Published online Sep 16, 2021. doi: 10.12998/wjcc.v9.i26.7704
Peer-review started: February 7, 2021
First decision: May 11, 2021
Revised: May 19, 2021
Accepted: August 2, 2021
Article in press: August 2, 2021
Published online: September 16, 2021
Processing time: 214 Days and 18.3 Hours
Maternal sepsis is a major cause of gestational morbidity and neonatal mortality worldwide and particularly in China.
To evaluate the etiology of maternal sepsis and further identify its risk factors.
In this retrospective study, we evaluated 70698 obstetric patients who were admitted to the Third Affiliated Hospital of Guangzhou Medical University between January 1, 2009 and June 30, 2018. Subjects were divided into sepsis group and non-sepsis group based on the incidence of sepsis. Data about medical history (surgical and obstetric history) and demographic information were collected. The Mann-Whitney U test was used to compare patient age, gestational age and duration of hospitalization between the two groups. Univariate and multivariate logistic regression models were used to analyze the etiology and the risk factors for maternal sepsis. Unadjusted and adjusted odds ratios (OR) are reported.
A total of 561 of 70698 obstetric patients were diagnosed with infection; of the infected patients, 492 had non-sepsis associated infection (87.7%), while 69 had sepsis (12.3%). The morbidity rate of maternal sepsis was 9.76/10000; the fatality rate in the sepsis group was 11.6% (8/69). Emergency admission (OR = 2.183) or transfer (OR = 2.870), irregular prenatal care (OR = 2.953), labor induction (OR = 4.665), cervical cerclage (OR = 14.214), first trimester (OR = 6.806) and second trimester (OR = 2.09) were significant risk factors for maternal sepsis.
Mode of admission, poor prenatal care, labor induction, cervical cerclage, first trimester and second trimester pregnancy were risk factors for maternal sepsis. Escherichia coli was the most common causative organism for maternal sepsis, and the uterus was the most common site of infection.
Core Tip: This study evaluated the etiology of maternal sepsis and identified its risk factors in Guangzhou, China. The results show that emergency admission or transfer, irregular prenatal care, labor induction, cervical cerclage, first trimester and second trimester were significant risk factors for maternal sepsis. The most common causative organism for maternal sepsis was Escherichia coli, and the most common site of infection was the uterus.
- Citation: Lin L, Ren LW, Li XY, Sun W, Chen YH, Chen JS, Chen DJ. Evaluation of the etiology and risk factors for maternal sepsis: A single center study in Guangzhou, China. World J Clin Cases 2021; 9(26): 7704-7716
- URL: https://www.wjgnet.com/2307-8960/full/v9/i26/7704.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i26.7704
Sepsis is the third leading cause of maternal mortality in the world[1]. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) define sepsis as life-threatening organ dysfunction caused by dysregulated host response to infection[2,3]. The World Health Organization definition (2017) refers to maternal sepsis as a life-threatening disease; it is defined as organ dysfunction resulting from infection during pregnancy, childbirth, post-abortion or postpartum period[4]. In the absence of timely diagnosis and treatment, sepsis may lead to maternal death, neonatal infection and other adverse outcomes.
Developing countries tend to have higher maternal mortality rates due to sepsis as compared to developed countries[1,5,6]. In China, the overall maternal mortality rate has declined from 141.7 per 100000 live births in 1990 to 17.2 per 100000 live births in 2013; however, sepsis related maternal morbidity and neonatal mortality remain high[1,7].
Studies conducted in Europe and the United States have identified several risk factors for maternal sepsis including multiparity, caesarean section, anemia, genitourinary infection and uterine infection[8-12]; however, few studies have investigated the risk factors for maternal sepsis in mainland China. Therefore, the aim of this study was to analyze the etiology of maternal sepsis and to identify the associated risks factors. Our findings may help strengthen perinatal health education, facilitate prompt recognition of maternal sepsis and improve maternal and neonatal outcomes.
This study was approved by the ethics committee of the Third Affiliated Hospital of the Guangzhou Medical University. Written informed consent was obtained from all subjects prior to their enrollment.
Data were collected from the obstetric center at the Third Affiliated Hospital of Guangzhou Medical University. This hospital is a referral center for obstetric patients and caters to a catchment population of approximately 14.044 million. We selected the study population through the hospital electronic data system and perinatal medical database. All patients with a clinical diagnosis of sepsis or septic shock were identified from the database based on the International Classification of Diseases Tenth Revision, Clinical Modification codes; the definition of maternal sepsis was based on the World Health Organization statement[3].
