 213 | 242 | 4.2 | 1.3 | 0.54 | 181 | Glutathione, ursodeoxycholic acid, ornithine monohydrate |
| 9 mo | 202 | 332 | 3.5 | 0.1 | 0.16 | 74 | Glutathione, compound glycyrrhizin |
| 11 mo | 388 | 515 | - | - | - | - | Glutathione, compound glycyrrhizin |
| 12 mo | 167 | 111 | 3.12 | 0.37 | 0.36 | 142 | Glutathione, compound glycyrrhizin, ornithine monohydrate |
| 19 mo | 128 | 196 | 2.16 | 0.32 | 0.09 | 95 | Bicyclol, compound glycyrrhizin |
V-ATPase is a proton pump that transports H+ via active transport and provides a homeostatic pH environment for various cellular activities[5]. For example, maintaining the pH gradient from the cis to the trans Golgi apparatus ensures proper protein post-translational modifications and targeting[3,6]. V-ATPase of eukaryotes is widely distributed in the membranes of various subcellular organelles (such as Lysosomes, Golgi, clathrin-coated vesicles, platelet dense granules, and chromaffin granules) and on cell membranes[3,7]. The human V-ATPase consists of two structural domains, V0 and V1. The V0 domain is embedded in the membrane and participates in proton transportation. It is composed of a1, d1, e1, RNaseK, c, c’’, and ATP6AP1 and ATP6AP2 subunits. The soluble V1 complex is responsible for catalyzing the hydrolysis of ATP and is composed of eight different subunits (A, B, C, D, E, F, G, and H)[8].
CDG is a growing group of monogenic diseases caused by disorders in the glycosylation process of proteins and lipids[9]. Glycosylation is a post-translational modification of proteins that occurs in the Golgi apparatus[10]. There are autosomal recessive, autosomal dominant, and X-linked modes of inheriting these diseases[11]. To have a better understanding of the relationship between mutations and diseases, the latest nomenclature for these diseases is "name of the mutated gene-CDG" (e.g., ATP6AP1-CDG as reported in this article)[9].
The mechanism by which ATP6AP1-CDG occurs is complex. One explanation is that missense mutations in ATP6AP1 affect the folding and assembly of V-ATPase, leading to reduced enzyme function and an inability to maintain Golgi pH homeostasis. This results in incorrect transportation of the glycosylase in the Golgi, prompting mislocalization of the enzyme. Mislocalization causes impaired glycosylation, which leads to CDG[3,8].
A total of 19 CDG patients with eight mutation types in the ATP6AP1 gene (M248I, L144P, E346K, Y313C, Y217N, L181R, L74P, and L311E) have been reported so far[4,12-15], all with missense mutations. Five of the patients who died had mutation types L74P, L311E, and E346K[4,13,14]. This paper reports on a Chinese patient carrying one of these mutation types, E346K (c.1036G>A). The mutant locus was not included in the East Asian population of the "gnomAD" database.
Tables 3 and 4 summarize some of the reported clinical data and ancillary findings in children with ATP6AP1-CDG. Patients with ATP6AP1-CDG all showed abnormal liver function. The liver biopsy results of our patient suggested chronic hepatitis and nodular cirrhosis. The severity of liver damage varies between patients with different mutation loci. Severity ranges from minor changes in transaminases to hepatomegaly, hepatocellular steatosis, or fatty liver, to severe cirrhosis or even death from liver failure. As shown in Table 3, all children carrying the c.1036G>A (p.E346K) mutation in the ATP6AP1 gene had more severe liver manifestations. All six children with clinical data documenting this mutation developed hepatosplenomegaly and cirrhosis, and two of them died of liver failure with a high mortality rate. Two other patients with mutations in other loci also died from liver failure.
