Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7196
Peer-review started: February 23, 2021
First decision: June 15, 2021
Revised: June 17, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: August 26, 2021
Processing time: 181 Days and 5 Hours
Lateral facial clefts are atypical with a low incidence in the facial cleft spectrum. With the development of ultrasonography (US) prenatal screening, such facial malformations can be detected and diagnosed prenatally rather than at birth. Although three-dimensional US (3DUS) can render the fetus' face via 3D reconstruction, the 3D images are displayed on two-dimensional screens without field depth, which impedes the understanding of untrained individuals. In contrast, a 3D-printed model of the fetus' face helps both parents and doctors develop a more comprehensive understanding of the facial malformation by creating more interactive aspects. Herein, we present an isolated lateral facial cleft case that was diagnosed via US combined with a 3D-printed model.
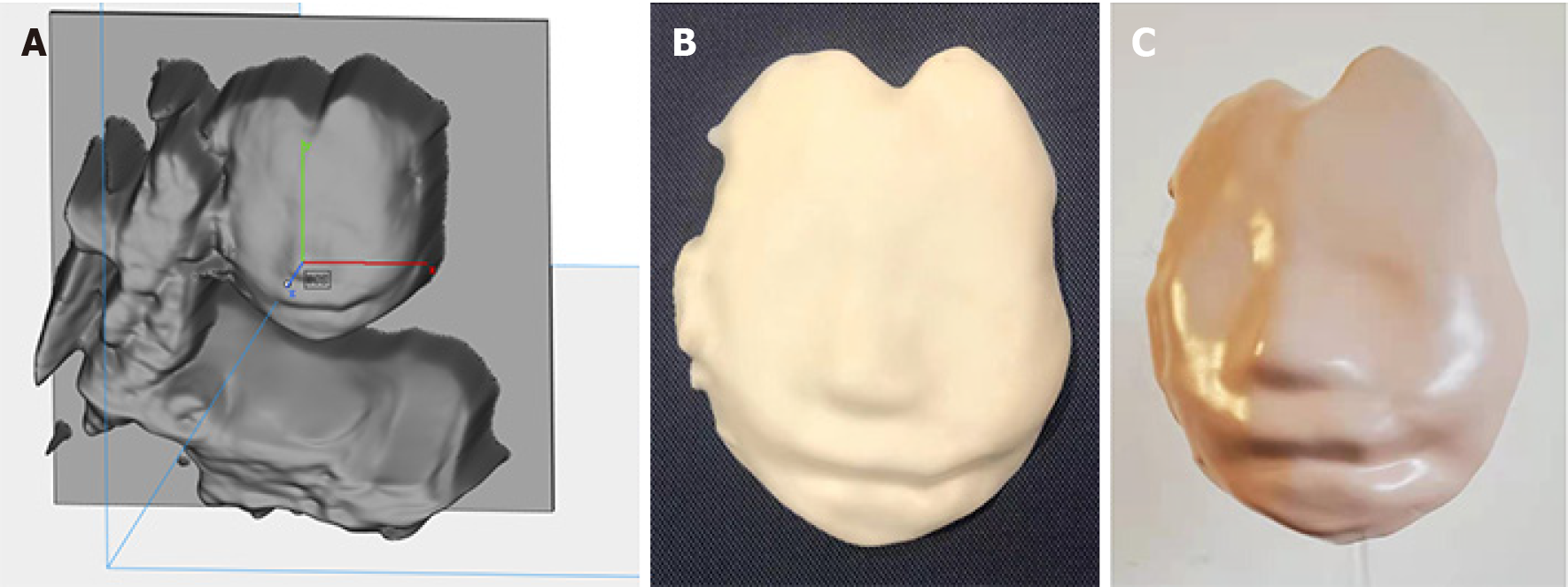
A 31-year-old G2P1 patient presented for routine prenatal screening at the 22nd wk of gestation. The coronal nostril-lip section of two-dimensional US (2DUS) demonstrated that the fetus' bilateral oral commissures were asymmetrical, and left oral commissure was abnormally wide. The left oblique-coronal section showed a cleft at the left oral commissure which extended to the left cheek. The results of 3DUS confirmed the cleft. Furthermore, we created a model of the fetal face using 3D printing technology, which clearly presented facial malformations. The fetus was diagnosed with a left lateral facial cleft, which was categorized as a No. 7 facial cleft according to the Tessier facial cleft classification. The parents terminated the pregnancy at the 24th wk of gestation after parental counseling.
