Published online Aug 26, 2021. doi: 10.12998/wjcc.v9.i24.7085
Peer-review started: April 29, 2021
First decision: May 23, 2021
Revised: May 30, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: August 26, 2021
Processing time: 116 Days and 11.5 Hours
Lymphangioleiomyomatosis (LAM) is a rare cystic lung disease characterized by the proliferation, metastasis, and infiltration of smooth muscle cells in the lung and other tissues, which can be associated with tuberous sclerosis complex (TSC). The disorder of TSC has a variable expression, and there is great phenotypic variability.
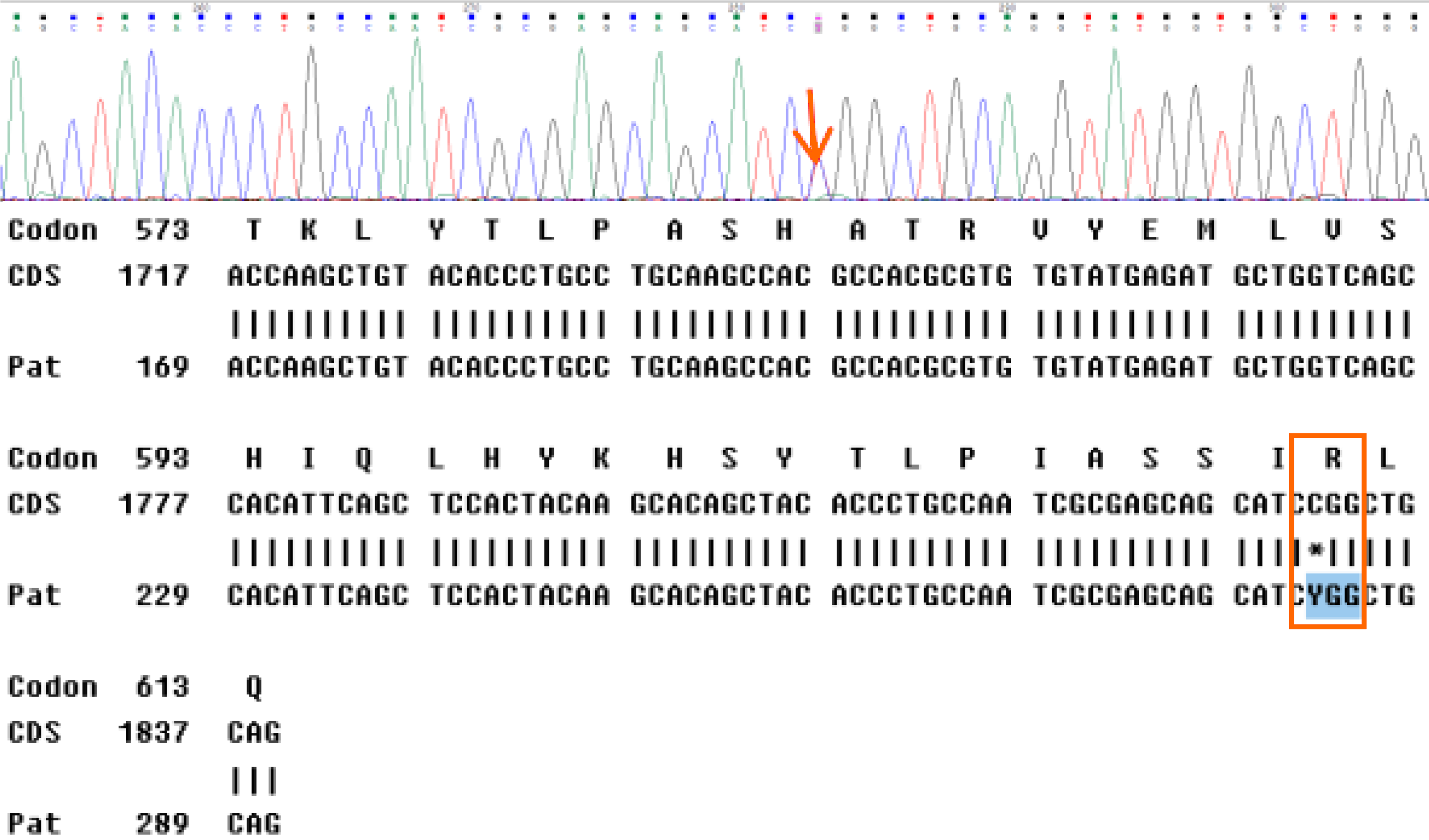
A 32-year-old Chinese woman with a history of multiple renal angioleiomyolipoma presented with a productive cough persisting for over 2 wk. High-resolution chest computed tomography revealed interstitial changes, multiple pulmonary bullae, bilateral pulmonary nodules, and multiple fat density areas of the inferior mediastinum. Conventional and contrast ultrasonography revealed multiple high echogenic masses of the liver, kidneys, retroperitoneum, and inferior mediastinum. These masses were diagnosed as angiomyolipomas. Pathology through thoracoscopic lung biopsy confirmed LAM. Furthermore, high-throughput genome sequencing of peripheral blood DNA confirmed the presence of a heterozygous mutation, c.1831C>T (p.Arg611Trp), of the TSC2 gene. The patient was diagnosed with TSC-LAM.