| Family | Case | Initial diagnosis/death age | cDNA mutation | Connective tissue abnormalities | Infections | Neurological symptoms | Hepatosplenomegaly |
| 1[4] | 1.1 | 20 yr | c.1284G>A | Bilateral inguinal hernias | + | - | Hepatomegaly |
| 1.2 | 12 yr | Bilateral inguinal hernias | + | +/- | - | ||
| 1.3 | 34 yr | Bilateral inguinal hernias | + | - | - | ||
| 2[4] | 2 | 14 yr | c.431T>C | NA | + | - | - |
| 3[4] | 3.1 | 8 yr | c.1036G>A | NA | + | + | + |
| 3.2 | Died 4 yr | NA | + | + | + | ||
| 4[4] | 4.1 | 23 yr | c.1036G>A | NA | + | + | + |
| 4.2 | 18 yr | NA | + | + | + | ||
| 5[4] | 5.1 | Died 12 mo | c.1036G>A | NA | + | + | + |
| 5.2 | 3 yr | NA | + | + | Hepatomegaly | ||
| 6[4] | 6 | 4 yr | c.938A>G | NA | + | - | Hepatomegaly |
| 7[15] | 7 | 5 mo | c.649T>A | CL, aortic root dilation, diaphragmatic hernia | + | - | + |
| 8[12] | 8 | 10 yr | c.542T>G | CL, joint hypermobility | + | +/- | + |
| 9[13] | 9.1 | Died 3 mo | c.221T>C | CL | - | - | + |
| 9.2 | Died 11 mo | CL | - | - | + | ||
| 10[14] | 10.1 | NA | c.923T>A | Ascending aorta dilation | - | - | + |
| 10.2 | Died 4 mo | Ascending aorta dilation, atrial septal defect | - | - | Hepatomegaly | ||
| 11[21] | 11 | 1.5 yr | NA | Joint hypermobility | + | - | + |
| 12 | 12 | 8 mo | c.1036G>A | - | + | + | + |
| Family | Case | Abnormal liver function | Hypogammaglobulinemia | Low serum copper/ ceruloplasmin | Liver biopsy |
| 1[4] | 1.1 | +/- | + | + | NA |
| 1.2 | +/- | + | + | NA | |
| 1.3 | +/- | + | NA | - | |
| 2[4] | 2 | +/- | + | + | Slight steatosis |
| 3[4] | 3.1 | +/- | + | + | Fibrosis, steatosis, cirrhosis |
| 3.2 | +/- | + | + | Steatosis, cirrhosis | |
| 4[4] | 4.1 | + | + | + | Micronodular cirrhosis |
| 4.2 | + | + | + | Micronodular cirrhosis | |
| 5[4] | 5.1 | + | + | + | Fibrosis, cirrhosis, steatosis, cholestasis |
| 5.2 | + | + | + | NA | |
| 6[4] | 6 | + | + | +/- | Fibrosis, cirrhosis, steatosis |
| 7[15] | 7 | + | - | + | Micronodular cirrhosis, steatosis |
| 8[12] | 8 | + | + | + | NA |
| 9[13] | 9.1 | + | - | + | Fibrosis, steatosis, cholestasis |
| 9.2 | + | - | + | Fibrosis, steatosis, cholestasis | |
| 10[14] | 10.1 | + | + | + | Fibrosis, steatosis, micronodular cirrhosis |
| 10.2 | + | - | + | Fibrosis, steatosis, micronodular cirrhosis | |
| 11[21] | 11 | + | + | + | Steatosis, cirrhosis |
| 12 | 12 | + | + | + | Chronic hepatitis, nodular cirrhosis |
Two infants with coagulation abnormalities have been previously reported[12,14]. One patient, who could not be a liver transplant candidate, died of respiratory failure due to pulmonary hemorrhage after palliative care failed[16]. Therefore, patients with this disease should have regular liver function tests and timely assessments for coagulation abnormalities, hepatic encephalopathy, ascites, and other related complications to avoid irreversible end-stage liver disease.
Most patients had symptoms of hypogammaglobulinemia (15/19) and infections (15/19). Infections in patients with ATP6AP1-CDG can involve multiple tissues or organs. They can manifest as purulent otitis media, pneumonia, gastrointestinal infections, urinary tract infections, and plantar abscesses. In our case, the infant was admitted to a local hospital for a cough and was diagnosed with bronchopneumonia during his hospitalization. It is now believed that immunodeficiencies are the main cause of the ATP6AP1-CDG patient's susceptibility to infection or recurrent infections.
Although the protein encoded by the ATP6AP1 gene is the Ac45 subunit of V-ATPase, cases of impaired liver function and immunodeficiency caused by genetic defects in V-ATPase itself have not been reported. The explanation for this phenomenon is that there may be tissue-specific processing of the Ac45 subunit in the liver, brain, and immune cells or that Ac45 may not only influence the pH-regulatory effects of V-ATPase[4]. Further studies are needed to investigate the mechanism of differential processing of Ac45 in different tissues.
As shown in Table 3, other abnormal clinical manifestations were connective tissue abnormalities (11/13) and neurological symptoms (9/19). Connective tissue abnormalities included CL, hernias, hyperlaxity of the joints, and aortic dilatation. No connective tissue lesions have been observed to date in the children reported in this study. Not all patients with CDG will have connective tissue abnormalities[17]. In some patients, this manifestation will gradually decrease with age[12]. All patients with documented laboratory tests had a decrease in ceruloplasmin or serum copper (19/19). The mechanism of the abnormal manifestation of connective tissue in ATP6AP1-CDG patients has not been fully investigated. The possible reason is that the reduction of copper in the body reduces the activity of lysyl oxidase (copper-dependent enzyme), which is responsible for linking collagen and elastin of the extracellular matrix. Some of these patients showed signs of CL[12,13,18]. A previous study reporting on the skin biopsy of one CL patient showed normal quantity and quality of elastin fibers, suggesting that the phenotype of CL may not be related to the quality and quantity of elastin[15]. The exact reasons for this are to be further investigated.
Neurological abnormalities may manifest as mental retardation, epilepsy, muscle weakness, progressive hearing loss, sensorineural deafness, hyperopia, and behavioral abnormalities. The intellectual development and cognitive backwardness of the patient in this study may be attributed to delayed brain myelination.
Any patient with unexplained multisystem involvement should be suspected of having CDG and undergo isoelectric focusing electrophoresis for transferrin or apolipoprotein C-III[19]. Since some patients were found to have genetic alterations with normal transferrin electrophoresis results, glycomics was used only as a screening tool. Sequencing of the genes is still required to confirm the diagnosis of CDG[9,16]. However, studying the altered glycosylation of proteins in different cellular environments and specific glycosylation sites may be a new thought for treating patients with CDG.
There is no specific treatment for the disease. Patients can mainly consider regular check-ups, monitoring, and symptomatic supportive treatment to improve the quality of life and prolong the life. Treatments include intravenous immunoglobulin to improve hypogammaglobulinemia. However, some patients respond poorly to immunoglobulin[4,20]. One case of a successful liver transplant was reported in a child with a good postoperative status[16]. It remains to be seen whether liver transplant can be used as a treatment for this disease.
Prenatal monitoring of the mother and fetus may be an effective means to prevent the disease. In one mother and fetus, pregnancy abnormalities have been reported, including elevated maternal serum alpha-fetoprotein, elevated alpha-fetoprotein and positive acetylcholinesterase in the amniotic fluid, and abnormal prenatal ultrasound[14]. The patient’s mother in this report had oligohydramnios, as did the mother of a patient diagnosed with CDG-Ih who also had the same symptoms[21]. It is not known whether these are characteristic manifestations of the disease during pregnancy, since there is a lack of data on the specific performance of the mother and fetus in the prenatal period. However, finding reliable prenatal indicators or clinical signs that can diagnose ATP6AP1-CDG may be needed and possible to detect the problem in time to avoid severe consequences.
In summary, 19 cases of CDG associated with ATP6AP1 gene mutations have been reported, with detailed clinical information. The infant’s clinical manifestations reported in this paper were hepatosplenomegaly, hypogammaglobulinemia, and developmental delay. All were consistent with the clinical features of previously described ATP6AP1-CDG cases. The diagnosis of this 8-mo-old infant with ATP6AP1-CDG was promptly clarified based on the clinical manifestations and trio-based whole exome gene analysis, which has not been reported in China. It enriched the clinical phenotype spectrum of this disease. The clinical manifestations of children with ATP6AP1-CDG are highly variable. The mortality rate of children with some mutated loci is high. Gene sequencing can provide evidence for early diagnosis, intervention, and treatment of this disease.
We are grateful to the patient and his family for their participation and support. We also appreciate the professional genetic sequencing by the Gene Corporation (Wuhan, China).