In the diagnostic course of the current case, in addition to the traditional application of 2D and 3DUS, we created a 3D-printed model of the fetus, which enhanced diagnostic evidence, benefited the education of junior doctors, improved parental counseling, and had the potential to guide surgical planning.
Core Tip: In this study, we present a case with rare facial anomaly of isolated lateral facial cleft. In the prenatal diagnostic course, in addition to the traditional screening using two-dimensional and three-dimensional (3D) ultrasonography, we created a 3D-printed model of the fetus which enhanced diagnostic evidence, benefited the education of junior doctors, improved parental counseling, and had the potential to guide surgical planning.
- Citation: Song WL, Ma HO, Nan Y, Li YJ, Qi N, Zhang LY, Xu X, Wang YY. Prenatal diagnosis of isolated lateral facial cleft by ultrasonography and three-dimensional printing: A case report. World J Clin Cases 2021; 9(24): 7196-7204
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7196.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7196
Lateral or transverse facial cleft, categorized as a No. 7 facial cleft in the Tessier facial cleft classification, is an atypical facial cleft with a low incidence in the facial cleft spectrum[1]. It is defined as a facial cleft extending from unilateral or bilateral oral commissures, through the cheek(s), and towards the external ear(s), which results in macrostomia of the involved side(s)[2]. It may arise mildly as an isolate extending of the oral commissure(s) alone, or more severely associated with skeletal disorders[3]. It can also present as a systemic malformation of systemic disorders, including Treacher-Collins syndrome (TCS) and oculo-auriculo-vertebral spectrum (OAVS)[4,5]. Lateral facial clefts can be detected and diagnosed via ultrasonography (US), which is routinely performed as an essential part of prenatal screening.
In the last decade, three-dimensional (3D) printing has developed rapidly in several medical fields, allowing the interaction between clinicians and tissue models. Recently, Nicot et al[6] created 3D-printed models of fetuses with facial deformities based on the surface rendering of 3DUS, which altered the traditional visualization of 3D reconstructions on two-dimensional (2D) screens, and led to improvement in parental understanding of fetal malformations. This innovation suggests the success of the expansion of 3D printing in the field of prenatal diagnosis.