We highlight a rare case of TSC-LAM and the first report of a mediastinum lymphangioleiomyoma associated with TSC-LAM.
Core Tip: Lymphangioleiomyomatosis is a rare cystic lung disease that can be associated with tuberous sclerosis complex (TSC). The disorder of TSC has a variable expression and there is great phenotypic variability. A 32-year-old Chinese woman with a history of multiple renal angioleiomyolipoma since the age of 14 was diagnosed with TSC-lymphangioleiomyomatosis by high-resolution computed tomography, conventional and contrast ultrasonography, pathology through thoracoscopic lung biopsy, and high-throughput genome sequencing of peripheral blood DNA.
- Citation: Chen HB, Xu XH, Yu CG, Wan MT, Feng CL, Zhao ZY, Mei DE, Chen JL. Tuberous sclerosis complex-lymphangioleiomyomatosis involving several visceral organs: A case report. World J Clin Cases 2021; 9(24): 7085-7091
- URL: https://www.wjgnet.com/2307-8960/full/v9/i24/7085.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i24.7085
Lymphangioleiomyomatosis (LAM) is a rare, cystic lung disease. It belongs to a family of neoplasms with perivascular epithelioid differentiation that causes a multisystemic disorder characterized by the proliferation, metastasis, and infiltration of smooth muscle cells in the lungs and other tissues. The disorder is thought to be caused by the loss of the tuberous sclerosis complex-2 (TSC2) gene heterozygosity in the 16p13 chromosome[1]. LAM can occur either sporadically or in association with TSC disease (TSC-LAM) (13%-40% of cases)[1,2]. TSC-LAM is characterized by the presence of pulmonary cysts and other lesion sites, including the brain, heart, kidneys, and skin[1,3]. Symptoms and severity of TSC differ widely between individuals and there is great phenotypic variability because, despite the nearly complete penetrance, this disorder has a variable expression[3]. We hereby present a rare case of TSC-LAM with skin, kidney, liver, retroperitoneum, inferior mediastinum, and lung involvement.
A 32-year-old woman was admitted to hospital with a 2 wk history of a productive cough.
The patient produced white foamy sputum that began 2 wk ago.
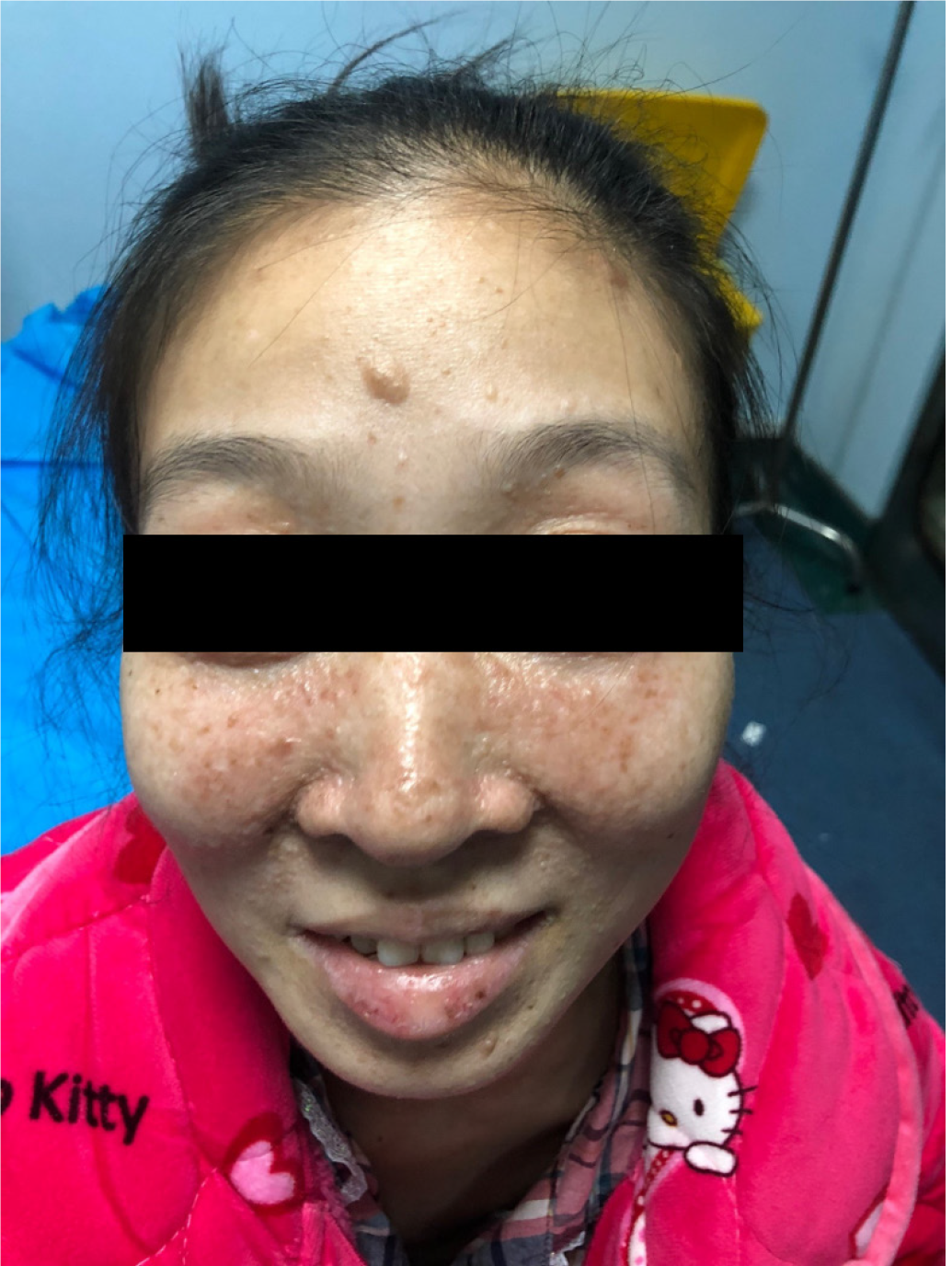
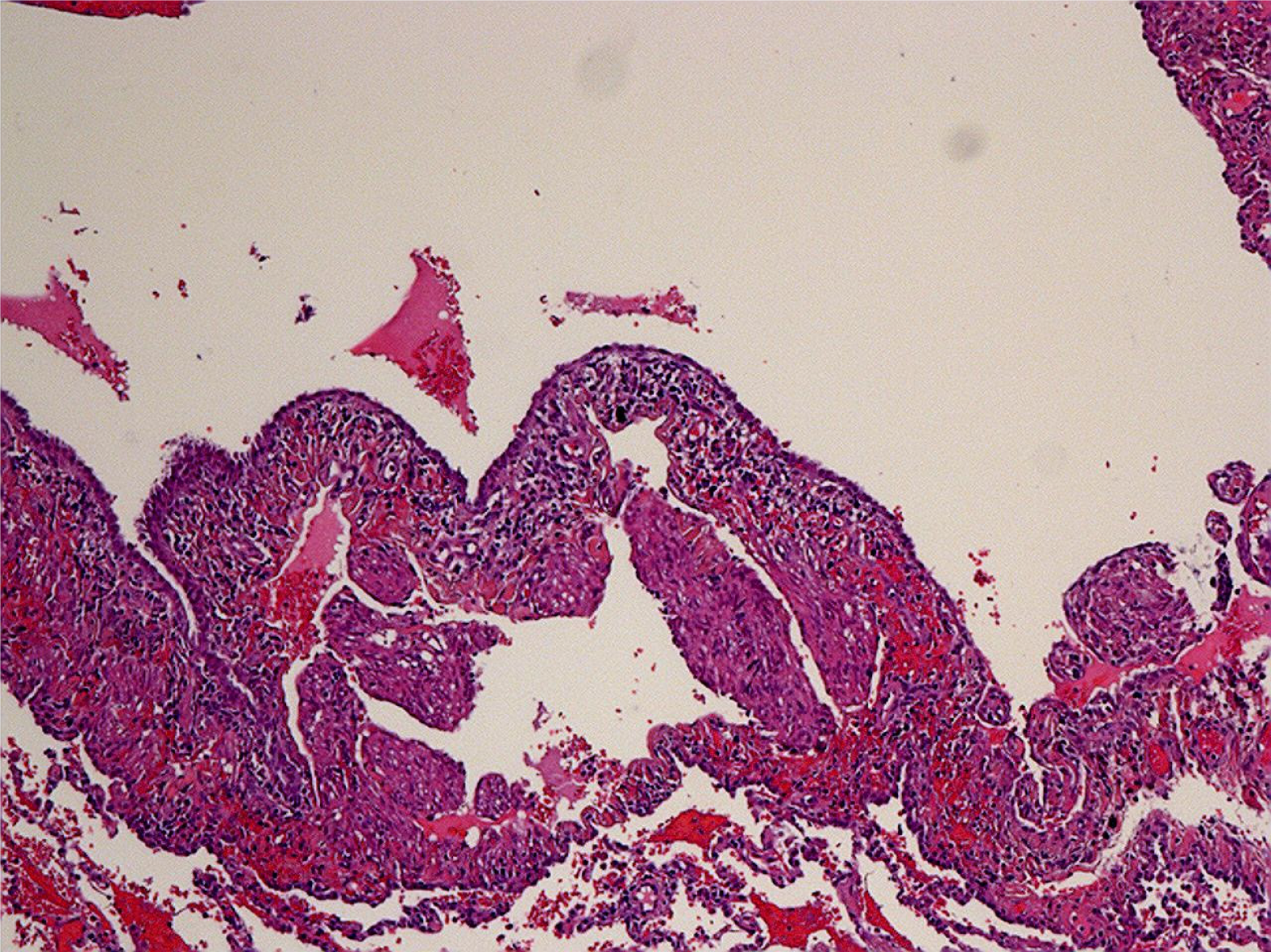
The patient was diagnosed with multiple renal angioleiomyolipomas in 2002 when she was 14 years old. In 2002 and 2008, she had multiple renal angioleiomyolipomas excised (Figure 1). After she was pregnant and had a cesarean section in 2013, cutaneous facial angiofibroma developed (Figure 2). In 2016, she was hospitalized for chylothorax. Bilateral pulmonary nodules were found, and thoracic surgery was performed (Table 1).
| Year | Summary from initial and follow-up visits | Diagnostic testing | Intervention |
| 2002 | Multiple renal angioleiomyolipomas | Ultrasonography | Angioleiomyolipoma resection |
| 2008 | Multiple renal angioleiomyolipoma | Ultrasonography | Angioleiomyolipoma resection |
| 2013 | Facial angiofibroma after pregnancy and cesarean section | None | None |
| 2016 | Chylothorax | HRCT: Bilateral pulmonary nodules | Thoracic operation |
| 2019 | TSC-LAM and pulmonary infection | HRCT; Thoracoscopic lung biopsy; High-throughput genome sequencing of peripheral blood DNA; Conventional and contrast-enhanced ultrasonography | Anti-infective symptomatic supportive treatment |
The patient had no family history of similar illnesses.
Tachycardia, hypertension, and low breath sounds throughout the bilateral chest field were observed. There were no other significant findings.
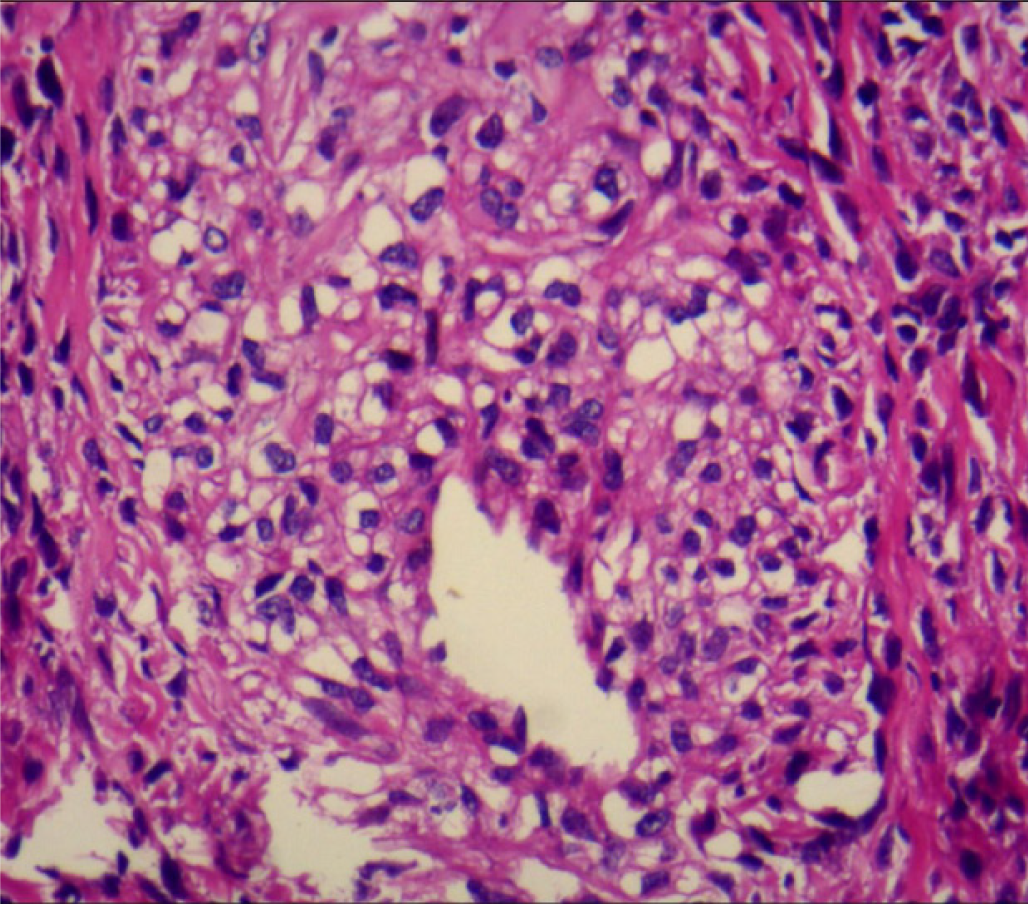
Thoracoscopic lung biopsy was performed. Pathology confirmed LAM, and immunohistochemistry showed human melanoma black-45 (+, sporadic), smooth muscle actin (+), Ki67 (+, 3%), and melan-A (+) (Figure 3). Furthermore, high-throughput genome sequencing of peripheral blood DNA confirmed the presence of the c.1831C>T (p.Arg611Trp) heterozygous mutation in the TSC2 gene (Figure 4).
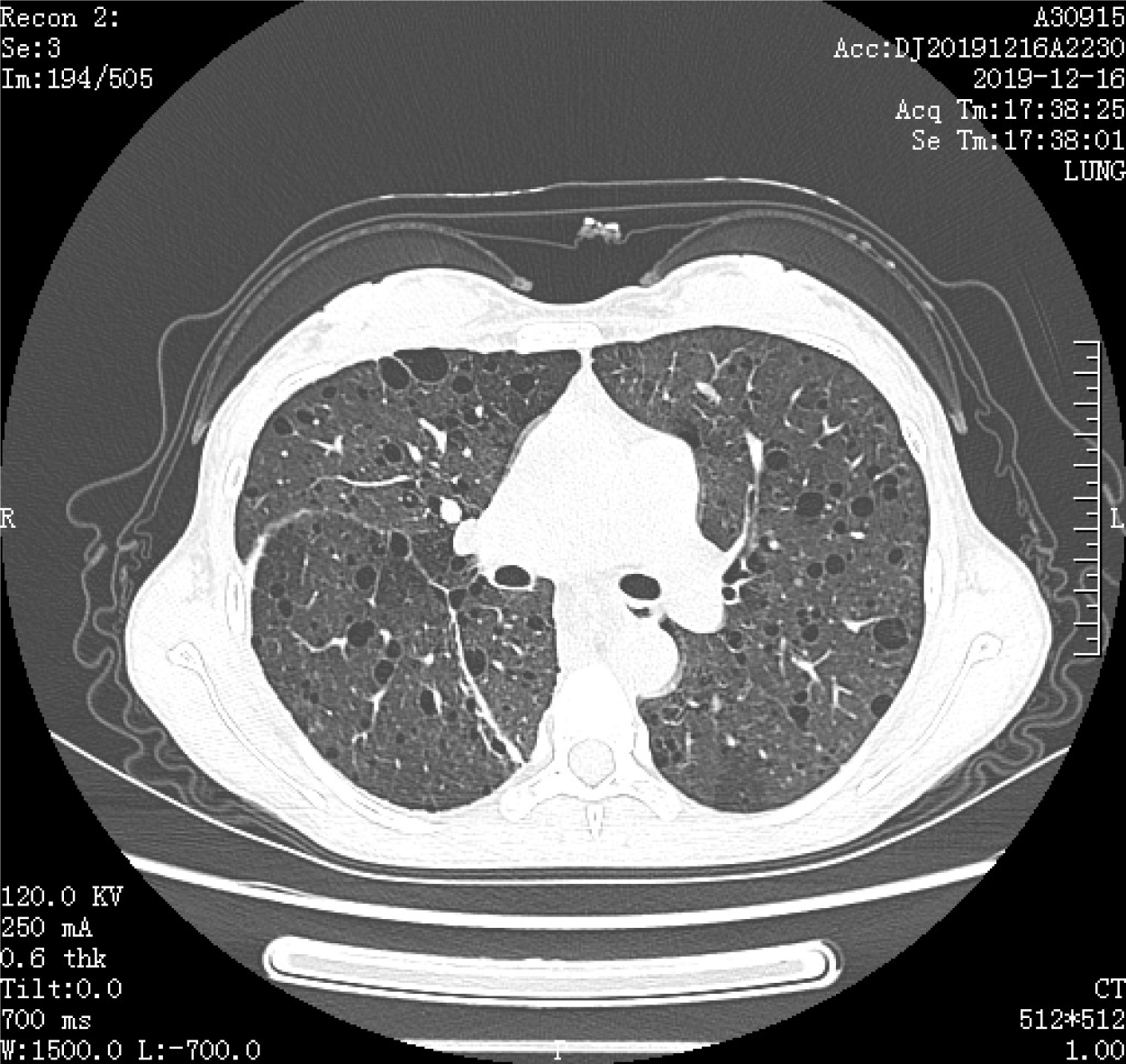
High-resolution chest computed tomography revealed interstitial changes, multiple pulmonary bullae, bilateral pulmonary nodules, and multiple fat density areas in the inferior mediastinum (Figure 5). Pulmonary function tests showed that the diffusing capacity of the lung for carbon monoxide decreased mildly (67%), forced expiratory volume in 1 s was reduced, and ratio of forced expiratory volume to forced vital capacity decreased, which contributed to severe obstructive ventilatory syndrome.
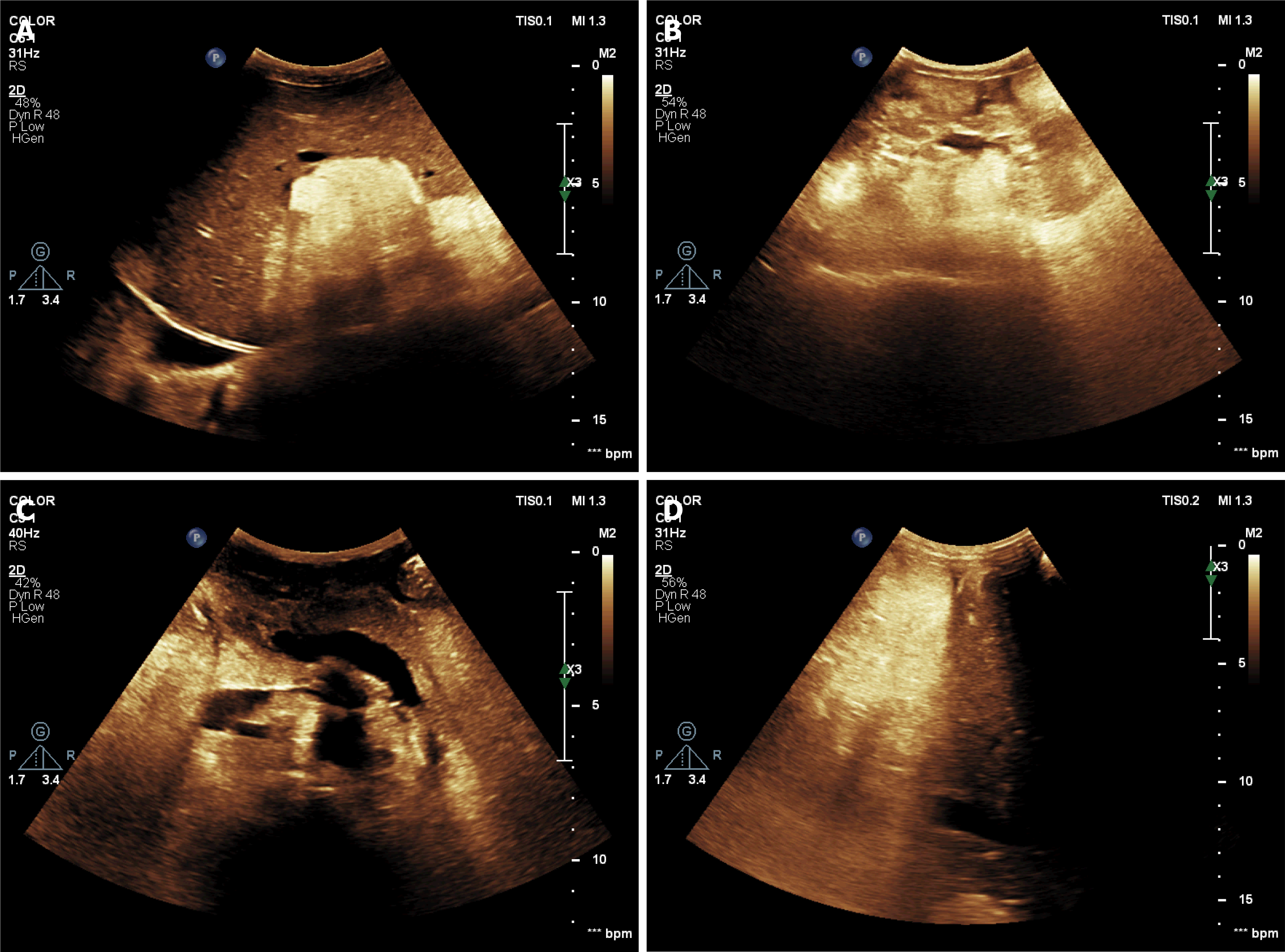
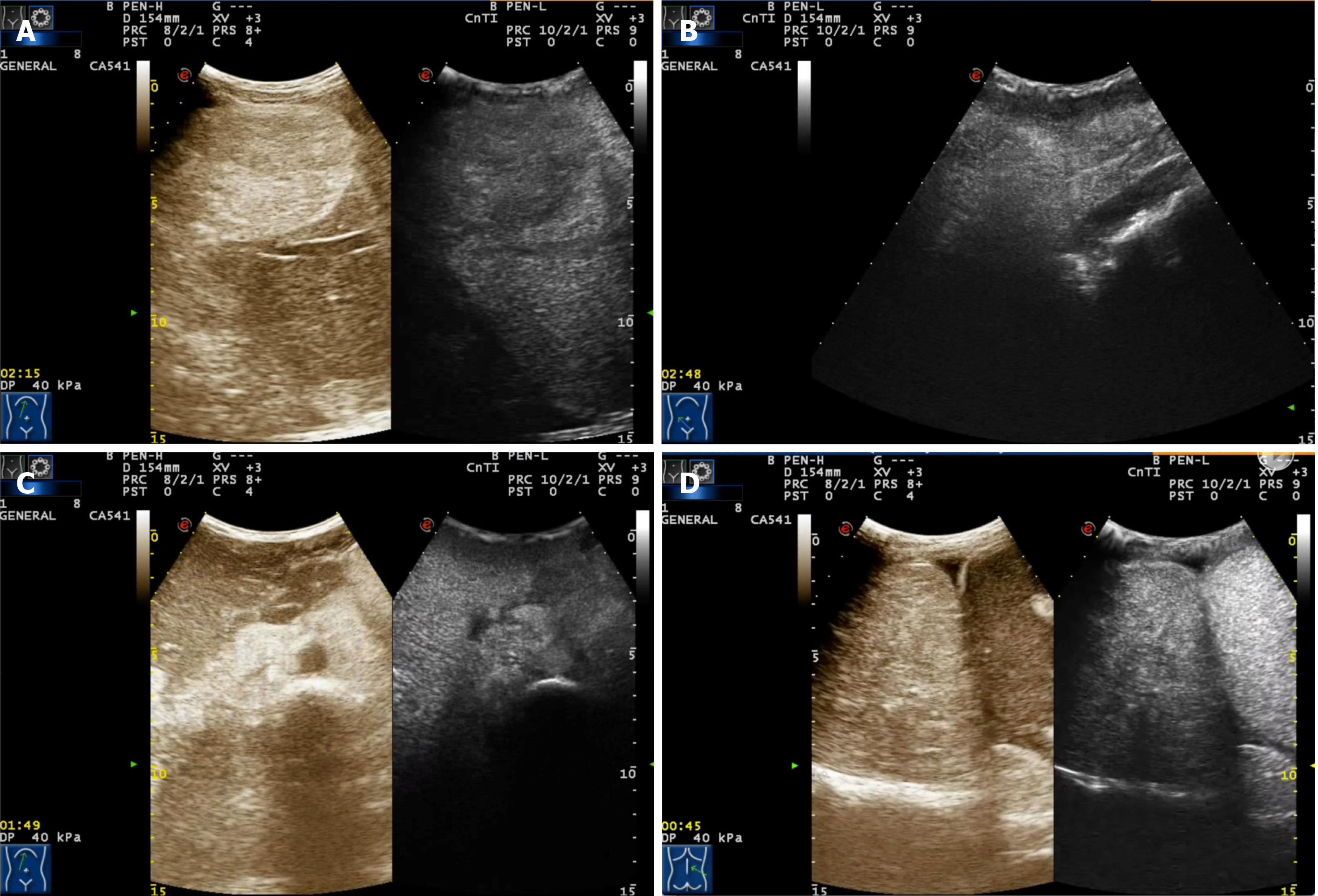
Conventional (Figure 6) and contrast ultrasonography (Figure 7) revealed multiple high echogenic masses in the liver, kidney (bilateral), retroperitoneum, and right thorax (inferior mediastinum). The masses were diagnosed as angiomyolipomas.
TSC-LAM.
The patient was referred for specific consultation and treatment with sirolimus after recovery of the pulmonary infection.
The patient’s symptoms decreased after drug treatment but did not resolve completely. We followed the patient regularly during sirolimus treatment, including ultrasound and lung function evaluation every 3 mo and computed tomography every 6 mo. At the 1-year follow-up, the effects of TSC-LAM on the skin, kidneys, liver, retroperitoneum, inferior mediastinum, and lungs were still observable by imaging analyses, and nothing had changed.
LAM is a rare interstitial lung disease[4]. It has an unknown etiology and is characterized by cystic destruction of the lung caused by infiltration of smooth muscle cells. It predominantly affects women in their reproductive years. The average age at diagnosis is 41 years old[2]. The patient in this case was 32 years old, which is much younger than the average age. Although LAM is predominantly a lung disorder, extrapulmonary involvement, such as renal angiomyolipomas and retroperitoneal masses, can occur in up to 75% of cases. A rare finding of abdominal aneurysm was reported before[5].
This patient was previously diagnosed with renal angiomyolipomas but no pulmonary involvement was documented until 2016. Angiomyolipomas or lymphangioleiomyomas of the inferior mediastinum, liver, and retroperitoneum were observed during the patient’s most recent hospitalization. To our knowledge, this is the first report of a patient with mediastinum lymphangioleiomyomas associated with TSC-LAM or sporadic LAM. The conventional and contrast ultrasonography imaging of the inferior mediastinum masses were similar to the renal angiomyolipoma imaging. The pathogenesis of mediastinum lymphangioleiomyomas in LAM may be similar to the mechanism of retroperitoneal lymphangioleiomyoma pathogenesis, but further research is needed to confirm this hypothesis.
The prognosis of patients with TSC-LAM is poor. Respiratory insufficiency and death often occurs within 5 years from onset of symptoms, while positive management of the disease and its complications would improve the long-term survival of these patients. The patient in this case accepted a complete evaluation first, and then received a specific consultation and treatment with sirolimus after recovery of her pulmonary infection.
The diagnosis of TSC-LAM in this patient was multisystemic. Affected organs included the skin, kidneys, liver, retroperitoneum, mediastinum, and lungs. This is the first reported case of a patient with a mediastinum lymphangioleiomyoma associated with TSC-LAM. High-resolution chest computed tomography and ultrasonography are critical to detect abnormalities, which may require specific treatment to prevent disease progression and poor outcomes.
| 1. | Harari S, Torre O, Moss J. Lymphangioleiomyomatosis: what do we know and what are we looking for? Eur Respir Rev. 2011;20:34-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, Reynaud-Gaubert M, Boehler A, Brauner M, Popper H, Bonetti F, Kingswood C; Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 365] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Caban C, Khan N, Hasbani DM, Crino PB. Genetics of tuberous sclerosis complex: implications for clinical practice. Appl Clin Genet. 2017;10:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | De Pauw RA, Boelaert JR, Haenebalcke CW, Matthys EG, Schurgers MS, De Vriese AS. Renal angiomyolipoma in association with pulmonary lymphangioleiomyomatosis. Am J Kidney Dis. 2003;41:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Chérrez-Ojeda I, Espinoza-Plaza J, Cottin V, Chérrez S. A Rare Case of an Abdominal Aneurysm in a Patient with Lymphangioleiomyomatosis: A Case Report. Perm J. 2019;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oley MH S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Li JH