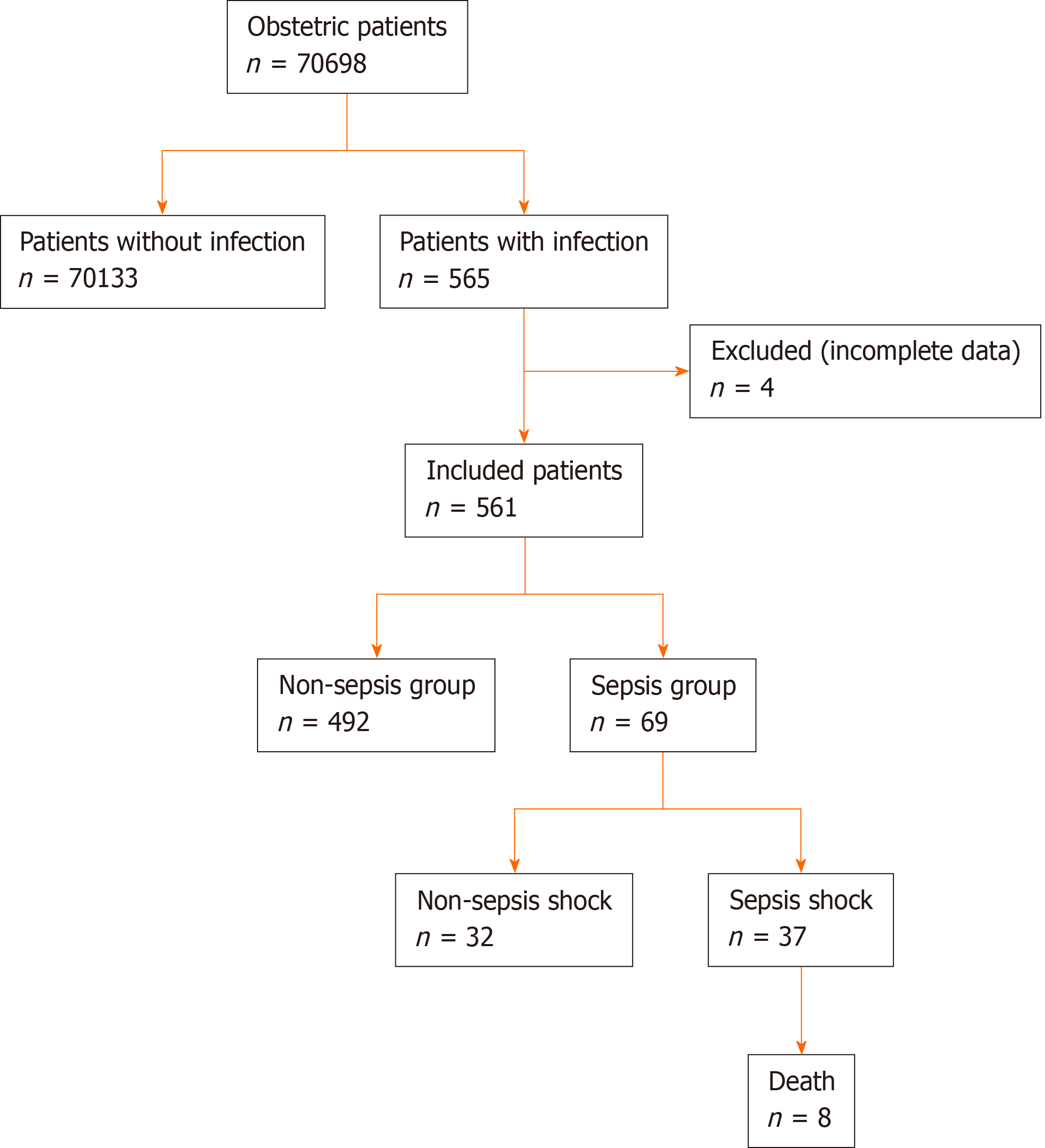
All obstetric patients within 42 d of pregnancy, intrapartum, abortion and postpartum admitted to the Third Affiliated Hospital of Guangzhou Medical University between January 1, 2009 and June 30, 2018 were eligible for inclusion. According to the database, 565 of the total maternal patients (70698) were diagnosed as “infection.” Four cases were excluded due to incomplete data. Based on the type of infection, the total study objects were divided into the sepsis group (n = 69, 12.3%) and non-sepsis group (n = 492, 87.7%).
Patients with ectopic pregnancy, hydatidiform mole and patients for whom complete data were not available were excluded.
There are two recommended approaches for the diagnosis of sepsis and septic shock[2]. The diagnostic criteria for sepsis and septic shock are shown in Table 1. Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. The assessment of sequential organ failure (SOFA) was used for the clinical identification of sepsis. SOFA scores ≥ 2 were considered indicative of organ dysfunction.
| Score | |||||
| System | 0 | 1 | 2 | 3 | 4 |
| Breath | |||||
| PaO2/FiO2, mmHg (kPa) | ≥ 400 (53.3) | < 400 (53.3) | < 300 (40.0) | < 200 (26.7) need breathing support | < 100 (13.3) |
| Clotting factor | |||||
| Platelets | ≥ 150 | < 150 | < 100 | < 50 | < 20 |
| Liver | |||||
| Bilirubin mg/dL (µmol/L) | < 1.2 (20) | 1.2–1.9 (20–32) | 2.0–5.9 (33–101) | 6.0–11.9 (102–204) | > 12.0 (204) |
| Cardiovascular | MAP ≥ 70 mmHg | MAP < 70 mmHg | Dopamine < 5.0 | Dopamine = 5.1–15.0 | Dopamine > 15.0 |
| Or dobutamine (any dose)1 | Or adrenaline ≤ 0.1 | Or adrenaline> 0.1 | |||
| Or noradrenaline ≤ 0.11 | Or noradrenaline >0.11 | ||||
| Central nerve system | |||||
| Glasgow | 15 | 13–14 | 10–12 | 6–9 | < 6 |
| Kidney | |||||
| Creatinine mg/dL (µmol/L) | < 1.2 (110) | 1.2–1.9 (110–170) | 2.0–3.4 (171–299) | 3.5–4.9 (300–440) | > 5.0 (440) |
| Urine output mL/d | < 500 | < 200 | |||
When laboratory data is not available or in emergency settings, adult patients with suspected infection can be identified using the quick SOFA method. According to this method, the presence of at least two of the following criteria is considered indicative of sepsis: Respiratory rate ≥ 22/min; altered mentation (Glasgow score < 13); and systolic blood pressure ≤ 100 mmHg. It is generally considered that the presence of two of the above three criteria is equivalent to SOFA score ≥ 2.
Patients with septic shock can be clinically identified by a requirement for vasopressor therapy to maintain a mean arterial pressure of ≥ 65 mmHg and serum lactate level > 2 mmol/L (> 18 mg/dL) in the absence of hypovolemia.
Infection refers to the invasion and multiplication of microorganisms such as bacteria, viruses and parasites that are not normally present within the body.
Regular prenatal care implies an obstetric checkup every 4 wk for 20-36 wk of gestation and every week after 36 wk of gestation. Irregular perinatal care implies a lack of adherence to the above protocol. Surgical history was an exposure factor.
SPSS 24.0 software (IBM, Armonk, NY, United States) was used for statistical analysis. Normally distributed variables are presented as mean ± SD, while non-normally distributed variables are presented as median and interquartile range. Between group differences were assessed using the Mann-Whitney U test with two independent samples, Pearson, likelihood ratio, continuous correction, Fisher’s exact and χ2 test (including the Monte Carlo method). Binary logistic regression analysis was used for analyzing risk factors. Univariate logical regression analysis was performed initially; variables that showed a significant association in univariate logistic regression analysis were included in the multivariate logistic regression model. P values < 0.05 were considered statistically significant difference.
A total of 70698 obstetric patients were admitted to the Third Affiliated Hospital of Guangzhou Medical University during the study reference period. Of these, 561 patients with infection qualified the inclusion criteria. The incidence rate of infection was 79.35/10000. Out of 561 patients, 69 were diagnosed with sepsis; the incidence rate of sepsis was 9.76/10000. These included 37 patients with septic shock. Eight patients died due to sepsis, which corresponded to a fatality rate of 11.6 % (Figure 1). The diagnostic criteria for sepsis and septic shock are shown in Table 1.
There were significant differences between the two groups with respect to proportion of migrant population, employment status, marital status, insurance status, education level, type of admission, prenatal care status, uterus scar condition, proportion of primiparous women, pregnancy history, gestational age (< 14 wk and ≥ 28 wk), presence of fever during pregnancy, surgical history, prevalence of gestational hypertensive disorder and placenta previa or placenta accrete (P < 0.05 for all) (Table 2).
| Infected maternal patients, n = 561 | Sepsis group, n = 69 | Non-sepsis group, n = 492 | Statistical value | P value | |
| Demographic information | |||||
| Age [yr, (IQR)] | 29 (25, 33) | 28 (24, 33) | 29 (26, 33) | z = -0.604 | 0.546 |
| Migrant population | |||||
| Yes | 105 | 21 (30.4) | 84 (17.1) | χ2 = 7.101 | 0.008 |
| No | 456 | 48 (69.4) | 408 (82.9) | ||
| Employment status | |||||
| No | 105 | 31 (44.9) | 74 (15.0) | χ2 = 35.529 | < 0.001 |
| Yes | 456 | 38 (55.1) | 418 (85.0) | ||
| Marital status | |||||
| Married | 524 | 57 (82.6) | 467 (94.9) | χ2 = 12.954 | < 0.001 |
| Single or divorced | 37 | 12 (17.4) | 25 (5.1) | ||
| Ethnicity | |||||
| Han | 553 | 66 (95.7) | 487 (99.0) | χ2 = 4.778 | 0.063 |
| Minorities | 8 | 3 (4.3) | 55 (1.0) | ||
| Education level | |||||
| > 12 yr | 156 | 11 (15.9) | 145 (29.5) | ||
| 7-12 yr | 365 | 48 (69.6) | 317 (64.4) | χ2 = 9.656 | 0.008 |
| ≤ 6 yr | 40 | 10 (14.5) | 30 (6.1) | ||
| Insurance status | |||||
| Yes | 193 | 14 (20.3) | 179 (36.4) | χ2 = 6.944 | 0.008 |
| Self-pay | 368 | 55 (85.1) | 313 (63.6) | ||
| Type of admission | |||||
| Outpatient | 251 | 15 (21.7) | 236 (48.0) | ||
| ER | 75 | 10 (14.5) | 65 (13.2) | χ2 = 18.369 | < 0.001 |
| Transfer | 235 | 41 (63.8) | 191 (38.8) | ||
| Prenatal care | |||||
| Regular | 310 | 20 (29.0) | 290 (58.9) | χ2 = 22.257 | < 0.001 |
| Irregular | 125 | 23 (33.3) | 102 (20.7) | ||
| Lack of prenatal care | 126 | 26 (37.3) | 100 (20.3) | ||
| Gestational age | 32.00 (26.15, 38.00) | 29.20 (21.05, 38.35) | 32.00 (27.23, 38.00) | Z = -2.296 | 0.022 |
| Gestational age | |||||
| < 14 | 18 | 5 (7.2) | 13 (2.6) | ||
| 14–27+6 | 144 | 27 (39.1) | 117(23.8) | χ2 = 11.652 | 0.003 |
| ≥ 28 | 399 | 37 (53.6) | 362 (73.6) | ||
| Premature rupture of membranes | |||||
| No | 422 | 51 (73.9) | 371(75.4) | χ2 = 0.072 | 0.788 |
| Yes | 139 | 18 (26.1) | 121 (24.6) | ||
| Surgical history | |||||
| No | 406 | 42 (60.9) | 364 (74.0) | χ2 = 5.205 | 0.023 |
| Yes | 155 | 27 (39.1) | 128 (26.0) | ||
| Fever | |||||
| Antepartum | 272 | 38 (55.1) | 234 (47.6) | ||
| Intrapartum | 31 | 2 (2.9) | 29 (5.9) | χ2 = 55.986 | < 0.001 |
| Postpartum | 111 | 29 (42.0) | 82 (16.7) | ||
| No | 147 | 0 (0) | 147 (29.9) | ||
| Gestational complications | |||||
| Multiple pregnancy | |||||
| No | 494 | 63 (91.3) | 431 (87.6) | χ2 = 0.789 | 0.374 |
| Yes | 67 | 6 (8.7) | 61 (12.4) | ||
| Gestational diabetic or diabetes | |||||
| No | 498 | 65 (94.2) | 433 (88.0) | χ2 = 2.329 | 0.127 |
| Yes | 63 | 4 (5.8) | 59 (12.0) | ||
| Gestational hypertension | |||||
| No | 479 | 65 (94.2) | 414 (84.1) | χ2 = 4.904 | 0.027 |
| Yes | 82 | 4 (5.8) | 78 (15.9) | ||
| Placenta previa or accrete | |||||
| No | 513 | 54 (78.3) | 459 (93.3) | χ2 = 17.476 | < 0.001 |
| Yes | 48 | 15 (21.7) | 33 (6.7) | ||
| Thyroid disease | |||||
| No | 539 | 65 (94.2) | 474 (96.3) | χ2 = 0.277 | 0.391 |
| Yes | 22 | 4 (5.8) | 18 (3.7) | ||
Emergency admission [odds ratio (OR) = 2.183] or transfer (OR = 2.870), irregular prenatal care (OR = 2.953), labor induction (OR = 4.665), cervical cerclage (OR = 14.214), first trimester (OR = 6.806) and second trimester (OR = 2.090) were found to be significant risk factors for maternal sepsis (Table 3).