Herein, we present an isolated lateral facial cleft diagnosed prenatally by 2D and 3DUS at the 22nd wk of gestation. Additionally, we created a 3D-printed model of the fetus' facial malformation, which enhanced diagnostic confidence and the understanding of inexperienced doctors and improved communication with parents during parental counseling.
A 31-year-old patient (G2P1) presented to our center for routine prenatal examination at the 22nd wk of gestation.
The patient was healthy, and her previous pregnancy resulted in laboring a healthy child.
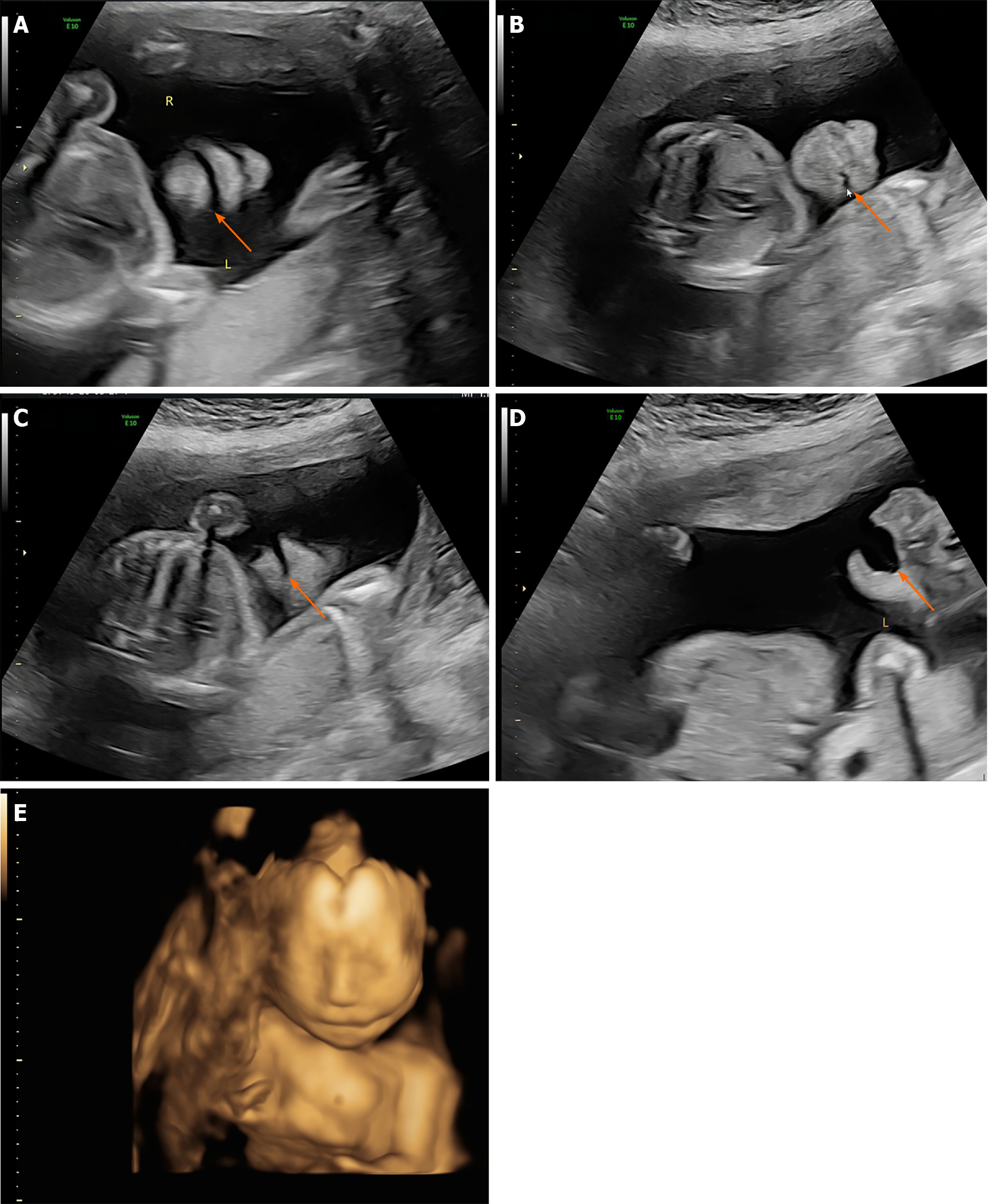
The patient received regular prenatal examinations beginning at the 6th wk of gestation, and US screening of the first trimester was unremarkable. During US screening, 2DUS examination showed normal results for fetal status. In the coronal lip-nostril plane, the fetus's lips, nose tip, nostrils, and mandible could be identified, and the edge of the upper lip appeared continuous. However, the oral commissures appeared asymmetric in the coronal plane, and a cleft was identified at the left oral commissure which extended to the left cheek (Figure 1A and B). The cleft was significantly enlarged while the mouth was open. The mid-sagittal plane was normal, while the left oblique-coronal section indicated the facial cleft extending from the left oral commissure through the left cheek towards the left ear (Figure 1C and D). Subsequently, the 3D surface rendering images were taken for further clarification, and the result showed the left lateral facial cleft, which resulted in unilateral macrostomia (Figure 1E). No other defects, e.g., signs of amniotic band syndrome, were identified on further investigations.
According to the findings, the patient was diagnosed with isolated unilateral facial cleft (Tessier No. 7 facial cleft).
The parents decided to terminate the pregnancy at 24 wk and 2 d of gestation after parental counseling and providing informed consent. They declined further genetic testing and postpartum anatomical examination. During parental counseling, the model was presented to the parents along with the US images, which enhanced their understanding of the fetus’ malformation.