| 1. | Verheijen J, Tahata S, Kozicz T, Witters P, Morava E. Therapeutic approaches in Congenital Disorders of Glycosylation (CDG) involving N-linked glycosylation: an update. Genet Med. 2020;22:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol. 2006;209:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 459] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 3. | Vasanthakumar T, Rubinstein JL. Structure and Roles of V-type ATPases. Trends Biochem Sci. 2020;45:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Jansen EJ, Timal S, Ryan M, Ashikov A, van Scherpenzeel M, Graham LA, Mandel H, Hoischen A, Iancu TC, Raymond K, Steenbergen G, Gilissen C, Huijben K, van Bakel NH, Maeda Y, Rodenburg RJ, Adamowicz M, Crushell E, Koenen H, Adams D, Vodopiutz J, Greber-Platzer S, Müller T, Dueckers G, Morava E, Sykut-Cegielska J, Martens GJ, Wevers RA, Niehues T, Huynen MA, Veltman JA, Stevens TH, Lefeber DJ. ATP6AP1 deficiency causes an immunodeficiency with hepatopathy, cognitive impairment and abnormal protein glycosylation. Nat Commun. 2016;7:11600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Toei M, Saum R, Forgac M. Regulation and isoform function of the V-ATPases. Biochemistry. 2010;49:4715-4723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Linders PTA, Peters E, Ter Beest M, Lefeber DJ, van den Bogaart G. Sugary Logistics Gone Wrong: Membrane Trafficking and Congenital Disorders of Glycosylation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Song Q, Meng B, Xu H, Mao Z. The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases. Transl Neurodegener. 2020;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Wang L, Wu D, Robinson CV, Wu H, Fu TM. Structures of a Complete Human V-ATPase Reveal Mechanisms of Its Assembly. Mol Cell. 2020;80:501-511.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Theodore M, Morava E. Congenital disorders of glycosylation: sweet news. Curr Opin Pediatr. 2011;23:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Ng BG, Freeze HH. Perspectives on Glycosylation and Its Congenital Disorders. Trends Genet. 2018;34:466-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 11. | Jaeken J, Péanne R. What is new in CDG? J Inherit Metab Dis. 2017;40:569-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Dimitrov B, Himmelreich N, Hipgrave Ederveen AL, Lüchtenborg C, Okun JG, Breuer M, Hutter AM, Carl M, Guglielmi L, Hellwig A, Thiemann KC, Jost M, Peters V, Staufner C, Hoffmann GF, Hackenberg A, Paramasivam N, Wiemann S, Eils R, Schlesner M, Strahl S, Brügger B, Wuhrer M, Christoph Korenke G, Thiel C. Cutis laxa, exocrine pancreatic insufficiency and altered cellular metabolomics as additional symptoms in a new patient with ATP6AP1-CDG. Mol Genet Metab. 2018;123:364-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ondruskova N, Honzik T, Vondrackova A, Stranecky V, Tesarova M, Zeman J, Hansikova H. Severe phenotype of ATP6AP1-CDG in two siblings with a novel mutation leading to a differential tissue-specific ATP6AP1 protein pattern, cellular oxidative stress and hepatic copper accumulation. J Inherit Metab Dis. 2020;43:694-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Tvina A, Thomsen A, Palatnik A. Prenatal and postnatal phenotype of a pathologic variant in the ATP6AP1 gene. Eur J Med Genet. 2020;63:103881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Witters P, Breckpot J, Foulquier F, Preston G, Jaeken J, Morava E. Expanding the phenotype of metabolic cutis laxa with an additional disorder of N-linked protein glycosylation. Eur J Hum Genet. 2018;26:618-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Gumm AJ, Basel DG, Thakrar P, Suchi M, Telega G. Liver failure and x-linked immunodeficiency type 47. Pediatr Transplant. 2020;24:e13808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 432] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Marklová E, Albahri Z. Screening and diagnosis of congenital disorders of glycosylation. Clin Chim Acta. 2007;385:6-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Woods AG, Woods CW, Snow TM. Congenital disorders of glycosylation. Adv Neonatal Care. 2012;12:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Vesela K, Honzik T, Hansikova H, Haeuptle MA, Semberova J, Stranak Z, Hennet T, Zeman J. A new case of ALG8 deficiency (CDG Ih). J Inherit Metab Dis. 2009;32 Suppl 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Quelhas D, Martins E, Azevedo L, Bandeira A, Diogo L, Garcia P, Sequeira S, Ferreira AC, Teles EL, Rodrigues E, Fortuna AM, Mendonça C, Fernandes HC, Medeira A, Gaspar A, Janeiro P, Oliveira A, Laranjeira F, Ribeiro I, Souche E, Race V, Keldermans L, Matthijs G, Jaeken J. Congenital Disorders of Glycosylation in Portugal-Two Decades of Experience. J Pediatr. 2021;231:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Miyoshi E, Sawada K S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX