Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7840
Peer-review started: March 18, 2021
First decision: June 24, 2021
Revised: July 9, 2021
Accepted: July 23, 2021
Article in press: July 23, 2021
Published online: September 16, 2021
Processing time: 175 Days and 18.5 Hours
Drug extravasation is a complication of totally implantable access port (TIAP) use and could cause tissue injury and sustained organ dysfunction. Therefore, the clinical management of children with TIAP is challenging.
This was a case of extravasation of a chemotherapeutic drug (paclitaxel) from an implantable infusion port in a 23-mo old child. After fully evaluating the skin at the site of extravasation, the nurse continued to use the infusion port to complete the follow-up chemotherapeutic course. The skin around the infusion port was red, and showed no ulceration, swelling, or induration at discharge.
Since children are more active and often noncompliant, it is necessary to appropriately train pediatric nurses caring for individuals with TIAPs, and any abnormal situation should be timely addressed.
Core Tip: This was a case of extravasation of a chemotherapeutic drug (paclitaxel) from an implantable infusion port in a 23-mo old child. After fully evaluating the skin at the site of extravasation, the nurse continued to use the infusion port to complete the follow-up chemotherapeutic course. The skin around the infusion port was red, and showed no ulceration, swelling, or induration at discharge.
- Citation: Lv DN, Xu HZ, Zheng LL, Chen LL, Ling Y, Ye AQ. Extravasation of chemotherapeutic drug from an implantable intravenous infusion port in a child: A case report. World J Clin Cases 2021; 9(26): 7840-7844
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7840.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7840
Totally implantable access port (TIAP) is an infusion device that can be left in the body for a long time after subcutaneous implantation, for safe delivery of chemotherapeutics, transfusion of blood and blood products, and laboratory sample collection[1]. With growing occurrence of childhood tumors, TIAPs are increasingly employed in children. Despite the associated convenience, the complications of TIAPs cannot be ignored, and include port-site and pocket infection, catheter malposition and kinking, and drug extravasation[2]. Extravasation of necrotic anticancer drugs occurs in 0.1%-6.5% of patients[3]. Meanwhile, extravasation of chemotherapeutics might result in tissue injury and sustained organ dysfunction[4]. Therefore, managing pediatric patients with TIAPs is challenging and might require specialized nurses[5]. Here, we report a pediatric case of paclitaxel extravasation due to needle removal from the infusion port without injury. After emergency treatment, chemotherapy was continued and completed through the infusion port, with no obvious skin abnor
Vaginal mouth tumor for more than half a month.
Half a month ago, the patient’s parents accidentally found a tumor in the vaginal orifice of the child, which was about the size of broad beans and soft, had a protruding surface, and caused no pain, no fever, no general activity change, no abnormal stool, and no urgency or pain. More than 10 d ago, the child's local tumor was broken and a small amount of bloody liquid, about the size of soybeans, was discharged. The remaining characteristics were the same as before. She went to a local hospital without treatment. In the urology department of our hospital, a vaginal mass of about 5.5 cm × 3 cm was found, as well as vaginal and intrauterine effusion by vaginal ultrasound. For further treatment, the patient was admitted in good spirits, with a clear mind, normal reactions, intact appetite, good sleep, normal stool and urine, and no weight gain.
The patient was born following a full term with natural labor, and she was vaccinated as planned and had no history of trauma surgery or food or drug allergy.
The parents were healthy and had no history of familial genetic diseases.
The body was clean, and the patient was in good spirits. Vital signs were stable; heart auscultation was not noisy. Rale was not reached, the abdomen was flat and soft, and no tenderness or rebound pain was found. No obvious package was found. There was a swelling in the vaginal mouth, about 1 cm × 0.5 cm in size, with soft texture, unclear boundary, no tenderness, and no skin swelling and rupture. General activity and physiological and pathological reflexes were unremarkable.
The routine blood examination was normal. The levels of tumor markers (female) were: Alpha-fetoprotein, 87866 ng/mL; carcinoembryonic antigen, 6.37 ng/mL; carbohydrate antigen 199, 52.05 μ/mL; and neuron-specific enolase, 23.94 ng/mL.
After admission, vaginal B-ultrasound showed a vaginal mass of about 5.5 cm × 3 cm, with vaginal and intrauterine effusion; pelvic enhanced computed tomography showed that the vaginal mass had intrauterine effusion as well as an endodermal sinus tumor.
Postoperative pathology showed a vaginal malignant germ cell tumor (endodermal sinus tumor).
On August 22, 2018, a right internal jugular vein infusion port was implanted under general anesthesia. The operation was successful, and the contraindications for chemotherapy were excluded. Bleomycin + etoposide + carboplatin regimen was administered for a total of nine cycles. During the treatment period, the nurse maintained the infusion port normally, and there was no obvious abnormality. During the fifth chemotherapy, the skin around the infusion port showed a red rash, which was considered a skin problem caused by allergy to medical adhesive. After each treatment, the nurse used skin protectants to protect the local skin around the infusion port before bandage. After three cycles of chemotherapy, the skin allergy and rash were not obvious and the infusion port was not damaged; in addition, the needle was fixed properly, and the original nine cycles of preoperative chemotherapy planned were successfully completed. On May 17, 2019, the patient underwent vaginal tumor resection under general anesthesia, and recovered well after operation. After excluding chemotherapy contraindications, the patient received paclitaxel + cisplatin + ifosfamide chemotherapy.
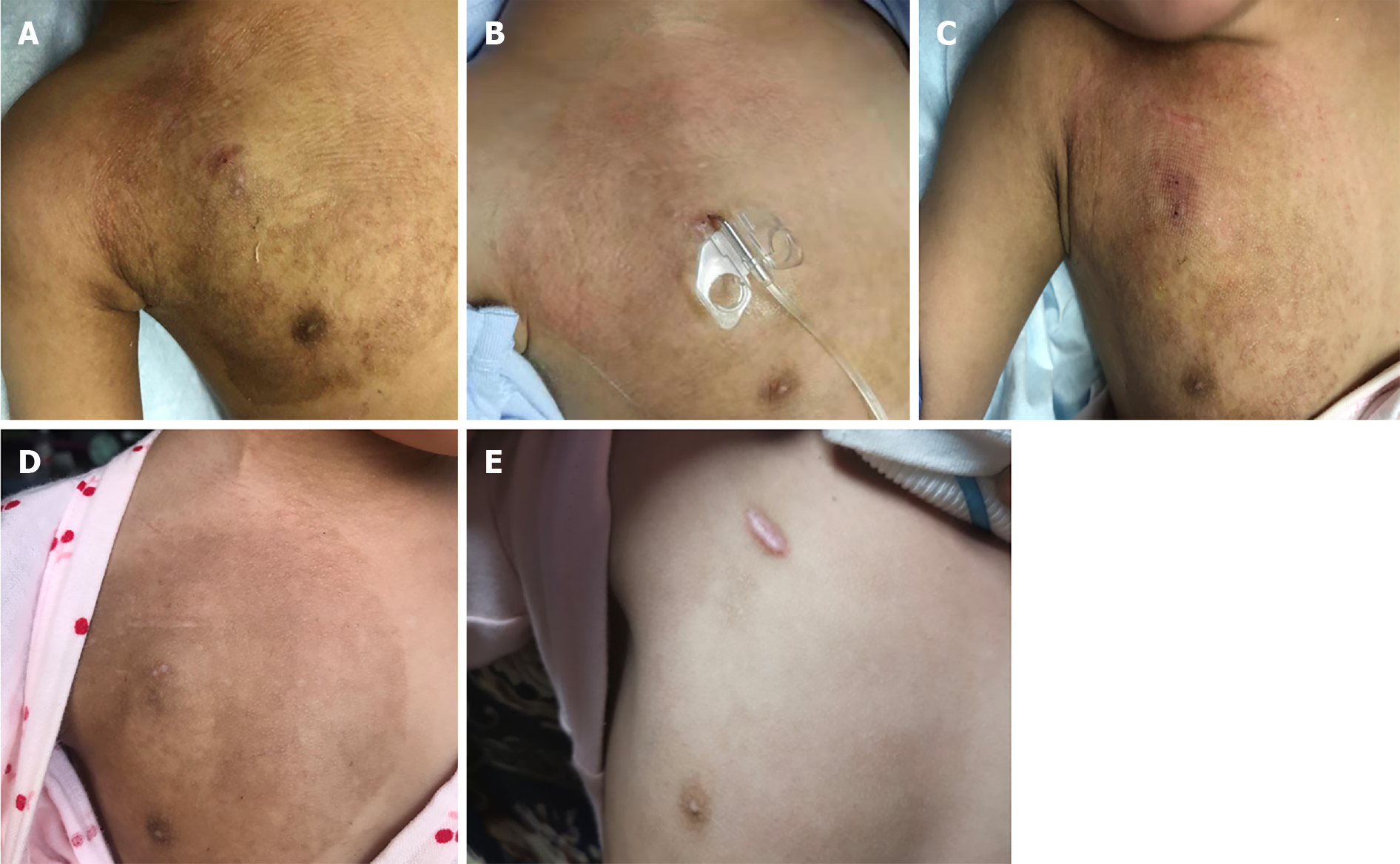
At present, the local skin of the infusion port has completely recovered, all treatments have been completed, and regular follow-up is needed (Figure 1).
Venous access for administering therapeutics and blood sampling is challenging in pediatrics. Assessing 29 patients aged 2-24 years, Golladay and Mollitt[6] identified issues, including extravasation (n = 2), suspected sepsis (n = 1), and hematoma (n = 2). In another set of 29 patients (5 mo-16 years), five catheters were removed with complications, including suspected infection (n = 1), proven infection (n = 1), extravasation (n = 2), and spontaneous extrusion/thrombosis (n = 1)[7].
Compared with adults, children's skin has incompletely developed epidermal and dermal layers, and is more susceptible to damage by viruses and physicochemical and environmental factors. Changes in skin structure cause abnormal skin barrier function[8]. Meanwhile, long-term dressing results in local temperature elevation, and metabolites on the skin surface cannot be timely removed, potentially inducing allergies[9]. In the non-injury needle puncture operation, skin protective agents and anti-allergic patches are used to protect the local skin in children, but redness and itching around the port body could still occur, causing frequent scratching and subsequently affecting the fixation firmness of the needle in the infusion port. Second, young children often show restlessness, fear, and other psychological states, and may not cooperate during the infusion process. Third, paclitaxel infusion takes a long time, and an infusion pump is used to control the flow rate. During the inspection period, the nurse may not check whether the infusion is smooth. Fourth, drug exudation could occur at night, with relatively limited nursing staff. Finally, the content of health education is not specific enough, and the caregiver is changed frequently; therefore, the caregiver might be unaware of the complications of the infusion port, with the non-injury needle slippage not timely found.
Once drug extravasation was found in the current patient, the infusion was stopped immediately, and dexamethasone + lidocaine was administered for local block. The non-injury needle was removed, the liquid was squeezed out, and the local skin was observed. The extent of drug extravasation was evaluated, and symptomatic treatment was used. The patient had exudation of paclitaxel, an antitumor molecule from plants, and cold compress was recommended after exudation[10]. Taking into account the poor compliance of children, with local wet compress being more challenging, antipyretic paste for local multi-point cold compress was selected, and magnesium sulfate cold compress was applied at night to reduce swelling and pain. The FLACC method is commonly used to assess pain in infants and young children. In individuals with a pain score ≥ 4 points, analgesic drugs are used as directed by the physician, and the child's face, breathing, drug efficacy, side effects, etc. are closely monitored. In individuals with a pain score below 4 points, non-drug analgesia methods are applied such as playing music, telling stories, showing cartoons, etc. for distraction.
In addition, attention should be paid to the psychological care of children and accompanying persons. Children and their caregivers do not know enough about the degree of harm caused by the extravasation of chemotherapeutic drugs. They are surprised by the occurrence of abnormal conditions, not knowing whether the infusion port should be further used, which causes anxiety and fear. The extravasation of chemotherapeutic drugs in children increases patient suffering as well as the financial burden on their parents. The medical staff should strictly follow the "chemotherapy drug extravasation treatment process", formulate a personalized nursing plan and observe the treatment effect closely, and choose an appropriate venous access to continue the treatment. The mechanism and treatment of extravasation of chemotherapeutic drugs should be explained to children and their caregivers to stabilize their emotions, and obtain their understanding and active cooperation. After discharge, the medical staff should provide guidance through telephone follow-up and psychological support to the children and their caregivers in a timely manner.
Infusion ports in children are increasingly used in cancer requiring multiple cycles of treatment. Since children are more active and often noncompliant, it is necessary to appropriately train pediatric nurses caring for individuals with TIAPs, and any abnormal situation should be timely addressed. Each step of clinical management should follow a standardized process to ensure that the intravenous infusion port is used appropriately and to guarantee child safety.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giordano G S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li X
| 1. | Shankar G, Jadhav V, Ravindra S, Babu N, Ramesh S. Totally Implantable Venous Access Devices in Children Requiring Long-Term Chemotherapy: Analysis of Outcome in 122 Children from a Single Institution. Indian J Surg Oncol. 2016;7:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Nagasawa Y, Shimizu T, Sonoda H, Mekata E, Wakabayashi M, Ohta H, Murata S, Mori T, Naka S, Tani T. A comparison of outcomes and complications of totally implantable access port through the internal jugular vein versus the subclavian vein. Int Surg. 2014;99:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Nakamura T, Sasaki J, Asari Y, Sato T, Torii S, Watanabe M. Complications after implantation of subcutaneous central venous ports (PowerPort®). Ann Med Surg (Lond). 2017;17:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Azaïs H, Bresson L, Bassil A, Katdare N, Merlot B, Houpeau JL, El Bedoui S, Meurant JP, Tresch E, Narducci F. Chemotherapy drug extravasation in totally implantable venous access port systems: how effective is early surgical lavage? J Vasc Access. 2015;16:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Biffi R, Pozzi S, Agazzi A, Pace U, Floridi A, Cenciarelli S, Peveri V, Cocquio A, Andreoni B, Martinelli G. Use of totally implantable central venous access ports for high-dose chemotherapy and peripheral blood stem cell transplantation: results of a monocentre series of 376 patients. Ann Oncol. 2004;15:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Golladay ES, Mollitt DL. Percutaneous placement of a venous access port in a pediatric patient population. J Pediatr Surg. 1986;21:683-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Wallace J, Zeltzer PM. Benefits, complications, and care of implantable infusion devices in 31 children with cancer. J Pediatr Surg. 1987;22:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Rosso JD, Zeichner J, Alexis A, Cohen D, Berson D. Understanding the Epidermal Barrier in Healthy and Compromised Skin: Clinically Relevant Information for the Dermatology Practitioner: Proceedings of an Expert Panel Roundtable Meeting. J Clin Aesthet Dermatol. 2016;9:S2-S8. [PubMed] |
| 9. | Xia Z, Pan YQ, Liu P. Effect of detail nursing on skin allergy caused by medical viscose on nursing satisfaction. Oriental Diet Ther Health Care. 2017;37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Sato J, Mori M, Nihei S, Kumagai M, Takeuchi S, Kashiwaba M, Kudo K. The effectiveness of regional cooling for paclitaxel-induced peripheral neuropathy. J Pharm Health Care Sci. 2016;2:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |