Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7805
Peer-review started: February 2, 2021
First decision: April 25, 2021
Revised: April 25, 2021
Accepted: August 10, 2021
Article in press: August 10, 2021
Published online: September 16, 2021
Processing time: 219 Days and 10.4 Hours
Chondrosarcomas of the larynx are malignant tumours that most commonly originate from the hyaline cartilage. Chondrosarcoma of the larynx, the most common type of low-grade tumour, accounts for 1% of all laryngeal neoplasms.
We present the case of a 60-year-old female patient who developed progressive hoarseness and shortness of breath over a 2-mo period. The patient had undergone resection of a laryngeal tumour 14 years before the aforementioned symptoms occurred, and histopathological analysis indicated that it was a chondroma. During the assessment of the patient, a submucosal, oval-shaped tumour was detected that was predominantly located on the left side of the larynx and was approximately 6 cm in size. The decision to perform left partial vertical laryngectomy was made. A pathohistological diagnosis of low-grade chondro
Chondrosarcoma of the larynx must be considered in the differential diagnosis of laryngeal submucosal tumours. It is crucial to carefully sample of tumour tissue, differentiate chondroma and chondrosarcoma, and consider the possibility of malignant changes from chondroma to chondrosarcoma.
Core Tip: Chondrosarcomas (ChSs) of the larynx are malignant tumours with a mesenchymal origin that most commonly originate from the hyaline cartilage. Although high-grade ChS of the larynx has also been described, low-grade tumours are the most common form of this disease. They are much more common in Caucasians and the men. This case highlights the utmost importance of recognizing mesenchymal tumours, especially those with a cartilage origin, during differential diagnosis in cases of intramural pathological processes in the laryngeal wall with a preserved mucosa, as these tumours are rare. It is crucial to carefully sample the tumour tissue, differentiate chondroma and chondrosarcoma, and consider the possibility of malignant changes from chondroma to chondrosarcoma.
- Citation: Vučković L, Klisic A, Filipović A, Popović M, Ćulafić T. Low-grade chondrosarcoma of the larynx: A case report. World J Clin Cases 2021; 9(26): 7805-7810
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7805.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7805
Chondrosarcomas (ChSs) of the larynx are malignant tumours with a mesenchymal origin that most commonly originate from the hyaline cartilage[1]. These tumours represent 0.1% of all tumours of the head and neck region and may occur in the nasal cavity, maxilla, and mandible in addition to the larynx. In regard to the larynx, ChS accounts for 1% of all laryngeal neoplasms[2,3]. Although these tumours rarely occur in the larynx, they have the second highest frequency after tumours originating from epithelial tissue, squamous cell carcinoma and adenocarcinoma[4]. Although high-grade ChS of the larynx has also been described, low-grade tumours are the most common form of the disease[1,5].
These tumours most commonly occur in the cricoid cartilage[6]. The tumours most commonly present on the anterior surface of the posterior lamina of the cricoid cartilage. Cases of occurrence in the epiglottis have also been described (3%)[7,8]. ChS of the larynx often occurs in elderly individuals, usually in the seventh decade of life (average age of 63 years)[1]. These tumours are much more common in Caucasians[1] and men (male-to-female ratio = 3.2:1). These tumours rarely progress to regional and distant metastases, even in the T4 stage[9]. Symptoms depend on the location and size of the tumour. Most often, the clinical symptoms are dominated by dyspnoea, dysphasia, dysphonia, and cough with hoarseness. The aetiology of tumour formation remains unclear[1,2]. In addition to clinical examinations, computed tomography and magnetic resonance imaging are recommended as diagnostic imaging methods. A definitive diagnosis is made by histopathological analysis of the tumour tissue. In terms of differential diagnosis, it is necessary to exclude chondroma in cases of low-grade ChS. In cases of high-grade ChS, other types of poorly differentiated malignant tumours (primarily other sarcomas and poorly differentiated cancers) should be excluded.