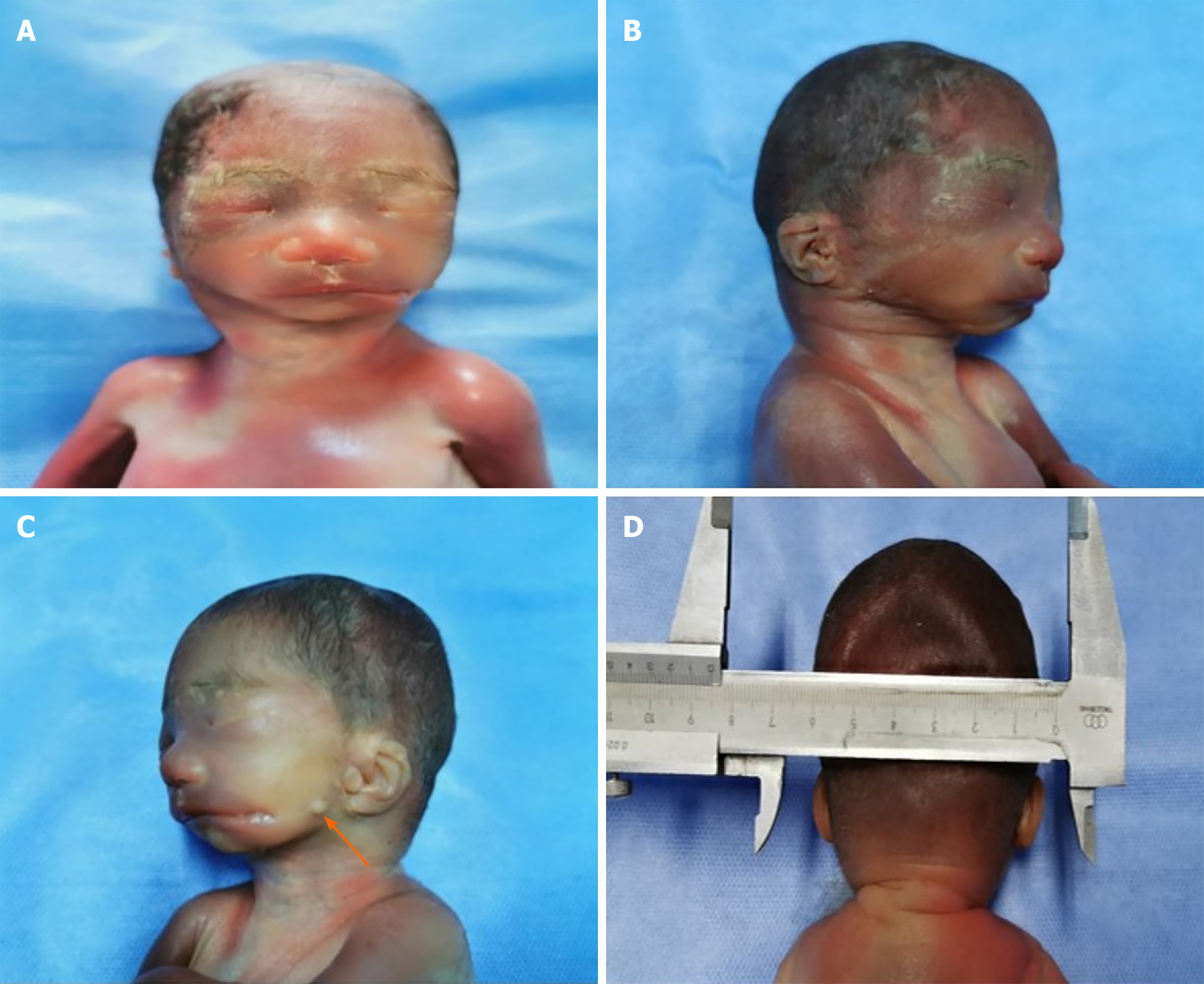
Post-inducing pathological examination confirmed the left lateral facial cleft, which presented as a left lateral facial cleft (Figure 2). However, a skin tag was identified anterior to the left external ear, which was not detected by US (Figure 3). Other tissues were normal in appearance.
Of all atypical facial clefts, lateral facial clefts are the most frequent, representing 0.3%-1.0% of the facial cleft spectrum with an incidence of 0.02% of all live births[7,8]. Similar to other atypical facial clefts, lateral facial clefts occur more frequently in male patients, and the bilateral form is six times rarer than unilateral clefts[2]. The pathogenesis of the lateral facial cleft remains unclear, and may be related to impaired migration of ectomesenchymal cells, palatal shelf disorders, embryonic hematoma formation due to stapedial artery stem disruption, and other disruptive factors such as amniotic bands[9,10]. In a study by Stelnicki et al[11], they used a lamb uterus model to demonstrate that cell migration impediment, ischemia, and cell apoptosis caused by disruptive restrictive forces resulted in the development of lateral facial cleft. Most lateral facial cleft cases were diagnosed via birth findings because of the unnoticeability of the presentation of lateral facial clefts under routine prenatal examination. Therefore, there have only been a few publications on the prenatal diagnosis of lateral facial clefts; instead, staged re-construction surgeries of lateral facial cleft cases have been abundantly documented[1].
Including the current report, a total of 11 cases with a lateral facial cleft have been diagnosed prenatally, all of which were initially screened or diagnosed by ultrasonography, which is a routine prenatal screening examination for the fetus. Among the reported cases, the average age of mothers was 31.5 years, and their obstetrical circumstances are listed below[12-14] (Table 1). The fetuses were initially suspected to have facial malformations between 21 wk and 33 wk of gestation, and six cases were diagnosed within the mid-trimester. For the detection approaches, six fetuses were suspected to have a lateral facial cleft under 2DUS; but for five patients, 2DUS reported no anomaly, while the results of 3DUS revealed malformation. Seven cases had lateral facial clefts, and the other four cases were later confirmed to be associated with other systemic disorders, including TCS (two cases) and OAVS (two cases). In terms of pregnancy outcomes, seven fetuses were live born (two were born via natural labor and five via cesarean section) and three families including the current case decided to terminate the pregnancy after consultation. In the present case, the fetus was detected with a unilateral facial cleft at the 22nd wk of gestation via 2D and 3DUS. This is the first time that a 3D-printed model was applied for the diagnosis of the lateral facial cleft.
| Ref. | Age | Gravida/para | Gestational age at the time of diagnosis | Bilateral or unilateral macrostomia | Method for first detection | Associated systemic disorders | Combined anomalies | Outcome |
| Cavaco-Gomes et al[15] | 35 | G4P1 | 21 wk | Unilateral | 2DUS | Negative | Low-set dysmorphological left external ear | Termination |
| Lee M et al[12] | 36 | G3P2 | 25 wk | Bilateral | 3DUS | Negative | Negative | Cesarean section |
| Chang et al[3] | 33 | G3P2 | 24 wk | Unilateral | 3DUS | Negative | Skin tag | Natural labor |
| Asai et al[21] | 27 | G1P0 | 31 wk | Bilateral | 3DUS | OAVS | Negative | Cesarean section |
| Pilu et al[13] | 29 | N/A | 21 wk | Unilateral | 2DUS | Negative | Single umbilical artery, skin tags | Termination |
| Witters et al[5] | 20 | N/A | 32 wk | Unilateral | 2DUS | OAVS | Single umbilical artery, cardiac atrial septum defect | Gestation intrauterine death |
| Troyano Luque et al[14] | 39 | G3P2 | 29 wk | Unilateral | 3DUS | Negative | Negative | Natural labor |
| Presti et al[10] | 33 | G3P0 | 26 wk | Bilateral | 2DUS | Negative | Negative | Cesarean section |
| Kubo et al[4] | 39 | G3P2 | 29 wk | Bilateral | 3DUS/4DUS | TCS | Negative except TCS | Cesarean section |
| Pereira et al[17] | 24 | G1P0 | 33 wk | Bilateral | 2DUS | TCS | Negative except TCS | Cesarean section |
| Current case | 31 | G2P1 | 22 wk | Unilateral | 2DUS | Negative | Skin tag | Termination |
In 1976, Paul Tessier published an anatomical classification of cranial-facial cleft ranging from 0-14 based on the cleft direction relative to the orbit[2]. As atypical facial clefts other than clefts of the lip and palate[10], lateral facial clefts have been categorized as Nos. 5-9 in the Tessier classification for their location lateral to the infraorbital foramen[2]. Among the five lateral types, Tessier No. 7 is the most lateral facial cleft without a relationship with the orbit[15]. Tessier No. 7 facial cleft or lateral facial cleft accounts for 0.3%-1.0% of infants with facial clefts[1]. The severity of Tessier No. 7 facial cleft is graded as: Grade I, slight widening of the oral commissure; Grade II, cleft reaches the anterior border of the masseter; and Grade III, cleft across the anterior masseter[16]. This grading system incorporates the macrostomia into the assessment; however, it is not the only presentation of Tessier No. 7 facial clefts. In addition to the facial sulcus, Tessier No. 7 facial clefts may present with or without other facial deformities, which can involve the external ear and other internal tissues such as the tongue, glands, and nerves[15]; additionally, bony deformities are commonly combined, including malformation of the zygoma, maxilla, and their attachments[7,17]. Woods et al[18] proposed a classification focused on the abnor
As a routine prenatal examination, US is cost-efficient, noninvasive, and radiation-free, and can provide fetal information in multiple aspects including appearance, circulation, and development. In China, it is performed regularly beginning at the 6th wk of gestation. In the reviewed literature, all cases underwent 2D and 3DUS, and most were diagnosed via ultrasonography in the second trimester. Before the popularization of 3DUS, 2DUS was the only method for ultrasonographic prenatal evaluation; however, accurate diagnosis of cleft using 2DUS is difficult even for the most skilled specialists[19]. To improve the sensitivity of 2DUS, the fetus must be assessed through various approaches, including frontal, sagittal, coronal, and oblique views across the midface[19]. The upper lip, median facial profile, orbits, and nostrils are important scanning marks and should be viewed under 2DUS[20]. In the coronal plane, subtle anomalies of the commissures and lips should be noted by operators. The mid-facial coronal plane can provide more valuable information on the lateral facial cleft, and abnormal widening of the commissure in the open-mouth view is conclusive evidence. In 2DUS, the amniotic fluid and membrane can provide diagnostic clues. Polyhydramnios has been reported to be associated with facial cleft due to impairment of the amniotic fluid intake mechanism[10,15,21]. Amniotic bands close to the fetus' head can also lead to facial cleft because of the restrictive force[11]. Hence, close attention is required when patients have such findings in 2DUS.
Several studies have reported that a combination of 2D and 3DUS was more accurate than 2DUS alone in prenatal examinations[19,22]. As a widely used examination, 3DUS is routinely performed by most prenatal screening centers. The surface rendering mode of 3DUS can provide more comprehensive information on fetal facial morphology, which helps compensate for the low sensitivity of 2DUS. In a prospective study of the diagnostic ability of 2D and 3DUS, the sensitivity and specificity for the detection of lip and palate anomalies reached 100% when using 2D + 3DUS, which showed significant improvements compared to those when only 2DUS was applied (80%)[19]. In the reported cases, five patients, who appeared normal under 2DUS, had facial clefts detected via subsequent 3DUS. Moreover, the visualization of fetal facial malformations, especially in cases of atypical facial cleft, is more specific and understandable for both practitioners and future parents.
However, although 3DUS may elevate the diagnostic accuracy, and the surface rendering of fetal appearance has become an essential tool for prenatal screening and surgical planning, it still has a major hardware limitation, which is the display of 3D reconstructions on a 2D interface[6]. Because the 2D picture lacks field depth, it represents a conceptual obstacle to complete morphologic understanding[23]. Recently, Nicot et al[6] and Schlund et al[24] successfully output the volume acquired from 3DUS and created 3D-printed models of the fetus to deliver more direct and interactive information to parents and doctors, who both developed a comprehensive understanding of the facial malformations of unborn children. In the current case, we detected a lateral facial cleft through 2DUS in the 22nd wk of gestation, and the suspicion was confirmed on subsequent 3DUS examination. Furthermore, we created a 3D-printed model of the fetus' face to facilitate the direct visualization of the lateral facial cleft.
3D-printing is a representative technology of additive manufacturing, which is one of the most important medical enabling technologies. Since 3D-printing allows us to fabricate all complicated device with least material, it was named as a “novel industrial revolution”[25]. Clinicians can transfer digital information of imaging examinations to computer-assisted design software, and further fabricate the model via a 3D-printier according to the designed STL documents. It enables individualized diagnosis and treatment by anatomical model fabrication, customized implantation, and tissue engineering[26,27]. The laboratory desktop 3D-printer and the material used in this study were not charged from the patient, and the cost including the charge of designing, material, and the printing was approximately 100USD; but if we send it to a professional workshop, the cost might be halved because of the share of machine cost was reduced by quantity production.
We found that the 3D-printed model was useful and contributed in at least three aspects. First, the 3D-printed model helped junior prenatal screening doctors to enhance their understanding and diagnostic capabilities of lateral facial clefts. For inexperienced junior doctors, it is difficult to confirm the diagnosis of atypical facial cleft due to its rarity and occultation. Under some circumstances, even with obvious abnormal findings in 2D/3DUS, we have to double-check the anomaly to ascertain the specificity of the diagnosis. The 3D-printed model is convincing diagnostic evidence that improves the doctors' understanding and diagnostic confidence of lateral facial clefts resulting in more definitive diagnoses. 3D-printed models can as well be applied in undergraduate student education and fill the gaps between theory and practice[28]. In the current case, we only used the model to explain the abnormality to some inexperienced doctors, which resulted in a favorable educational effect. They rapidly developed their understanding of lateral facial cleft by interacting with the model and comparing the model with the results of 2D and 3DUS. Second, the haptic 3D-printed model improved parental counseling. The model can help parents understand the anatomical abnormality of their child. Since parents are not familiar with ultrasonography and have resistance to accept the incidence of such abnormalities in their children, explanation of fetal malformations using a 2D image captured using 3D surface rendering may not be effective. With the haptic 3D-printed model, parents can obtain more specific knowledge by manipulating and observing the model from multiple angles, which leads to more direct and distinctive awareness of the deformity among parents[23]. Thus, the model greatly improved communication and helped mentally prepare the parents for their children[24]. Lastly, the 3D-printed model has the potential to guide surgeons in optimizing surgical planning. Although the parents selected to terminate the pregnancy, we noticed the model's potential to act as guidance for reconstructive surgery, similar to its role in orthopedics[29]. With the same technology, 3D-printed models of other spatial malformations, such as spinal and cardiac deformities, may be practical and helpful for future surgery planning.
The present case is the first reported application of 3D-printing technology to lateral facial clefts, but the study has several limitations. Differences were observed when comparing the pathology and 3D-printed model. Although the lateral facial cleft was revealed in US findings and the 3D-printed model, the skin tag was not detected until pathological examination. In our experience, the differences were mainly caused by the small size and concealed position of the lesion, and US results can often be affected greatly by fetal and utero conditions. The 3D-printed model was based on the data acquired by US, so it cannot remedy any misdiagnoses in US. As a result, since the lateral facial cleft may be combined with other facial deformities, comprehensive and elaborative US examination is essential when a cleft is spotted. There are some intrinsic limitations of case report, and more clinical investigation on 3D printing of prenatal diagnosis ought to be carried out to provide better evidence.
Lateral facial cleft is a rare facial malformation presenting as macrostomia with/ without other facial deformities. In prenatal screening, it can be diagnosed via 2DUS. Moreover, the supplementation of surface rendering with 3DUS has elevated the diagnostic accuracy. In this study, we created a 3D-printed model of the fetus, which enhanced diagnostic evidence, improved the understanding of junior doctors and parents, and has the potential to guide surgical planning.
| 1. | Khorasani H, Boljanovic S, Knudsen MAK, Jakobsen LP. Surgical management of the Tessier 7 cleft: A review and presentation of 5 cases. JPRAS Open. 2019;22:9-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 2. | Tessier P. Anatomical classification facial, cranio-facial and latero-facial clefts. J Maxillofac Surg. 1976;4:69-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 540] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Chang YL, Lien R, Chang SD, Chao AS. Prenatal 3D sonographic diagnosis of an isolated lateral facial cleft. J Clin Ultrasound. 2012;40:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kubo S, Horinouchi T, Kinoshita M, Yoshizato T, Kozuma Y, Shinagawa T, Ushijima K. Visual diagnosis in utero: Prenatal diagnosis of Treacher-Collins syndrome using a 3D/4D ultrasonography. Taiwan J Obstet Gynecol. 2019;58:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Witters I, Schreurs J, Van Wing J, Wouters W, Fryns JP. Prenatal diagnosis of facial clefting as part of the oculo-auriculo-vertebral spectrum. Prenat Diagn. 2001;21:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Nicot R, Couly G, Ferri J, Levaillant JM. Three-dimensional printed haptic model from a prenatal surface-rendered oropalatal sonographic view: a new tool in the surgical planning of cleft lip/palate. Int J Oral Maxillofac Surg. 2018;47:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 7. | Kuriyama M, Udagawa A, Yoshimoto S, Ichinose M, Suzuki H. Tessier number 7 cleft with oblique clefts of bilateral soft palates and rare symmetric structure of zygomatic arch. J Plast Reconstr Aesthet Surg. 2008;61:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Verheyden CN. Anatomical considerations in the repair of macrostomia. Ann Plast Surg. 1988;20:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Stein G, Stelzner A. [The clinical value of unspecific humoral immune parameters. Attempt at an immunogram. 1. Quantitative determination of IgG, IgM, IgA and IgD and demonstration of antibodies against cell and tissue bound antigens in patients with chronic pyelonephritis]. Z Gesamte Inn Med. 1979;34:117-123. [PubMed] |
| 10. | Presti F, Celentano C, Marcazzò L, Dolcetta G, Prefumo F. Ultrasound prenatal diagnosis of a lateral facial cleft (Tessier number 7). Ultrasound Obstet Gynecol. 2004;23:606-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Stelnicki EJ, Hoffman WY, Vanderwall K, Harrison MR, Foster R, Longaker MT. A new in utero model for lateral facial clefts. Craniofac Surg. 1997;8:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Lee M, Ko YB, Yang JB, Kang BH. Prenatal 3D sonography of an isolated lateral facial cleft. J Clin Ultrasound. 2018;46:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Pilu G, Visentin A, Ambrosini G, D'Antona D. Three-dimensional sonography of unilateral Tessier number 7 cleft in a mid-trimester fetus. Ultrasound Obstet Gynecol. 2005;26:98-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Troyano Luque JM, Padilla Pérez AI, Guerra Martín AL, Sánchez Peraza JJ, De La Rosa Rodríguez MA. A case of isolated Tessier 7 cleft in the newborn of a diabetic mother. J Obstet Gynaecol. 2011;31:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Cavaco-Gomes J, Duarte C, Pereira E, Matias A, Montenegro N, Merz E. Prenatal ultrasound diagnosis of Tessier number 7 cleft: Case report and review of the literature. J Obstet Gynaecol. 2017;37:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Makhija LK, Jha MK, Bhattacharya S, Rai A, Dey AB, Saha A. Transverse facial cleft: A series of 17 cases. Indian J Plast Surg. 2011;44:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Pereira DC, Bussamra LC, Araujo Júnior E, Drummond CL, Nardozza LM, Moron AF, Aldrighi JM. Prenatal diagnosis of treacher-collins syndrome using three-dimensional ultrasonography and differential diagnosis with other acrofacial dysostosis syndromes. Case Rep Obstet Gynecol. 2013;2013:203976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Woods RH, Varma S, David DJ. Tessier no. 7 cleft: a new subclassification and management protocol. Plast Reconstr Surg. 2008;122:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Mittermayer C, Blaicher W, Brugger PC, Bernaschek G, Lee A. Foetal facial clefts: prenatal evaluation of lip and primary palate by 2D and 3D ultrasound. Ultraschall Med. 2004;25:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez-Andrade E, Johnsen SL, Kalache K, Leung KY, Malinger G, Munoz H, Prefumo F, Toi A, Lee W; ISUOG Clinical Standards Committee. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2011;37:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 660] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 21. | Asai S, Tanaka M, Miyakoshi K, Kim SH, Minegishi K, Matsuzaki Y, Kosaki K, Ogata H, Yoshimura Y. A case of Tessier number 7 cleft with severe micrognathia: prenatal sonographic and three-dimensional helical computed tomographic images. Prenat Diagn. 2010;30:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Chmait R, Pretorius D, Jones M, Hull A, James G, Nelson T, Moore T. Prenatal evaluation of facial clefts with two-dimensional and adjunctive three-dimensional ultrasonography: a prospective trial. Am J Obstet Gynecol. 2002;187:946-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Nicot R, Druelle C, Hurteloup E, Levaillant JM. Prenatal craniofacial abnormalities: from ultrasonography to three-dimensional printed models. Ultrasound Obstet Gynecol. 2019;54:835-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Schlund M, Levaillant JM, Nicot R. Three-Dimensional Printing of Prenatal Ultrasonographic Diagnosis of Cleft Lip and Palate: Presenting the Needed "Know-How" and Discussing Its Use in Parental Education. Cleft Palate Craniofac J. 2020;57:1041-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Hossain N, Chowdhury MA, Shuvho MBA, Kashem MA, Kchaou M. 3D-Printed Objects for Multipurpose Applications. J Mater Eng Perform. 2021;1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Valverde I, Gomez-Ciriza G, Hussain T, Suarez-Mejias C, Velasco-Forte MN, Byrne N, Ordoñez A, Gonzalez-Calle A, Anderson D, Hazekamp MG, Roest AAW, Rivas-Gonzalez J, Uribe S, El-Rassi I, Simpson J, Miller O, Ruiz E, Zabala I, Mendez A, Manso B, Gallego P, Prada F, Cantinotti M, Ait-Ali L, Merino C, Parry A, Poirier N, Greil G, Razavi R, Gomez-Cia T, Hosseinpour AR. Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardiothorac Surg. 2017;52:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (1)] |
| 27. | Zamborsky R, Kilian M, Jacko P, Bernadic M, Hudak R. Perspectives of 3D printing technology in orthopaedic surgery. Bratisl Lek Listy. 2019;120:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Nicot R, Druelle C, Schlund M, Roland-Billecart T, Gwénaël R, Ferri J, Gosset D. Use of 3D printed models in student education of craniofacial traumas. Dent Traumatol. 2019;35:296-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Han Q, Zhang K, Zhang Y, Wang C, Yang K, Zou Y, Chen B, Wang J. Individual resection and reconstruction of pelvic tumor with three-dimensional printed customized hemi-pelvic prosthesis: A case report. Medicine (Baltimore). 2019;98:e16658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Usta I S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Li X