| OR | 95%CI | P value | ||
| Lowest | Highest | |||
| Immigrant population | 1.380 | 0.698 | 2.729 | 0.355 |
| Single/divorced | 2.347 | 0.958 | 5.748 | 0.062 |
| Ethnic groups | 2.888 | 0.504 | 16.556 | 0.234 |
| Education level | 0.540 | |||
| > 12 yr | Control | |||
| 7–12 yr | 0.961 | 0.426 | 2.168 | 0.924 |
| ≤ 6 yr | 1.615 | 0.523 | 4.985 | 0.405 |
| Employment | 0.212 | 0.112 | 0.399 | < 0.001 |
| Insured | 0.889 | 0.421 | 1.877 | 0.758 |
| Mode of admission | 0.025 | |||
| Outpatient | Control | |||
| ER | 2.183 | 0.834 | 5.715 | 0.112 |
| Transfer | 2.870 | 1.336 | 6.167 | 0.007 |
| Prenatal care | 0.029 | |||
| Regular | Control | |||
| Irregular | 2.953 | 1.324 | 6.586 | 0.008 |
| No prenatal care | 1.950 | 0.885 | 4.295 | 0.098 |
| Manual removal of placenta | 2.518 | 0.333 | 19.059 | 0.371 |
| Labor induction | 4.665 | 1.984 | 10.966 | < 0.001 |
| Cervical cerclage | 14.214 | 2.201 | 91.808 | 0.005 |
| Placenta previa or accrete | 2.158 | 0.931 | 5.003 | 0.073 |
| Gestational age | 0.002 | |||
| ≥ 28 | Control | |||
| 14–27+6 | 2.090 | 1.094 | 3.995 | 0.026 |
| < 14 | 6.806 | 2.021 | 22.919 | 0.002 |
Escherichia coli (E. coli) was the most common pathogenic bacterium, and intrauterine infection was the common cause of maternal sepsis (Table 4). Maternal sepsis occurred more often in patients with a history of manual removal of retained placenta, labor induction or cervical cerclage procedure. The proportion of patients who underwent labor induction was significantly different between the sepsis and the non-sepsis groups (P < 0.05).
| Infected maternal patients, n = 561 | Sepsis group, n = 69 | Non-sepsis group, n = 492 | Statistical value | P value | |
| Surgical history | |||||
| Placenta previa or accrete | 6 | 3 (4.7) | 3 (0.6) | χ2 = 7.991 | 0.027 |
| Labor induction | 57 | 15 (21.7) | 42 (8.2) | χ2 = 11.555 | 0.001 |
| Amniocentesis | 12 | 3 (4.3) | 9 (1.8) | χ2 = 0.828 | 0.363 |
| Cervical cerclage | 7 | 3 (4.3) | 4 (0.8) | χ2 = 6.136 | 0.043 |
| Cesarean section | 72 | 9 (13.0) | 63(12.8) | χ2 = 0.003 | 0.956 |
| Number of pathogenic bacterium | |||||
| 0 | 68 | 19 (27.5) | 49 (10.0) | ||
| 1 | 345 | 34 (49.3) | 311 (63.2) | χ2 = 23.873 | < 0.001 |
| 2 | 53 | 9 (13.0) | 44 (8.9) | ||
| ≥ 3 | 16 | 4 (5.8) | 12 (2.4) | ||
| Without pathogen detection | 79 | 3 (4.3) | 76 (15.4) | ||
| Category of pathogenic bacterium | |||||
| Escherichia coli | 101 | 25 (36.2) | 76 (15.4) | χ2 = 17.709 | < 0.001 |
| Staphylococcus | 32 | 8 (11.6) | 24 (4.9) | χ2 = 3.903 | 0.048 |
| GAS | 9 | 3 (4.3) | 6 (1.2) | χ2 = 2.032 | 0.154 |
| GBS | 10 | 1 (1.4) | 9 (1.8) | χ2 = 0.000 | 1 |
| Enterococcus faecalis | 29 | 4 (5.8) | 25 (5.1) | χ2 = 0.000 | 1 |
| Site of infection | |||||
| Other or unknown | 15 | 2 (2.9) | 13 (2.6) | ||
| Uterine | 217 | 32 (46.4) | 185 (37.6) | ||
| Respiratory | 133 | 12 (17.4) | 121 (24.6) | χ2 = 24.395 | < 0.001 |
| Urinary tract | 176 | 13 (18.8) | 163 (33.1) | ||
| Placental complications | 20 | 10 (14.5) | 10 (2.0) | ||
The common pathogenic bacteria for maternal sepsis were E. coli, Group A Staphylococcus, Enterococcus faecalis, and Group B Staphylococcus. Patients with multi-bacterial infection had higher morbidity (P < 0.05).
There was a significant between group difference with respect to the proportion of patients who underwent hysterectomy, intensive care unit (ICU) admission, tracheal intubation, transfusion of blood products, hemofiltration or plasma exchange (P < 0.01 for all). The duration of hospitalization and the fatality were also significantly different between the two groups (P < 0.01 for all). Out of the 69 patients in the sepsis group, 2 patients received medication for abortion without doctors’ instructions and 5 patients gave birth outside the hospital. Four of these five patients who gave birth outside the hospital died, which accounted for 50% of the fatalities. Two patients died due to severe hepatitis, one died due to hematological disease, and one died due to severe pneumonia. The between group difference with respect to maternal mortality was statistically significant (P < 0.01).
A total of 76 babies died of infection during the perinatal period. Of these, 26 babies were born of mothers in the sepsis group while 50 babies were born of mothers in the non-sepsis group. The perinatal mortality rate in the sepsis and non-sepsis groups was 37.7% and 10.2%, respectively (Table 5).
| Infected maternal patients, n = 561 | Sepsis group, n = 69 | Non-sepsis group, n = 492 | Statistical value | P value | |
| Maternal outcomes | |||||
| Hysterectomy | 21 | 13 (18.8) | 8 (1.6) | χ2 = 45.106 | < 0.001 |
| Intubation | 44 | 31 (44.9) | 13 (2.6) | χ2 = 149.697 | < 0.001 |
| Transfusion | 56 | 39 (56.5) | 17 (3.5) | χ2 = 189.643 | < 0.001 |
| Hemofiltration or plasma exchange | 17 | 16 (23.2) | 1 (0.2) | χ2 = 101.117 | < 0.001 |
| ICU admission | 108 | 58 (84.1) | 50 (10.2) | χ2 = 212.564 | < 0.001 |
| Death | 8 | 8 (11.59) | 0 | χ2 = 57.869 | < 0.001 |
| Duration of hospitalization | 8.0 (5.0, 11.0) | 11.0 (8.0, 14.5) | 8.0 (5.0, 10.0) | z = -4.971 | < 0.001 |
| Perinatal outcomes | |||||
| Survive | 309 | 37 (53.6) | 272 (55.3) | ||
| Death | 76 | 26 (37.7) | 50 (10.2) | χ2 = 46.753 | < 0.001 |
| Not yet delivered | 176 | 6 (8.7) | 170 (34.6) | ||
The description of sepsis can be traced back to the time of Hippocrates when it was referred to as the process of physical decay and wound ulceration[13]. Four components of sepsis have been described previously, i.e. systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis and septic shock. Subsequently, systemic inflammatory response syndrome and severe sepsis have been removed from the “sepsis–3,” which only refers to sepsis and its subset septic shock[2,3]. There are no separate gold standard diagnostic criteria for maternal sepsis; the diagnosis is based on the criteria used for the diagnosis of sepsis in non-pregnant women. In this study, we used the definition in the World Health Organization statement[4]. Accordingly, 69 maternal patients were diagnosed with sepsis. Of these, eight died due to sepsis-related diseases. The most significant risk factors for maternal sepsis in the Guangzhou area were mode of admission, hospital transfer, irregular prenatal care, labor induction, cervical cerclage, and first and second trimesters. E. coli was the most common pathogenic bacteria for maternal sepsis, and intrauterine infection was the most common cause of sepsis.
The incidence of sepsis in previous reports shows much variability owing to the inconsistent diagnostic criteria. Most of the available sepsis-related data were collected from high-income countries, while there is a paucity of data from low- and middle-income countries, which account for 87% of the world’s population[1,6,13]. In high-income countries, the incidence of pregnancy-related sepsis is 9–49 per 100000 deliveries[14]. In New Zealand, obstetric sepsis is the most common cause of admission to the intensive care unit and high-dependent care unit; the reported incidence rate of severe sepsis among obstetric women is 1.4/10000–5.0/10000 with a fatality rate of 1/75[15]. The morbidity and mortality attributed to sepsis are on the rise in the United States, and sepsis is considered to be the main cause of intensive care unit deaths. Maternal sepsis was shown to be associated with a 2.81-fold higher risk of premature delivery and 5.78-fold higher risk of perinatal mortality[8]. In low- and middle-income countries, maternal sepsis is associated with a fatality rate of approximately 4%–50%; maternal fatality rate in septic shock varies between 20%–28%[9]. Based on the diagnostic criteria of Sepsis-3, the incidence rate of maternal sepsis in our study was 9.76/10000, which is consistent with previous reports. The maternal fatality attributable to sepsis and septic shock in the present study was 11.6% and 21.6%, respectively. Although the maternal and perinatal outcomes are consistent with those reported by previous studies, the actual incidence and mortality in the region may be lower since this medical facility is a referral center.
Pregnancy-related structural, physiological and immunological changes render pregnant women more vulnerable to infection, especially urogenital tract infection, health care-related infection and other non-reproductive infections. Some systemic infections may be more severe or occur more frequently during pregnancy. The known risk factors for sepsis include elderly pregnant women, poor nutritional status, low socioeconomic status, lack of medical insurance, ethnic minorities, smoking history, obesity, history of invasive procedures (e.g., amniocentesis, multifetal reduction, cervical cerclage and especially emergency cervical cerclage), gestational complications (e.g., multiple vaginal examinations, prolonged labor, vaginal delivery surgery, cesarean section, premature delivery, premature rupture of membranes, multiple pregnancy, postpartum hemorrhage, placental retention, hysterectomy, blood transfusion, postpartum infection), immune factors (immune impairment or use of immunosuppressive drugs) and other medical diseases (e.g., diabetes, anemia)[16-20]. Our study confirmed some of the above-mentioned risk factors including history of invasive procedures and gestational complications. Some other risk factors that are uncommon in other regions were also identified in our study; these included mode of admission, hospital transfer, lack of regular prenatal care and gestational age (first and second trimesters). Not many studies have indicated gestational age as a risk factor for maternal sepsis.
Ethnic minorities are more likely to be a transient population in Guangzhou city. Pregnant women belonging to ethnic minority groups are at a high risk of sepsis due to unstable income, lack of medical insurance, low education level, poor awareness about health care, irregular prenatal care or even no prenatal care, lack of timely medical consultation when necessary and poor medical compliance. Women with high education levels tend to have a good knowledge of self-care. They actively seek information about perinatal health care and undergo prenatal examinations. This can facilitate early identification of infection symptoms and high-risk factors for sepsis, leading to timely intervention and improved prognosis of patients. Patients with sepsis are most likely to be referred due to the severity of the disease. The Third Affiliated Hospital of Guangzhou Medical University is a referral center for pregnant women. Therefore, the proportion of sepsis among referral patients was the largest, and referral was a high-risk factor for sepsis.
First and second trimester was another risk factor for sepsis; this may be because invasive procedures (such as cervical cerclage, abortion and amniocentesis) are more likely to be performed during this period. In our study, pregnant women showed a higher risk of sepsis, especially during the first and second trimesters. In addition, septic patients have a higher probability of transfer due to their serious condition; this makes hospital transfer as one of the correlates of maternal sepsis. In our study, transferred patients accounted for the largest proportion of patients in the sepsis group as our hospital is the referral center for obstetric cases.
In this study, 39.1% patients in the sepsis group had a history of intrapartum or postpartum invasive procedures. Although there was no significant between group difference with respect to cesarean section rate, labor induction and cesarean section accounted for most cases of maternal sepsis. Labor induction and cervical cerclage were identified as significant risk factors in our study, suggesting that sepsis may be related to intrauterine infection and ascending genital tract infection. Cesarean section has been reported to be associated with maternal sepsis as well as an increased risk for maternal sepsis-related death[11]. A large national cohort study in the Netherlands showed that 42.9% of women with postpartum sepsis applied cesarean delivery[12]. In addition, a multicenter study in the United States and Israel found that both prophylactic and urgent cervical cerclage were risk factors for maternal sepsis[19]. The relative risk of labor induction in sepsis was 5.2[16]. However, in a study by Knowles et al[17], use of preventive antibacterial drugs was shown to reduce the incidence of infection related to cesarean section from 85% to 5%. Due to the retrospective nature of the study, complete data pertaining to the use of antibiotics were not available; thus we were unable to draw any definitive conclusions in this regard.
Several studies have found that socio-economic and healthcare insurance status are risk factors for maternal sepsis[8,21]. In this study, health insurance status did not appear to be a significant risk factor for maternal sepsis. However, we found that unemployment was a risk factor for maternal sepsis. Risk factors in this study are not completely consistent with previous studies; this may be attributable to: (1) Small sample size of patients with sepsis; and (2) Differences with respect to study population and definition of sepsis.
In this study, uterine infection and urinary tract infection were the most common causes of infection in the non-sepsis group (incidence rate: 37.6% and 33.1%, respectively). The incidence of fever during the postpartal period was 2.9 times higher than that in the antenatal period. The most common cause of maternal sepsis was also uterine infection; among these, postpartum placental complications accounted for 14.5% of all cases; 97.1% of the cases had a fever before or after childbirth.
E. coli was the most common pathogen in sepsis followed by Staphylococcus, Enterococcus faecalis, Group A Staphylococcus, Group B Staphylococcus and multi-bacterial infection. In a study by Lepine et al[15], Group A Staphylococcus-related septic shock was the most common manifestation of maternal sepsis with a fatality rate of 8%-23%. Multi-bacterial infections accounted for 18.8% of the total bacterial infections; the prevalence of multi-bacterial infection in the sepsis group was greater than that in the non-sepsis group. However, the causative organism was not investigated for three patients. According to Sepsis-3 guidelines, two or more sets of blood culture (aerobic and anaerobic) are recommended prior to any new antibacterial therapy for patients with suspected sepsis or septic shock[3]. Therefore, etiological tests should be performed routinely for patients with suspected sepsis.
We analyzed the causes and risk factors of maternal sepsis in a fairly representative sample in Guangzhou area. We also identified some uncommon risk factors of maternal sepsis that are different from those reported from other regions. However, some limitations of our study should be acknowledged. All data in the pregnancy and perinatal medical database were entered manually, which may have introduced some errors. Second, all data were collected from a referral medical facility, which may have introduced an element of bias. Third, complete etiological test results were not available for three patients. Fourth, due to the relatively rare occurrence of maternal sepsis, some potential risk factors of sepsis could not be investigated. Fifth, data about the use of antibiotics was not included due to the retrospective nature of the study. Lastly, this was a single-center study. However, the sample size was relatively large and fairly representative of a large catchment area. Further multicenter clinical study is required to confirm our findings.
In conclusion, mode of admission, poor prenatal care, labor induction, cervical cerclage and gestational age (first trimester and second trimester) were risk factors for maternal sepsis. Improving residents’ health care awareness, standardization of the diagnosis of maternal sepsis and use of antibacterial drugs and timely identification of the risk factors are essential for the prevention of maternal sepsis and improvement of maternal and perinatal outcomes.
Globally, sepsis is the third leading cause of maternal mortality. China has relatively high rates of sepsis-related maternal morbidity and neonatal mortality. However, the risk factors for maternal sepsis in mainland China are not well-characterized.
We aimed to analyze the etiology of maternal sepsis and to identify the associated risk factors using the new definitions of sepsis and septic shock. The identified risk factors can be used to develop prediction models for early intervention.
To analyze the etiology of maternal sepsis and to identify the associated risk factors.
Data of obstetric patients with infection who were admitted to the Third Affiliated Hospital of Guangzhou Medical University between January 2009 and June 2018 were retrospectively analyzed. Patients were divided into the sepsis group and non-sepsis group based on the definition of sepsis. Patient characteristics, obstetric factors and duration of hospitalization were compared between the two groups. Risk factors for maternal sepsis were identified using multivariate logistic regression models.
The morbidity rate of maternal sepsis was 9.76/10000; the fatality rate in the sepsis group was 11.6%. Mode of admission, poor prenatal care, labor induction, cervical cerclage and first trimester and second trimester pregnancy were risk factors for maternal sepsis. Escherichia coli was the most common causative organism for maternal sepsis; the uterus was the most common site of infection.
This study determined the incidence and fatality rate of sepsis in pregnant women and identified the risk factors for sepsis in infected pregnant women.
The identified risk factors can be used to establish prediction models for early intervention against maternal sepsis.
Manuscript source: Unsolicited manuscript
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Behera B, Deshwal H, Inal V, Rodrigues AT S-Editor: Liu M L-Editor: Filipodia P-Editor: Li JH
| 1. | Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, Gonzalez-Medina D, Barber R, Huynh C, Dicker D, Templin T, Wolock TM, Ozgoren AA, Abd-Allah F, Abera SF, Abubakar I, Achoki T, Adelekan A, Ademi Z, Adou AK, Adsuar JC, Agardh EE, Akena D, Alasfoor D, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Al Kahbouri MJ, Alla F, Allen PJ, AlMazroa MA, Alsharif U, Alvarez E, Alvis-Guzmán N, Amankwaa AA, Amare AT, Amini H, Ammar W, Antonio CA, Anwari P, Arnlöv J, Arsenijevic VS, Artaman A, Asad MM, Asghar RJ, Assadi R, Atkins LS, Badawi A, Balakrishnan K, Basu A, Basu S, Beardsley J, Bedi N, Bekele T, Bell ML, Bernabe E, Beyene TJ, Bhutta Z, Bin Abdulhak A, Blore JD, Basara BB, Bose D, Breitborde N, Cárdenas R, Castañeda-Orjuela CA, Castro RE, Catalá-López F, Cavlin A, Chang JC, Che X, Christophi CA, Chugh SS, Cirillo M, Colquhoun SM, Cooper LT, Cooper C, da Costa Leite I, Dandona L, Dandona R, Davis A, Dayama A, Degenhardt L, De Leo D, del Pozo-Cruz B, Deribe K, Dessalegn M, deVeber GA, Dharmaratne SD, Dilmen U, Ding EL, Dorrington RE, Driscoll TR, Ermakov SP, Esteghamati A, Faraon EJ, Farzadfar F, Felicio MM, Fereshtehnejad SM, de Lima GM, Forouzanfar MH, França EB, Gaffikin L, Gambashidze K, Gankpé FG, Garcia AC, Geleijnse JM, Gibney KB, Giroud M, Glaser EL, Goginashvili K, Gona P, González-Castell D, Goto A, Gouda HN, Gugnani HC, Gupta R, Hafezi-Nejad N, Hamadeh RR, Hammami M, Hankey GJ, Harb HL, Havmoeller R, Hay SI, Pi IB, Hoek HW, Hosgood HD, Hoy DG, Husseini A, Idrisov BT, Innos K, Inoue M, Jacobsen KH, Jahangir E, Jee SH, Jensen PN, Jha V, Jiang G, Jonas JB, Juel K, Kabagambe EK, Kan H, Karam NE, Karch A, Karema CK, Kaul A, Kawakami N, Kazanjan K, Kazi DS, Kemp AH, Kengne AP, Kereselidze M, Khader YS, Khalifa SE, Khan EA, Khang YH, Knibbs L, Kokubo Y, Kosen S, Defo BK, Kulkarni C, Kulkarni VS, Kumar GA, Kumar K, Kumar RB, Kwan G, Lai T, Lalloo R, Lam H, Lansingh VC, Larsson A, Lee JT, Leigh J, Leinsalu M, Leung R, Li X, Li Y, Liang J, Liang X, Lim SS, Lin HH, Lipshultz SE, Liu S, Liu Y, Lloyd BK, London SJ, Lotufo PA, Ma J, Ma S, Machado VM, Mainoo NK, Majdan M, Mapoma CC, Marcenes W, Marzan MB, Mason-Jones AJ, Mehndiratta MM, Mejia-Rodriguez F, Memish ZA, Mendoza W, Miller TR, Mills EJ, Mokdad AH, Mola GL, Monasta L, de la Cruz Monis J, Hernandez JC, Moore AR, Moradi-Lakeh M, Mori R, Mueller UO, Mukaigawara M, Naheed A, Naidoo KS, Nand D, Nangia V, Nash D, Nejjari C, Nelson RG, Neupane SP, Newton CR, Ng M, Nieuwenhuijsen MJ, Nisar MI, Nolte S, Norheim OF, Nyakarahuka L, Oh IH, Ohkubo T, Olusanya BO, Omer SB, Opio JN, Orisakwe OE, Pandian JD, Papachristou C, Park JH, Caicedo AJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pereira DM, Pesudovs K, Petzold M, Poenaru D, Polanczyk GV, Polinder S, Pope D, Pourmalek F, Qato D, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, ur Rahman S, Raju M, Rana SM, Refaat A, Ronfani L, Roy N, Pimienta TG, Sahraian MA, Salomon JA, Sampson U, Santos IS, Sawhney M, Sayinzoga F, Schneider IJ, Schumacher A, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shakh-Nazarova M, Sheikhbahaei S, Shibuya K, Shin HH, Shiue I, Sigfusdottir ID, Silberberg DH, Silva AP, Singh JA, Skirbekk V, Sliwa K, Soshnikov SS, Sposato LA, Sreeramareddy CT, Stroumpoulis K, Sturua L, Sykes BL, Tabb KM, Talongwa RT, Tan F, Teixeira CM, Tenkorang EY, Terkawi AS, Thorne-Lyman AL, Tirschwell DL, Towbin JA, Tran BX, Tsilimbaris M, Uchendu US, Ukwaja KN, Undurraga EA, Uzun SB, Vallely AJ, van Gool CH, Vasankari TJ, Vavilala MS, Venketasubramanian N, Villalpando S, Violante FS, Vlassov VV, Vos T, Waller S, Wang H, Wang L, Wang X, Wang Y, Weichenthal S, Weiderpass E, Weintraub RG, Westerman R, Wilkinson JD, Woldeyohannes SM, Wong JQ, Wordofa MA, Xu G, Yang YC, Yano Y, Yentur GK, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Jin KY, El Sayed Zaki M, Zhao Y, Zheng Y, Zhou M, Zhu J, Zou XN, Lopez AD, Naghavi M, Murray CJ, Lozano R. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1090] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 2. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 18667] [Article Influence: 1866.7] [Reference Citation Analysis (3)] |
| 3. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3352] [Cited by in RCA: 4138] [Article Influence: 459.8] [Reference Citation Analysis (8)] |
| 4. | World Health Organization. Statement on maternal sepsis. [cited 10 January 2021]. Available from: https://apps.who.int/iris/handle/10665/2546082017. |
| 5. | Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2297] [Cited by in RCA: 2396] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 6. | Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4232] [Cited by in RCA: 3706] [Article Influence: 308.8] [Reference Citation Analysis (0)] |
| 7. | Chen XC, Yang YF, Wang R, Gou HF, Chen XZ. Epidemiology and microbiology of sepsis in mainland China in the first decade of the 21st century. Int J Infect Dis. 2015;31:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Al-Ostad G, Kezouh A, Spence AR, Abenhaim HA. Incidence and risk factors of sepsis mortality in labor, delivery and after birth: population-based study in the USA. J Obstet Gynaecol Res. 2015;41:1201-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Galvão A, Braga AC, Gonçalves DR, Guimarães JM, Braga J. Sepsis during pregnancy or the postpartum period. J Obstet Gynaecol. 2016;36:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Acosta CD, Harrison DA, Rowan K, Lucas DN, Kurinczuk JJ, Knight M. Maternal morbidity and mortality from severe sepsis: a national cohort study. BMJ Open. 2016;6:e012323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Acosta CD, Knight M, Lee HC, Kurinczuk JJ, Gould JB, Lyndon A. The continuum of maternal sepsis severity: incidence and risk factors in a population-based cohort study. PLoS One. 2013;8:e67175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Kramer HM, Schutte JM, Zwart JJ, Schuitemaker NW, Steegers EA, van Roosmalen J. Maternal mortality and severe morbidity from sepsis in the Netherlands. Acta Obstet Gynecol Scand. 2009;88:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med. 2017;377:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 909] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 14. | Bonet M, Souza JP, Abalos E, Fawole B, Knight M, Kouanda S, Lumbiganon P, Nabhan A, Nadisauskiene R, Brizuela V, Metin Gülmezoglu A. The global maternal sepsis study and awareness campaign (GLOSS): study protocol. Reprod Health. 2018;15:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Lepine S, Lawton B, Geller S, Abels P, MacDonald EJ. Severe maternal morbidity due to sepsis: The burden and preventability of disease in New Zealand. Aust N Z J Obstet Gynaecol. 2018;58:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Acosta CD, Bhattacharya S, Tuffnell D, Kurinczuk JJ, Knight M. Maternal sepsis: a Scottish population-based case-control study. BJOG. 2012;119:474-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Knowles SJ, O'Sullivan NP, Meenan AM, Hanniffy R, Robson M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG. 2015;122:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Bamfo JE. Managing the risks of sepsis in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2013;27:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Bauer ME, Housey M, Bauer ST, Behrmann S, Chau A, Clancy C, Clark EAS, Einav S, Langen E, Leffert L, Lin S, Madapu M, Maile MD, McQuaid-Hanson E, Priessnitz K, Sela HY, Shah A, Sobolewski P, Toledo P, Tsen LC, Bateman BT. Risk Factors, Etiologies, and Screening Tools for Sepsis in Pregnant Women: A Multicenter Case-Control Study. Anesth Analg. 2019;129:1613-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Mohamed-Ahmed O, Nair M, Acosta C, Kurinczuk JJ, Knight M. Progression from severe sepsis in pregnancy to death: a UK population-based case-control analysis. BJOG. 2015;122:1506-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Feng XL, Zhu J, Zhang L, Song L, Hipgrave D, Guo S, Ronsmans C, Guo Y, Yang Q. Socio-economic disparities in maternal mortality in China between 1996 and 2006. BJOG. 2010;117:1527-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |