Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7146
Peer-review started: January 4, 2021
First decision: April 25, 2021
Revised: April 27, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: August 26, 2021
Processing time: 231 Days and 8.2 Hours
Neuroendocrine carcinoma of the breast (NECB) is a rare type of malignant tumor. Due to the rarity of NECB, the relevant literature mostly comprises case reports. Available data on treatment options for NECB are very limited.
A 62-year-old woman presented to our hospital in October 2016 for intermittent vomiting and diarrhea and masses in the liver found on abdominal computed tomography (CT) imaging. She was diagnosed in July 2012 with neuroendocrine carcinoma of the right breast in local hospital. The patient initially presented with a painful lesion of the right breast. She then undergone surgical resection and adjuvant chemotherapy with pirarubicin and paclitaxel for four cycles as well as endocrine therapy. She was regularly followed every 3 mo after surgery. Enhanced abdominal CT imaging at our hospital revealed multiple suspicious masses in the liver with the largest lesion measuring 8.4 cm × 6.3 cm. Chest CT revealed masses in the anterior chest wall and lung. Core needle biopsy of the lesion revealed liver metastases of NECB. A bone scan showed right second anterior rib metastases. Upper endoscopy and colonoscopy did not provide any evidence of another possible primary tumor. She stopped receiving endocrine therapy and then received etoposide and cisplatin (EP) chemotherapy as a first-line treatment regimen for six cycles at our hospital after liver, bone, and lung metastases. On October 2017, the chemotherapy regimen was changed to S-1 (40 mg twice daily, days 1-14) combined with temozolomide (200 mg once daily, days 10-14) (STEM) every 21 d as a second-line treatment regimen due to disease progression. Progression-free survival (PFS) and adverse effects after treatment were analyzed, and the efficacy of the STEM regimen was assessed using RECIST version 1.1. This patient achieved a partial response after using the STEM regimen, with a PFS of 23 mo. Adverse effects included only grade 1 digestive tract reactions with no need for a reduction in chemotherapy.
This case report suggests that the STEM regimen may be effective and well tolerated as the second-line treatment for advanced NECB. STEM is still highly effective in patients who show disease progression with the EP regimen. More evidence is needed to prove the validity of STEM.
Core Tip: Neuroendocrine carcinoma of the breast (NECB) is a highly malignant tumor. There is no standard treatment protocol for NECB due to its rarity. We treated an NECB patient with the S-1 combined with temozolomide (STEM) regimen as a second-line treatment due to disease progression. The effect of the STEM regimen on the patient was good, and she achieved a progression-free survival of 23 mo. During the chemotherapy period, the patient achieved a partial response and suffered only grade 1 adverse reactions. This report can serve as a reference for clinical practice.
- Citation: Wang X, Shi YF, Duan JH, Wang C, Tan HY. S-1 plus temozolomide as second-line treatment for neuroendocrine carcinoma of the breast: A case report. World J Clin Cases 2021; 9(24): 7146-7153
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7146.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7146
Neuroendocrine carcinoma (NEC) constitutes a group of rare neuroendocrine neoplasms (NENs) that can be distributed throughout the body, but they are commonly found in the gastroenteropancreatic and respiratory systems[1]. NEC of the breast (NECB) is very rare, accounting for approximately 0.1% of all breast cancers and 1% of neuroendocrine tumours (NETs)[2]. Because of the high malignancy of NEC, it is prone to metastasis. Currently, there is no standard treatment for patients with advanced NECB. Capecitabine combined with temozolomide (CAPTEM) is the regimen used for poorly differentiated NEC[3-6]. Since S-1 is also a 5-fluorouracil (5-FU) prodrug that can increase anticancer activity and reduce drug toxicity, we administered S-1 combined with temozolomide (STEM) as a second-line treatment regimen to an advanced NECB patient after the failure of the first-line treatment with etoposide and cisplatin (EP). This patient achieved a good objective response with acceptable toxicities.
A 62-year-old woman presented to our hospital in November 2016 complaining of intermittent nausea, vomiting, and diarrhea and multiple masses in the liver found on routine abdominal computed tomography (CT) imaging.