Chondromas and ChSs are known to be found synchronously in the same tumour or metachronously as a consequence of the malignant transformation of chondroma into ChS, suggesting the enormous importance of adequate sampling of tumour tissue during a pathologic analysis[5]. Surgery is the treatment method of choice[7]. It is important to describe rare cases in clinical practice for the purpose of differential diagnostic assessment, with the aim of making an accurate diagnosis and selecting the proper treatment. Here, we present a case study of a patient treated for low-grade ChS of the larynx.
We present a case of a 60-year-old female patient who developed progressive hoarseness and shortness of breath.
Hoarseness and shortness of breath developed progressively over a 2-mo period.
The patient underwent resection of a laryngeal tumour 14 years before the aforementioned symptoms developed, and histopathological analysis indicated that it was a chondroma.
The patient had suffered from hypertension for many years, with no significant hereditary diseases. She worked in administrative jobs and never smoked cigarettes or consumed alcohol.
Palpatory examination of the neck during patient assessment revealed an oval-shaped tumour, which was predominantly located on the left side, approximately 6 cm in size, and fixed to the larynx wall. No enlarged lymph nodes were found.
By indirect laryngoscopy, the left hemilarynx was found to be immobile and displaced medially, and the respiratory space was reduced. The mucous membrane was unchanged.
Laboratory analyses were within the reference values.
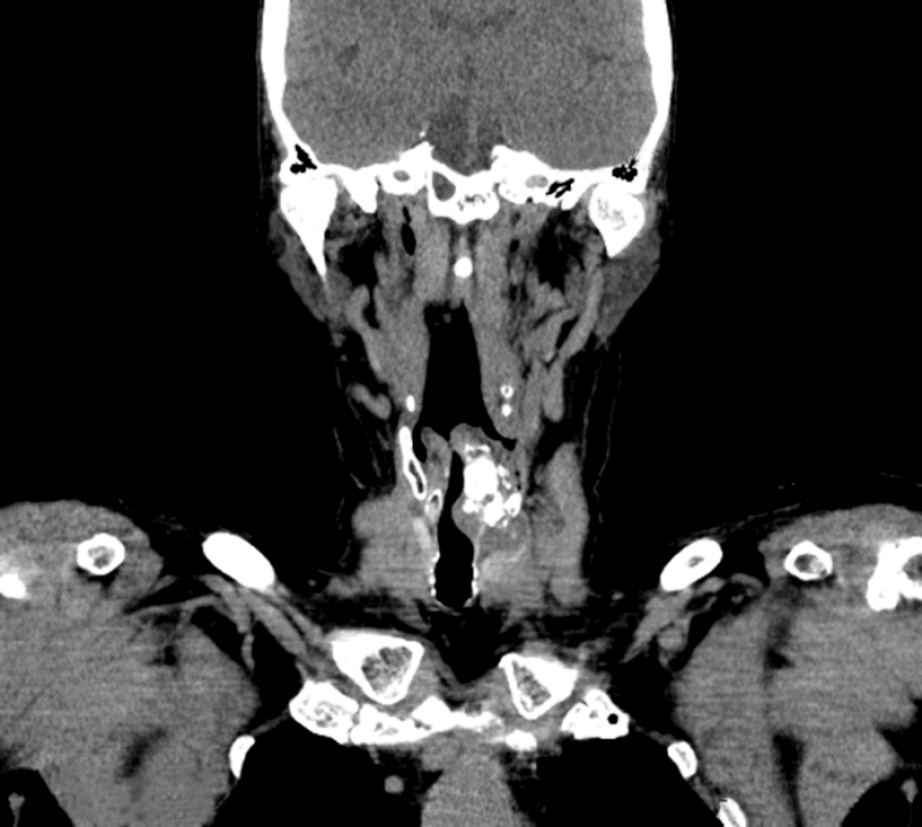
A computed tomography examination of the neck found a hypodense tumour, permeated with calcifications, up to 5.6 cm in diameter on the left, in the laryngeal wall in the subglottic segment, with no clear boundaries. The tumour infiltrated and partially destroyed the cricoid and thyroid cartilage. The tumour asymmetrically and significantly narrowed the lumen of the subglottic laryngeal segment (Figure 1). On computed tomography, no enlarged and pathologically altered lymph nodes were found, and there were no pathological changes in bone structure.