The patient underwent right-sided modified radical mastectomy including lymphadenectomy with nipple and areola preservation 4 years ago at a local hospital. No lymph node metastases were detected. Postoperative pathology revealed poorly-differentiated NEC of the right breast with a size of 1.5 cm × 1.5 cm × 1 cm. Immunohistochemical staining revealed expression of chromogranin A (CgA), synaptophysin (Syn), and hormone receptors [estrogen receptor (ER) and progesterone receptor (PR)]. Staining for human epidermal growth factor receptor 2 (HER-2) was negative. The Ki-67 index was 50%-75%. Curative resection was followed by four cycles of adjuvant chemotherapy with the pirarubicin and paclitaxel regimen. The patient had been receiving endocrine therapy after operation and regular follow-up every 3 mo.
The patient had a free previous medical history.
The patient dined any personal and family history.
The physical examination revealed no obvious abnormalities.
Laboratory examination revealed no obvious abnormalities.
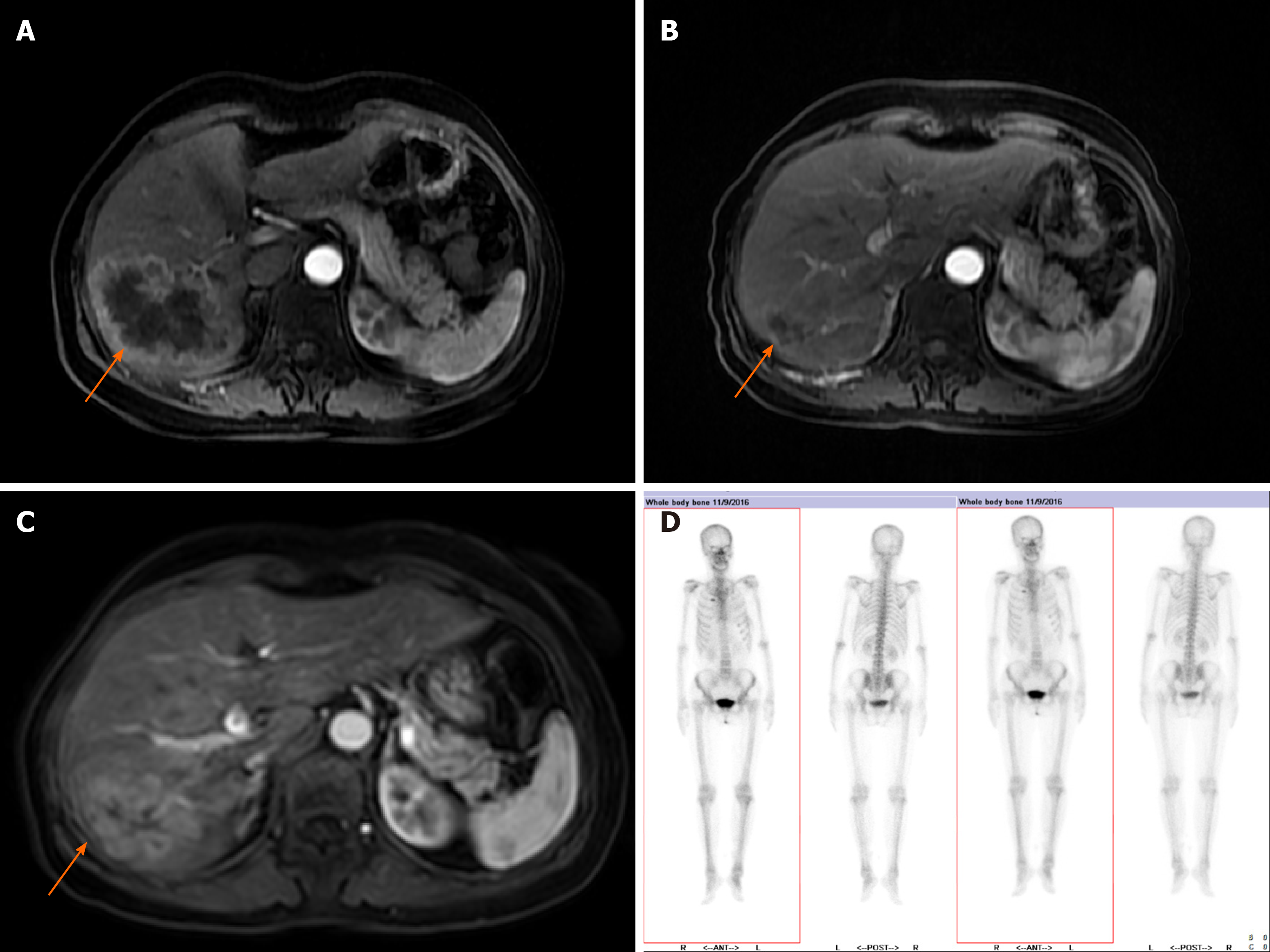
An initial imaging evaluation by enhanced abdominal CT revealed multiple masses in the liver, with the largest one measuring about 8.4 cm × 6.3 cm. Chest CT showed a mass on the right front chest wall and a small nodule in the upper lobe of the right lung.
The liver lesions were further evaluated by abdominal magnetic resonance imaging (MRI), which revealed multiple masses in the liver with the largest one measuring about 8.8 cm × 6.7 cm. A whole body bone scan revealed increased bone metabolism in the second anterior rib on the right, which was considered local bone invasion caused by chest wall masses combined with previous chest CT findings.
Further clinical work-up including upper endoscopy and colonoscopy did not reveal further pathological findings, not providing any evidence of another possible primary tumor.
The pathological consultation performed at our hospital of the primary breast lesion showed an NEC in the right breast with no metastases in the axillary lymph nodes. Immunohistochemical staining revealed expression of Syn, CgA, and hormone receptors (ER > 50%, slightly weaker expression of PR). Staining for HER-2 was negative. The Ki-67 index was approximately 50%.
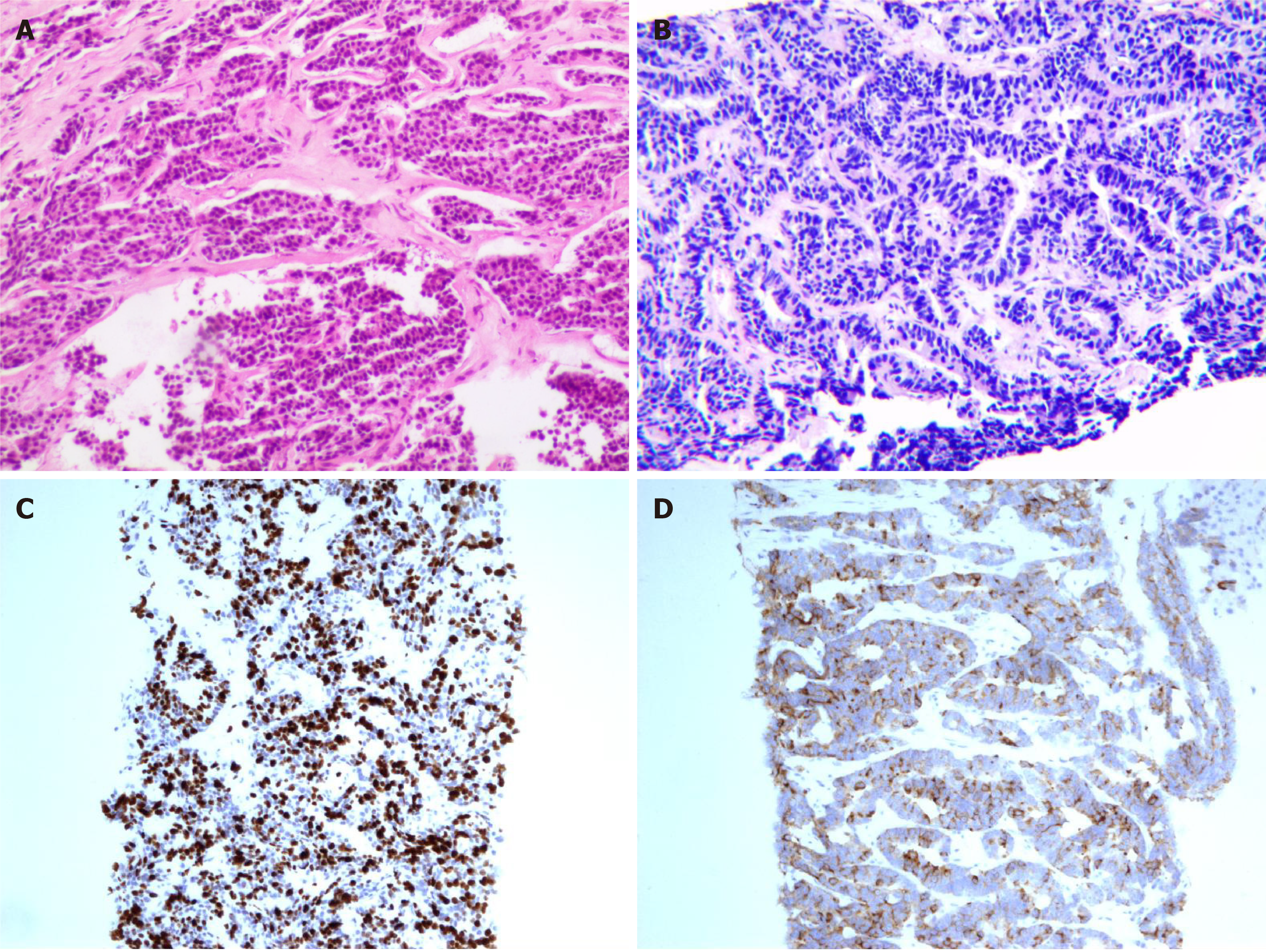
This patient underwent a liver and chest wall biopsy at our hospital. Liver and bone metastases of the NECB were detected. Immunohistochemical analysis of a biopsy taken from the lesion in the liver and chest wall showed an NEC with positive expression of CgA and Syn as well as strong expression for ER (> 95%). The expression of O6-methylguanine DNA methyltransferase (MGMT) and somatostatin receptor SSTR2 was negative. The Ki67 index was approximately 70% (Figure 1).
The final diagnosis of the presented case was stage IV NECB with liver, lung, and bone metastases.
Apparently, this patient presented with liver, lung, and bone metastases at 4 years after right modified radical mastectomy. Systemic chemotherapy was initiated using chemotherapeutic regimen based on etoposide (120 mg, days 1-3, intravenously) and cisplatin (40 mg, days 1-3, intravenously) every 21 d as a first-line treatment in November 2016. After administration of six cycles of chemotherapy in March 2017, the patient was referred to our hospital. CT imaging revealed a partial response.
At 6 mo after the cessation of EP chemotherapy, the disease progressed. Then, she began receiving S-1 (40 mg twice daily, days 1-14) combined with temozolomide (200 mg once daily, days 10-14) orally every 21 d beginning in October 2017. The last time that the patient received STEM chemotherapy was July 2019.
The patient underwent blood cell counts and creatinine and liver function tests at every cycle. Radiological assessment was performed every three cycles to evaluate the efficacy using RECIST version 1.1. The side effects were categorized according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Abdominal MRI analysis after one year of the STEM regimen showed a significant reduction in hepatic lesions until September 2019, when MRI analysis showed an increase in liver lesions, indicating disease progression (Figure 2). After receiving the STEM regimen, this patient achieved a partial response, with a progression-free survival (PFS) time of 23 mo. STEM treatment was well tolerated by the patient. Grade 1 digestive tract adverse reactions occurred, but a dose reduction was not needed (Table 1).
| April 2012 | July 2012 | 2012-2016 | October 2016 | October 2016 | November 2016-March 2017 | October 2017-July 2019 |
| Feel pain of the right breast | Right-sided modified radical mastectomy. Diagnosis of neuroendocrine carcinoma of the breast | Endocrine therapy + regular follow-up | Intermittent nausea, vomiting and diarrhea; multiple masses in the liver found on routine abdominal CT imaging | Diagnosis of neuroendocrine carcinoma of the breast stage IV with liver, lung, and bone metastases | Etoposide and cisplatin chemotherapyfor 6 cycles | S-1 combined with temozolomide chemotherapy |
| Local hospital | Local hospital | Our hospital | Our hospital | Our hospital |
The patient experienced twice hepatic artery embolization afterwards. She then orally received a small molecule inhibitor of multiple receptor tyrosine kinases, with inhibitory effects on tumor angiogenesis and growth. The therapy is still being continued and the patient is still alive.
NENs are a rare and heterogeneous group of tumors that can be divided into well-differentiated NETs and poorly-differentiated NEC. NEC is associated with a poor prognosis and rapid progression with a high Ki-67 proliferation index of > 20%. According to the 2012 World Health Organization, breast tumors with neuroendocrine features are divided into three categories: Well-differentiated NETs, poorly differentiated NEC, and invasive carcinoma with neuroendocrine differentiation. Interestingly, poorly differentiated NECs are morphologically identical to small-cell lung cancer (SCLC)[7]. NECB is rare in both breast cancer and extrapulmonary NEC. Based on the data collected from the Surveillance, Epidemiology And End Results (SEER) database, Wang et al[8] reported that from 2003 to 2009, there were only 142 cases of primary NECB among 381786 cases of invasive breast carcinoma.
NECB usually expresses neuroendocrine markers such as CgA, Syn, and CD56, tends to express hormone receptors such as ER and PR, and is usually negative for HER-2[9]. Imaging examinations such as mammography or MRI are necessary, but a definitive diagnosis depends on the pathology examination of the tissue after surgery or biopsy. In this case, immunohistochemical staining of the lesions in the breast, liver, and chest revealed expression of CgA, Syn, and hormone receptors. Staining for HER-2 was negative.
NECB is not different from other types of breast cancer in terms of its clinical characteristics. Most patients initially present with a hard breast lump. It is reported to be more common in older women[8]. In our case, the patient’s age was 62 years, which is consistent with the findings of previous reports. The most common distant metastatic sites are the liver and bone[10]. Long-term follow-up is necessary because NECB can metastasize to many sites, even after many years of treatment[11]. In this patient, liver and right-rib metastases occurred approximately 4 years after surgery.
There is no standard treatment protocol for NECB due to its rarity. Radical mastectomy and axillary clearance are the only curative methods for early NECB. However, NECB is highly malignant and prone to metastasis. Chemotherapy is needed for patients with a high risk of recurrence or advanced unresectable tumors. Chemotherapy for NECB generally conforms to the principles of chemotherapy for other types of breast cancer or SCLC[12,13], which include anthracyclines and taxanes or platinum-based regimen. Etoposide combined with cisplatin or carboplatin (EP/EC) is recommended as the first-line treatment option for patients with unresectable advanced NEC. However, NEC is heterogeneous, and NEC tumors at different sites respond differently to platinum-based chemotherapy[14]. A retrospective study[14] of 252 patients with advanced gastrointestinal NEC showed that patients who were treated with EP or EC as the first-line regimen had a response rate of approximately 30% in terms of achieving stable disease. The median PFS was 4 mo. We administered EP as the first-line treatment for our NECB patient, and she achieved a partial response with a PFS of 11 mo.
Second-line treatments for NEC have been reported, such as 5-FU combined with oxaliplatin or irinotecan (FOLFOX/FOLFIRI) and a temozolomide-based regimen[15-18]. However, there are no related reports on NECB. S-1 is a novel oral 5-FU prodrug comprising three components. Considering that S-1 is also a fluoropyrimidine antimetabolite agent that can increase anticancer activity and significantly reduce drug toxicity[19], we treated this patient with the STEM regimen as second-line treatment. The effect of the STEM regimen on the patient was good, and she achieved a PFS of 23 mo. During the chemotherapy period, the patient achieved a partial response and suffered only grade 1 adverse reactions. It is important to note that this patient, for whom the previous chemotherapy regimen had failed, still responded to the STEM regimen. This patient was amenable to the oral chemotherapy regimen due to its convenience and relatively few side effects.
Patients positive for somatostatin receptors can benefit from treatment with somatostatin analogues (SSAs) and peptide receptor radionuclide therapy[20,21]. In addition, novel targeted therapies may provide additional treatment options for NECB[22].
Tumor size and stage, hormone receptor status, and the Ki67 proliferation index are independent prognostic factors[8,23]. Oral treatment with the STEM regimen was highly effective in this case, which may be related to the relatively low Ki67 index and negative MGMT expression[22].
Our understanding of treatment efficacy for NECB is limited due to its rarity. As shown in this case, the STEM regimen is a promising alternative therapy that elicits few side effects and has a high curative effect. This report can serve as a reference for clinical practice. However, the efficacy of this regimen as a second-line solution for NECB requires further exploration.
| 1. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1203] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 2. | Ogawa H, Nishio A, Satake H, Naganawa S, Imai T, Sawaki M, Yamamoto E, Miyata T. Neuroendocrine tumor in the breast. Radiat Med. 2008;26:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Rogowski W, Wachuła E, Gorzelak A, Lebiedzińska A, Sulżyc-Bielicka V, Iżycka-Świeszewska E, Żołnierek J, Kos-Kudła B. Capecitabine and temozolomide combination for treatment of high-grade, well-differentiated neuroendocrine tumour and poorly-differentiated neuroendocrine carcinoma - retrospective analysis. Endokrynol Pol. 2019;70:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Chatzellis E, Angelousi A, Daskalakis K, Tsoli M, Alexandraki KI, Wachuła E, Meirovitz A, Maimon O, Grozinsky-Glasberg S, Gross D, Kos-Kudła B, Koumarianou A, Kaltsas G. Activity and Safety of Standard and Prolonged Capecitabine/Temozolomide Administration in Patients with Advanced Neuroendocrine Neoplasms. Neuroendocrinology. 2019;109:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Fine RL, Gulati AP, Krantz BA, Moss RA, Schreibman S, Tsushima DA, Mowatt KB, Dinnen RD, Mao Y, Stevens PD, Schrope B, Allendorf J, Lee JA, Sherman WH, Chabot JA. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Ramirez RA, Beyer DT, Chauhan A, Boudreaux JP, Wang YZ, Woltering EA. The Role of Capecitabine/Temozolomide in Metastatic Neuroendocrine Tumors. Oncologist. 2016;21:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Tan PH, Schnitt SJ, van de Vijver MJ, Ellis IO, Lakhani SR. Papillary and neuroendocrine breast lesions: the WHO stance. Histopathology. 2015;66:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Wang J, Wei B, Albarracin CT, Hu J, Abraham SC, Wu Y. Invasive neuroendocrine carcinoma of the breast: a population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer. 2014;14:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | López-Bonet E, Alonso-Ruano M, Barraza G, Vazquez-Martin A, Bernadó L, Menendez JA. Solid neuroendocrine breast carcinomas: incidence, clinico-pathological features and immunohistochemical profiling. Oncol Rep. 2008;20:1369-1374. [PubMed] |
| 10. | Wei B, Ding T, Xing Y, Wei W, Tian Z, Tang F, Abraham S, Nayeemuddin K, Hunt K, Wu Y. Invasive neuroendocrine carcinoma of the breast: a distinctive subtype of aggressive mammary carcinoma. Cancer. 2010;116:4463-4473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Valente I, Tringali G, Martella EM, Pallavera L, D'Aloia C. Primary neuroendocrine carcinoma of the breast: A case report of liver and lymph node metastases after eight years from diagnosis. Breast J. 2020;26:505-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, Caplin M, O'Toole D, Perren A; Vienna Consensus Conference participants. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 13. | Inno A, Bogina G, Turazza M, Bortesi L, Duranti S, Massocco A, Zamboni G, Carbognin G, Alongi F, Salgarello M, Gori S. Neuroendocrine Carcinoma of the Breast: Current Evidence and Future Perspectives. Oncologist. 2016;21:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 741] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 15. | Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117:4617-4622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Hentic O, Hammel P, Couvelard A, Rebours V, Zappa M, Palazzo M, Maire F, Goujon G, Gillet A, Lévy P, Ruszniewski P. FOLFIRI regimen: an effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer. 2012;19:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Hadoux J, Malka D, Planchard D, Scoazec JY, Caramella C, Guigay J, Boige V, Leboulleux S, Burtin P, Berdelou A, Loriot Y, Duvillard P, Chougnet CN, Déandréis D, Schlumberger M, Borget I, Ducreux M, Baudin E. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer. 2015;22:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Lamarca A, Frizziero M, Barriuso J, McNamara MG, Hubner RA, Valle JW. Urgent need for consensus: international survey of clinical practice exploring use of platinum-etoposide chemotherapy for advanced extra-pulmonary high grade neuroendocrine carcinoma (EP-G3-NEC). Clin Transl Oncol. 2019;21:950-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Wang D, Yu X, Wang X. High/positive expression of 5-fluorouracil metabolic enzymes predicts better response to S-1 in patients with gastric cancer: a meta-analysis. Int J Biol Markers. 2016;31:e101-e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Terlević R, Perić Balja M, Tomas D, Skenderi F, Krušlin B, Vranic S, Demirović A. Somatostatin receptor SSTR2A and SSTR5 expression in neuroendocrine breast cancer. Ann Diagn Pathol. 2019;38:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Savelli G, Zaniboni A, Bertagna F, Bosio G, Nisa L, Rodella C, Biasiotto G, Bettinsoli G, Migliorati E, Peli A, Falchi R, Giuffrida F, Giubbini R. Peptide Receptor Radionuclide Therapy (PRRT) in a Patient Affected by Metastatic Breast Cancer with Neuroendocrine Differentiation. Breast Care (Basel). 2012;7:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Vranic S, Palazzo J, Sanati S, Florento E, Contreras E, Xiu J, Swensen J, Gatalica Z. Potential Novel Therapy Targets in Neuroendocrine Carcinomas of the Breast. Clin Breast Cancer. 2019;19:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Cloyd JM, Yang RL, Allison KH, Norton JA, Hernandez-Boussard T, Wapnir IL. Impact of histological subtype on long-term outcomes of neuroendocrine carcinoma of the breast. Breast Cancer Res Treat. 2014;148:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mitra S S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Li JH