A lung X-ray and abdominal ultrasound were performed, and no distant metastases were found.
A portion of the larynx wall and a resected portion of the trachea were submitted for pathohistological analysis, with tumour tissue with a lobulated appearance, 6 cm × 4 cm × 2 cm in size, a whitish colour, homogeneous structure, and moderately solid consistency.
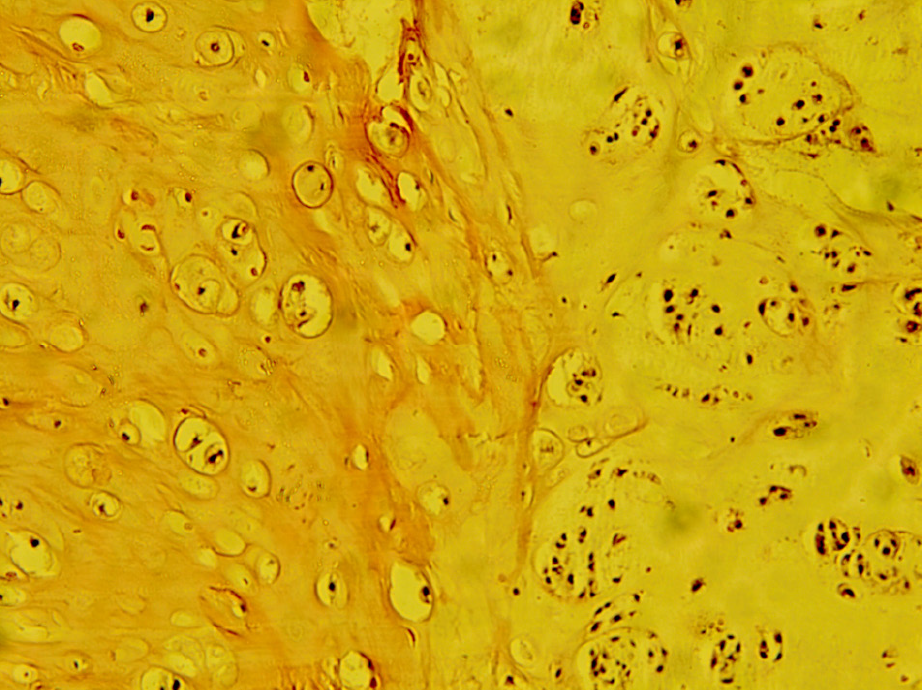
The histology of the tumour tissue was dominated by a moderately abundant basophilic extracellular matrix, part of which had a myxoid appearance. The cellularity of the tumour tissue was moderately focal, with atypical, individual or organized smaller clusters and smaller chondrocytes with rare mitoses present in extracellular matrix lacunae (Figure 2). The tumour growth was nodulo-infiltrative.
A final diagnosis of low-grade ChS was made based on the morphological characteristics of the tumour tissue.
The tumour tissue was located focally on the resection line of the surgical specimen.
The decision to perform left partial vertical laryngectomy was made based on the previous diagnosis of chondroma and the size of the tumour, with the aim of preserving larynx function. Intraoperatively, a hard, nodular-infiltrative tumour was found, including the subglottis from the medial line to the left, as well as the first two tracheal rings. Subsequently, the tumour tissue infiltrated the left subglottis back to the medial line as well as up to the inferior horn of the infiltrated left thyroid cartilage. The tumour tissue was resected en bloc, including the surrounding larynx tissue (up to 5 mm wide), the left half of the trachea to the third ring, back along the medial line and up to the lower edge of the thyroid cartilage, with horn resection. A reconstruction of the defect was made with the perichondrium of the left thyroid plate and local mucous membrane.
In consideration of the results of the pathohistological analysis and positive margins of the resection, a total laryngectomy was performed based on the decision of a multidisciplinary team. Low-grade ChS microscopic focuses were found in the rest of the larynx wall. The operative and postoperative courses were regular.
During the 12-mo follow-up, no local relapse or regional or distant metastases were detected in the patient.
Here, we present a case of a 60-year-old female patient who was diagnosed with low-grade ChS 14 years after the diagnosis of laryngeal chondroma of the cricoid cartilage. Considering that these tumours are extremely rare, it is of the utmost importance to recognize these types of cartilage-origin neoplasms. These facts should be taken into account when considering the differential diagnosis in cases of intramural pathological processes with a preserved laryngeal mucosa.
ChS is characterized primarily by the infiltration of surrounding tissues, although lymphogenic and haematogenous metastases have been reported in approximately 10% of cases. Metastases to the lymph nodes, lungs, bones, kidney, spleen and spinal cord have been described[5].
The risk factors are unknown. Recurrent laryngeal trauma, radiotherapy, Teflon injections and irregular ossification of the laryngeal cartilage are reported as possible causes. Recurrent ischaemia in chondroma has also been reported as a possible cause for the onset of this tumour[1]. In addition to abnormal cartilage ossification, Gao and co-workers proposed two additional theories: that the tumour is caused by congenital cartilage residues or that chondroplasia occurs due to chronic inflammation. In the literature review, this group of authors cited a case of the malignant transformation of chondroma into ChS within 6 years[10]. Wang et al[7] stated that no correlation between alcohol consumption and cigarette smoking with the onset of this tumour has been demonstrated.
The patient described in this study had been treated for hypertension for many years. She was a non-smoker and did not consume alcohol.
The clinical manifestations of laryngeal ChS may be nonspecific[2]. Symptoms of laryngeal ChS include dyspnoea (if intraluminal tumour growth is dominant), dysphagia (if pharynx infiltration is dominant), and progressive hoarseness. Considering that this is a slow-growing tumour, the patient can tolerate the narrowing airways for a long time, and therefore acute respiratory failure may be the first symptom to occur. In ChS with a thyroid cartilage origin, the clinical picture may also be dominated by a painless tumour mass[5].
The clinical manifestations of this patient were dominated by hoarseness and respiratory problems.
When a submucosal mass is found during endoscopic examination, most commonly infraglottic and posterior, soft tissue laryngeal tumours should be considered. Although the mucosa is usually smooth, it can be ulcerated in the case of larger tumours. Computed tomography and magnetic resonance imaging are recommended as radiological examinations. Each of these methods has advantages in the analysis of tumour tissue, as well as in the evaluation of the relationship of tumour tissue to the surrounding structures of the laryngeal wall[5,7]. Surgical excision is the treatment method of choice for ChS. Tumour resections must have clear margins. Based on the fact that cervical lymph node metastases are rare, neck dissection should be reserved for clinically or radiologically suspected lymph node metastases. The complex function of the larynx depends on the conservation of the cricoid cartilage[11]. Surgery that preserves laryngeal function is recommended whenever possible. Since 75% of laryngeal ChS forms in the cricoid cartilage, which is considered crucial for laryngeal function, its preservation is often not possible. Total laryngectomy is recommended in cases where more than half of the cricoid cartilage is destroyed by tumour tissue, as well as in cases involving relapse[2,7]. Nonsurgical treatment modalities are rarely used for the treatment of this tumour[8]. Radiotherapy does not seem to play a significant role in the treatment of laryngeal ChS. In the past, it was used to provide local disease control in cases of unresectable or recurrent disease. Chemotherapy has not been shown to be effective for laryngeal ChS, especially low-grade ChS[5,10]. Most authors report relapses in 16% to 50% of cases[1,2]. Regarding the prognosis of this disease, the expected one-year survival rate is 96.5%, the five-year survival rate is 88.6% and the ten-year survival rate is 84.8% of patients[1]. Definitive diagnosis is made by histopathological analysis. The histology of the tumour is dominated by a basophilic acellular matrix (on haematoxylin-eosin stained preparations) containing lacunae with cells[5]. Cell characteristics, nuclear characteristics, cellularity, and the mitotic index are important for histological analysis[1,7]. Macroscopic tumour tissue is solid and hard in consistency. However, it is possible to find softer and even cystic parts due to degenerative changes. In cases of resection of the laryngeal wall, smooth mucosa is often located above the tumour tissue[5].
Since most laryngeal ChSs are tumours with low-grade differentiation, they are characterized by a lobular-infiltrative growth pattern with cartilage destruction. Given that the most common case in practice is low-grade laryngeal ChS, the differential diagnosis of chondroma and low-grade ChS is very important[5]. Microscopic chondromas are hypocellular (30 to 40 nuclei/HPF), with a homogenous structure and lobular growth pattern, with no cellular atypia or mitosis. Increased cellularity, cellular atypia, binucleation in lacunae, hyperchromasia, a prominent nucleolus, mitoses, and pleomorphism all suggest ChS. It is especially important to analyse parts of the tumour in the border zone compared to the preserved portions of the laryngeal wall[1,5,7]. Immunohistochemical analysis is rarely required. ChS tumour cells are positive for vimentin, S100 and D2-40[1,5]. In less differentiated ChS, cellularity increases and nuclear pleomorphism, hyperchromasia, and mitotic activity are increased[1]. In cases of poorly differentiated laryngeal ChSs, chondroblastic osteosarcoma, fibrosarcoma, myxoid liposarcoma, embryonic rhabdomyosarcoma, spindle cell squamous cell carcinoma with cartilaginous metaplasia should be included in differential diagnosis[5].
It is important that mesenchymal tumours, especially those with a cartilage origin, are considered during differential diagnosis in cases of intramural pathological processes in the laryngeal wall with a preserved mucosa. Laryngeal ChS case reports are important because these tumours are rare. It is crucial to carefully sample the tumour tissue, differentiate chondroma and ChS, and consider the possibility of malignant changes from chondroma to ChS.
Manuscript source: Unsolicited manuscript
Specialty type: Otorhinolaryngology
Country/Territory of origin: Montenegro
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li XY S-Editor: Liu M L-Editor: A P-Editor: Liu JH
| 1. | El-Naggar A, Chan JKC, Grandis JR, Tkata T, Slootweg P. WHO Classification of head and neck tumours. 4th ed. Lyon: IARC, 2017. |
| 2. | Zhou HW, Wang J, Liu Y, Zhang HM. Recurrent chondrosarcoma of the larynx: A case report and literature review. Medicine (Baltimore). 2016;95:e4118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Cleven AHG, Schreuder WH, Groen E, Kroon HM, Baumhoer D. Molecular findings in maxillofacial bone tumours and its diagnostic value. Virchows Arch. 2020;476:159-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Saraydaroglu O, Narter S, Ozsen M, Coskun H. Non-epithelial tumors of the larynx: case series of 12 years. Eur Arch Otorhinolaryngol. 2019;276:2843-2847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Ferlito A, Devaney KO, Mäkitie AA. Differing characteristics of cartilaginous lesions of the larynx. Eur Arch Otorhinolaryngol. 2019;276:2635-2647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Magliocca KR, Edgar MA, Corey A, Villari CR. Dedifferentiated chondrosarcoma of the larynx: Radiological, gross, microscopic and clinical features. Ann Diagn Pathol. 2017;30:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Wang Q, Chen H, Zhou S. Chondrosarcoma of the larynx: report of two cases and review of the literature. Int J Clin Exp Pathol. 2015;8:2068-2073. [PubMed] |
| 8. | Chin OY, Dubal PM, Sheikh AB, Unsal AA, Park RC, Baredes S, Eloy JA. Laryngeal chondrosarcoma: A systematic review of 592 cases. Laryngoscope. 2017;127:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Dubal PM, Svider PF, Kanumuri VV, Patel AA, Baredes S, Eloy JA. Laryngeal chondrosarcoma: a population-based analysis. Laryngoscope. 2014;124:1877-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Gao CP, Liu JH, Hou F, Liu H, Xu WJ. Low-grade chondrosarcoma of the cricoid cartilage: a case report and review of the literature. Skeletal Radiol. 2017;46:1597-1601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Rovó L, Bach Á, Sztanó B, Matievics V, Szegesdi I, Castellanos PF. Rotational thyrotracheopexy after cricoidectomy for low-grade laryngeal chrondrosarcoma. Laryngoscope. 2017;127:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |