Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.5830
Peer-review started: January 1, 2021
First decision: January 24, 2021
Revised: March 4, 2021
Accepted: June 17, 2021
Article in press: June 17, 2021
Published online: July 26, 2021
Processing time: 200 Days and 9.2 Hours
Anterior ischemic optic neuropathy (AION) is a group of ophthalmic diseases in which the optic nerve is injured causing blindness. However, the pathogenesis, clinical manifestations, and clinical treatments of AION are yet elusive. Only a few related experimental or clinical reports are available on the disease. In this study, spectral domain optical coherence tomography (SD-OCT) was used to examine the morphology of thickness swelling and atrophic changes of macular ganglion cell complex (mGCC) in the different stages of AION that were then compared with the visual fields. Thus, the clinical value of mGCC examination was alleged to be similar to that of the visual field.
To explore the mGCC injury at different stages in AION and the clinical signi
Cases with AION were analyzed in a retrospective study. SD-OCT was used to analyze the correlation between mGCC and peripapillary retinal nerve fiber layer thicknesses at different stages of AION and the changes in the corresponding stages of visual fields.
A total of 21 cases (28 eyes) presented AION. The onset time of AION was defined as early stage (within 3 wk of onset), middle stage (from 3 wk to 2 mo), and late stage (disease span > 2 mo). In the early stage, the mGCC thickness of SD-OCT was within the normal high limit, and the perioptic nerve fibers thickness was more than the normal. The changes in the visual field in early stage were not consistent with the swelling changes in mGCC and peri-disc nerve fibers. In addition, atrophy and thinning appeared in mGCC, and the perioptic nerve fibers were swollen. However, the thickness was lower in the middle period than that in the early stage. The change in visual field was consistent with that of mGCC in this period. In the late stage, mGCC shrank and thinned, and the thickness of the nerve fibers around the optic disc in the corresponding region shrank and thinned.
The changes in mGCC thickness in patients with AION showed early, middle, and late stages of development by SD-OCT. Although the early stage visual field changes of AION were not consistent with the swelling changes of mGCC, the horizontal delimitation or annular atrophy of mGCC was consistent with that in the middle and late stage of the disease. The atrophy of peripheral nerve fibers was later than that of the mGCC atrophy.
Core Tip: This study to explore the macular ganglion cell complex (mGCC) injury at different stages in anterior ischemic optic neuropathy and the clinical significance. The onset time of anterior ischemic optic neuropathy was defined as early stage, middle stage, and late stage. The early stage visual field changes of anterior ischemic optic neuropathy were not consistent with the swelling changes of mGCC. The horizontal delimitation or annular atrophy of mGCC was consistent with that in the middle and late stage of the disease. The atrophy of peripheral nerve fibers was later than that of the mGCC atrophy.
- Citation: Zhang W, Sun XQ, Peng XY. Macular ganglion cell complex injury in different stages of anterior ischemic optic neuropathy. World J Clin Cases 2021; 9(21): 5830-5839
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/5830.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.5830
Anterior ischemic optic neuropathy (AION) is a group of ophthalmic diseases in which the optic nerve is injured causing blindness. Clinical practice is not uncommon. AION can be divided into inflammatory and non-inflammatory diseases according to the causes. However, the pathogenesis, clinical manifestations, and clinical treatments of AION are yet elusive[1,2]. Based on the previous clinical cases, the present study aimed to analyze the changes in ganglion cells, perioptic nerve fibers, and the visual fields in different stages of AION in order to further understand the clinical pathogenesis and pathogenesis of AION. Presently, only a few related experimental or clinical reports are available on the disease[3-5], which indicates the changes in macular ganglion cell complex (mGCC) thickness as assessed by neuro-ophthalmology and mGCC atrophic injury caused by chiasma opticum, visual radiation, and visual cortical diseases[6-9]. In this study, spectral domain optical coherence tomography (SD-OCT) was used to examine the morphology of thickness swelling and atrophic changes of mGCC in the different stages of AION that were then compared with the visual fields[10]. Thus, the clinical value of mGCC examination was alleged to be similar to that of the visual field.
A total of 21 cases (28 eyes) of AION including 13 males and 8 females undergoing ambulatory treatment in our hospital from 2012 to 2015 were analyzed in this retrospective study. The age of the patients was 29-69 (average: 50) years. The cohort comprised of 7 binoculus cases and 14 monocular cases (right eye of 14 cases and left eye of 14 cases). Also, 7 bilateral cases of optic nerve atrophy, which occurred months or years ago, were identified at the time of treatment. Almost all cases had different degrees of optic papillary edema. Fundus fluorescein angiography (FFA) results showed that the optic disc had different degrees of fluorescein leakage and optic disc staining in the late stage. In addition to optic disc staining, no abnormalities were detected in the retinal fluorescence angiography. Except for a few cases with severe optic disc edema, staining of the serous retinal detachment was observed in the macular area. Moreover, none of the patients showed optic nerve injury caused by glaucoma, intracranial disease, trauma, heredity, and toxic damage. Also, the patients with AION in this study were not accompanied by inflammatory or non-inflammatory disease. The patients were tested for erythrocyte sedimentation rate, anti-O, G protein reaction, anti-cardiolipin antibody, chest fluoroscopy or radiography, and liver and kidney functions. A majority of the patients presented non-arteritic AION (NAION). However, whether AION has inflammatory or non-inflammatory differentiation was investigated to understand the etiology of the disease and the treatment and prognostic significance. Because the present case had damaged ganglion cells, it was not elucidated clearly. This research was conducted with Beijing Aier Intech Eye Hospital Infirmary Institutional Review Board approval and adhered to the tenets of the Declaration of Helsinki.
In this study, 2005 or 2010 Carl Zeiss Meditec visual field analyzer was used for detecting the AION cases. A few cases were treated with OCTOPUS visual field analyzer, and all underwent a 30° central visual field examination. Topcon frequency domain OCT-1000 MARK II was used to analyze the retinal thickness of the macular area, mGCC thickness, and perioptic nerve fiber thickness topographic map (peripapillary retinal nerve fiber layer, pRNFL) on the topographic maps and the probability analysis of the clinically significant lesion, respectively, was conducted. Among them, the mGCC thickness topographic map and the probability analysis of the clinically significant lesion were dominant, followed by the coordinated analysis of macular retinal thickness and pRNFL with the clinically significant lesion probability analysis. The examination time was at the beginning of onset, within 3 wk of onset, from 3 wk to 2 mo after onset, and after 2 mo of onset.
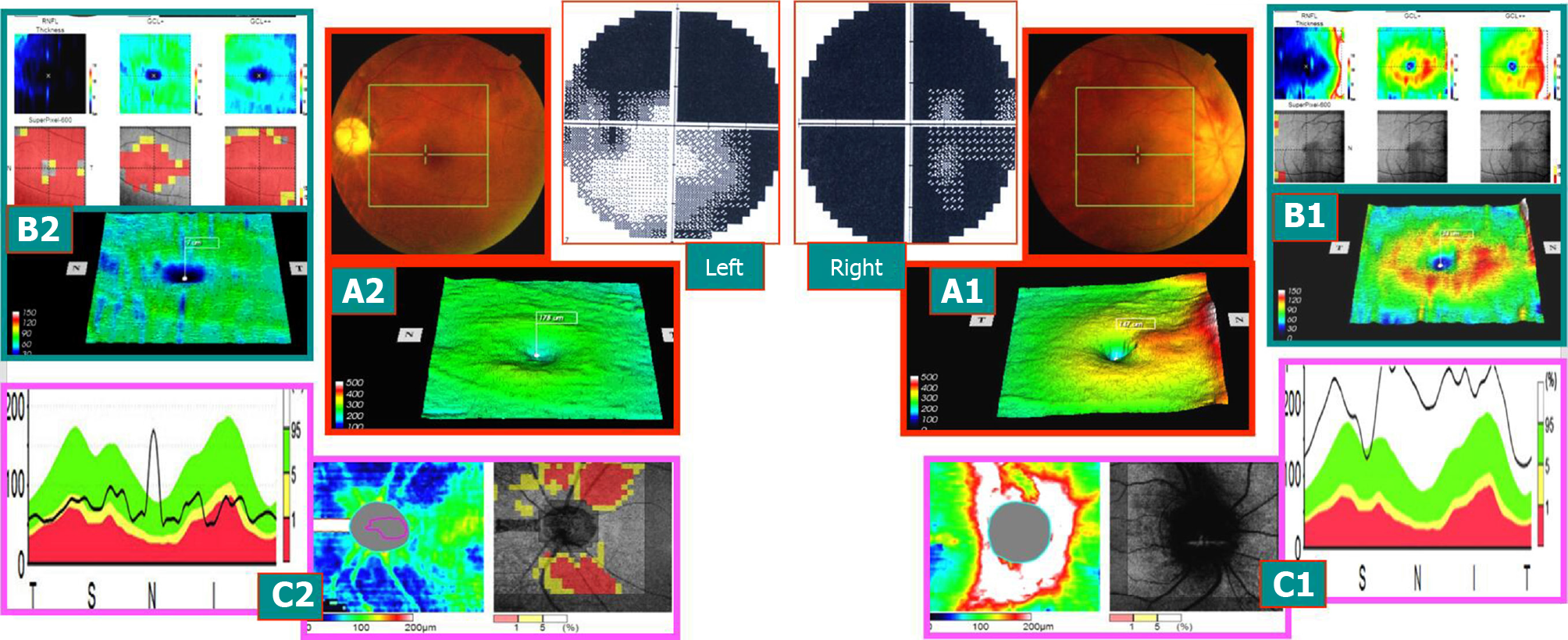
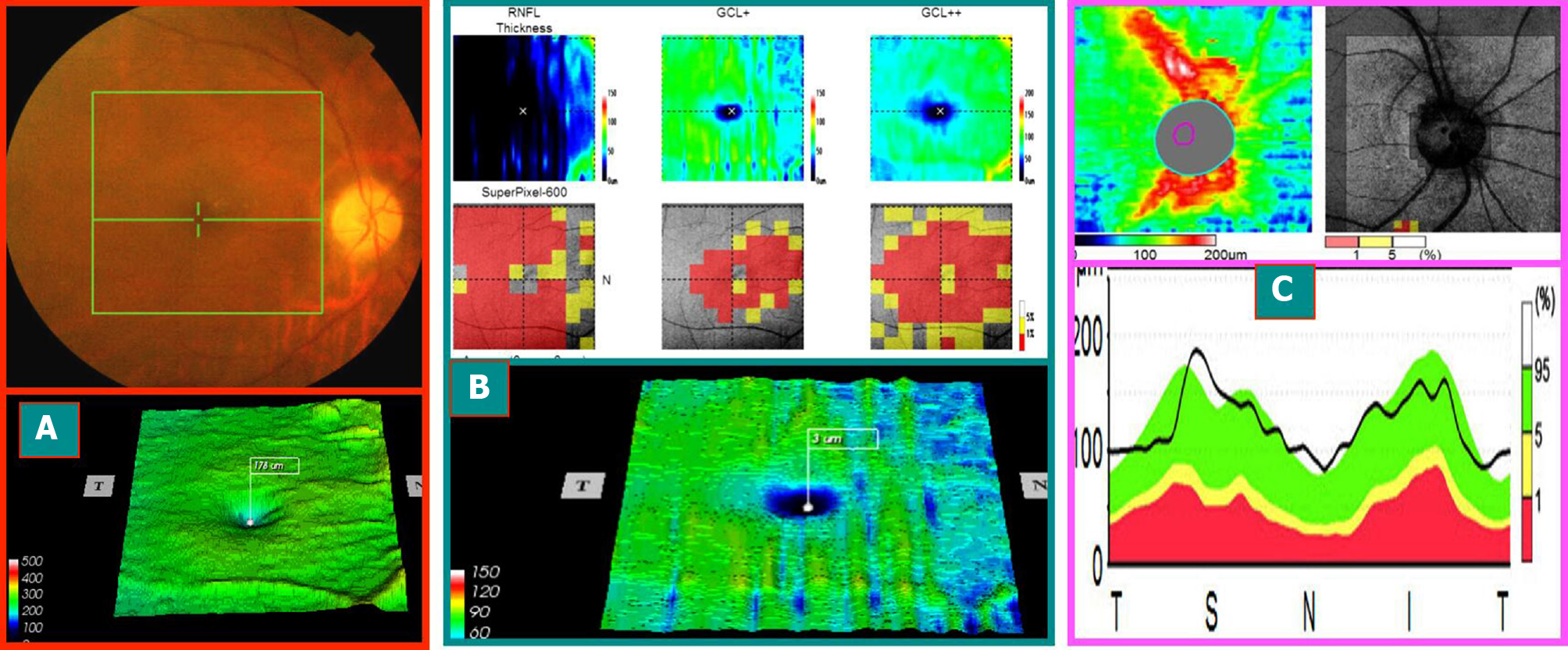
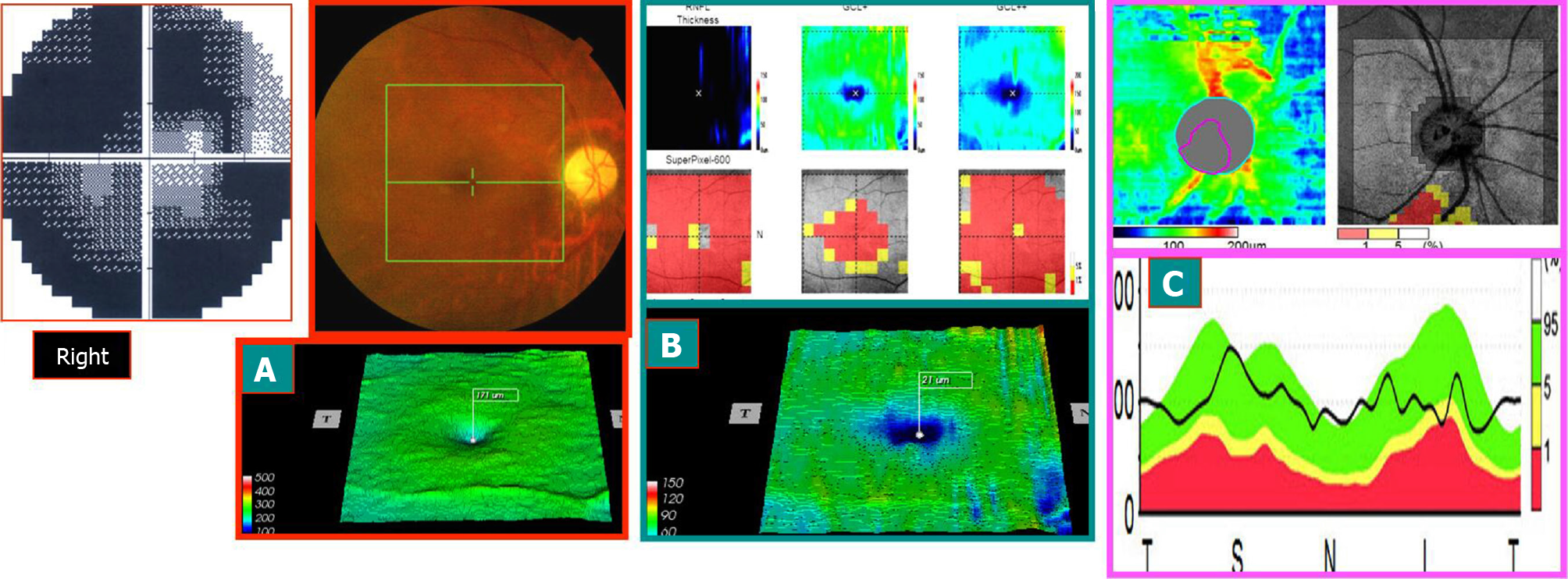
Case 1: A 58-year-old male patient, due to “binocular vision decline, right eye for 1 wk, and left eye for > 1 year,” visited the hospital. The patient had a history of hypertension, and oral drug was used to regulate the blood pressure. More than a year ago, he had left eye disease and was administered oral hormone, vitamins, and injected nerve growth factor after the treatment of stable visual acuity after discharge. The outpatient ophthalmological examination revealed that the best corrected visual acuity was 0.1 in the right eye and 0.5 in the left eye. Fundus: the right optic disc edema with hemorrhage of the optic disc, macular central light reflex disappeared; the left optic disc was light, the retinal artery was thin, and macular central light reflex disappeared. FFA: right optic disc edema and leakage and double late stage optic disc staining. Visual field and SD-OCT examination is described in Figures 1-3.
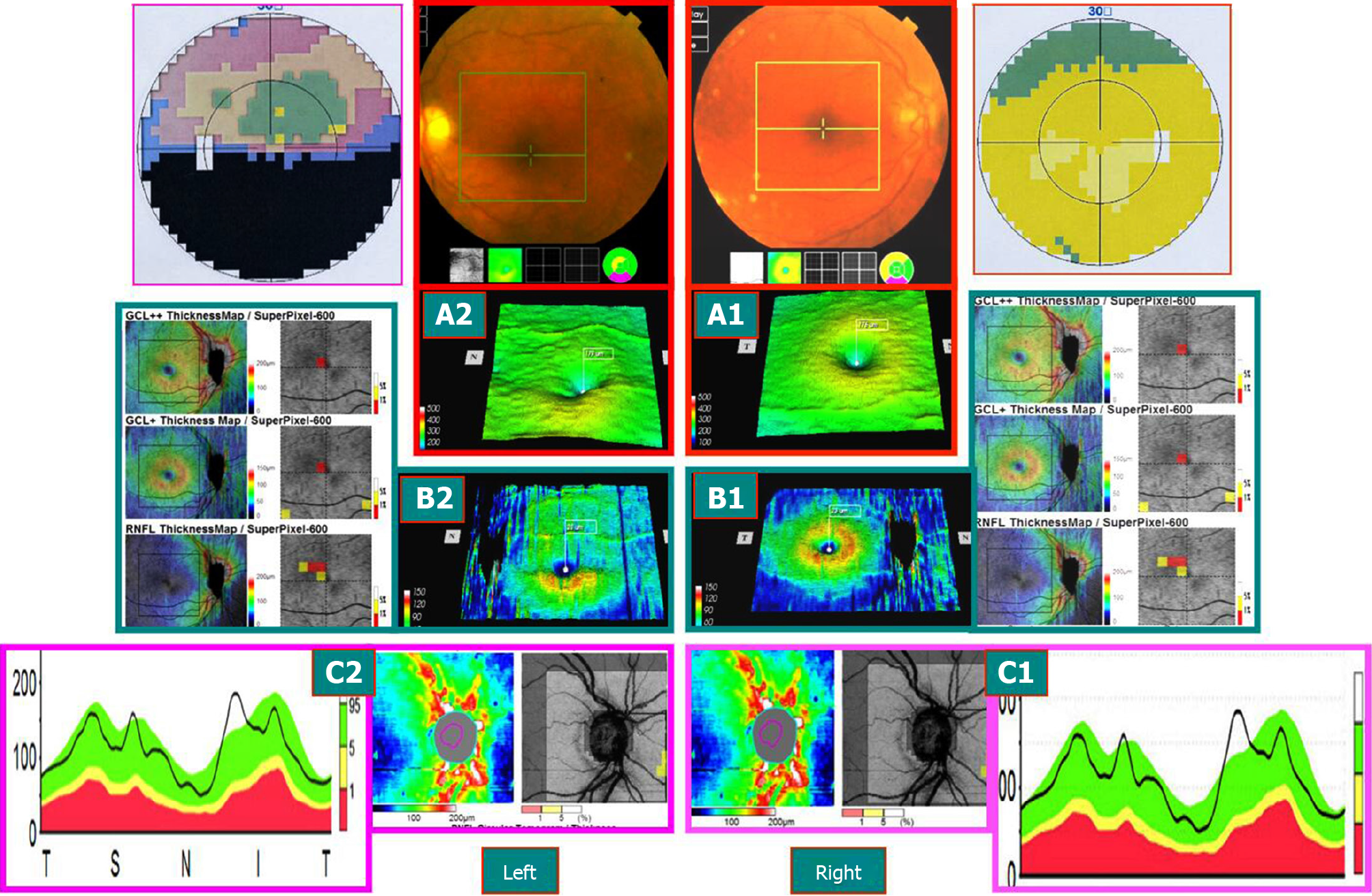
Case 2: A 58-year-old female patient visited the hospital because of the requirement of fundus examination. She had a history of Sjögren’s syndrome and was orally administered hormones and immunosuppressive agents for a prolonged period and occasionally hydroxychloroquine. Ophthalmological examination: Best corrected visual acuity: Binocular 1.0, normal binocular vision field; SD-OCT examination: Bilateral mGCC and peripheral optic disc nerve fiber thickness slightly increased. After 8 mo, the patient revisited the hospital because of “left eye sudden inferior visual field occlusion for 2 mo.” After the onset of the disease, the patient did not show any significant improvement in the symptoms after treatment for 2 mo. Best corrected visual acuity: right eye 1.0, left eye 0.2. Medical records and color fundus images obtained from the outside hospital showed edema of the optic disc of the left eye with a small amount of bleeding. The diagnosis of AION by FFA late optic disc leakage staining was conclusive. The SD-OCT examination is described in Figure 4.
The onset time of AION was defined as early stage (within 3 wk of onset), middle stage (course of disease of 3 wk to 2 mo), and late stage (course of disease > 2 mo). The visual field of 28 eyes from 21 patients with AION showed the level or quadrant defect associated with the optic disc, and SD-OCT showed different changes at various time points. The examination results of the visual field and SD-OCT in 28 eyes of 21 patients with AION were summarized as follows:
Early stage: The visual acuity of the affected eyes decreased markedly, the visual field disappeared sharply, and the FFA late optic disc was stained. The mGCC thickness of SD-OCT was within the normal high limit. The perioptic nerve fiber thickness exceeded the normal thickness. The early visual field changes were not consistent with the mGCC and swelling changes in the peridisc nerve fibers.
Medium stage: Visual acuity and visual field improved slightly. mGCC showed partial atrophy and thinning (exhibiting horizontal suture or a ring). Although the nerve fibers surrounding the optic disc were swollen, the thickness was lower than that in the early stage. The change in the visual field was consistent with the altered mGCC.
Late stage: The visual acuity and visual field changes were stable. mGCC shrank and thinned, and the thickness of the nerve fibers around the optic disc shrank in the corresponding area. The visual field changes were consistent with that of mGCC.
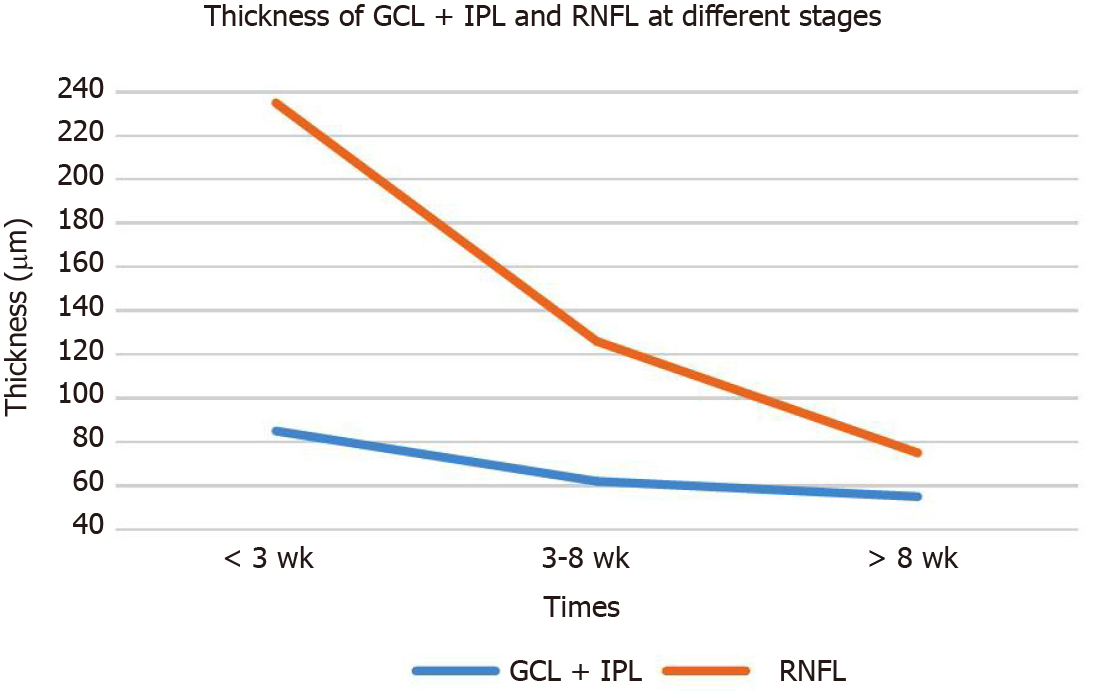
The contralateral non-diseased eye visual field was normal in 19/21 cases with AION in this study. However, SD-OCT revealed that the mGCC and pRNFL thicknesses were in the high or partially high limit of the normal range, and the FFA late optic disc was stained. Therefore, we proposed the concept of “subnormal eyes.” It is characterized by bilateral mGCC swelling (in normal high limit range or beyond high limit thickness) and pRNFL swelling (referring to high or beyond high thickness in normal range). Also, the visual acuity and visual field were found to be normal or stable [thickness of ganglion cell layer (GCL) + inner plexus layer (IPL) and RNFL at different stages, Table 1 and Figure 5].
| Thickness in μm | < 3 wk | 3-8 wk | > 8 wk | |
| Affected eyes | GCL + IPL | 85 ± 8 | 62 ± 10 | 55 ± 11 |
| RNFL | 235 ± 72, P = 0.001 | 126 ± 45, P = 0.03 | 75 ± 9, P = 0.095 | |
| Unaffected eyes | GCL + IPL | 89 ± 6 | 89 ± 6 | 88 ± 5 |
| RNFL | 99 ± 11 | 98 ± 11 | 100 ± 12 |
A total of 50% of the retinal ganglion cells (RGC) were concentrated in the macular area, especially in the annular area around the central fovea of the macula. The thickness of mGCC demonstrated that the thickness in the annular area was almost evenly distributed, showing a yellowish ring. However, the temporal thickness of the ring was slightly thicker than that of the nasal side. The ring appearing intensely red from reddish-pink-crimson indicated that the mGCC thickness was gradually increasing. The “subnormal eye” was a clinical phenomenon often observed by the authors during mGCC examination based on the four main features: bilateral mGCC swelling thickening or normal high limit range; bilateral optic disc nerve fiber swelling thickening or normal high limit range; bilateral FFA late optic disc staining; and visual field was normal or stable (clinical disease cured eyes). The subnormal eyes may represent the preclinical latent state of mGCC swelling-related ophthalmopathy (such as fundus disease, optic nerve disease, and glaucoma)[11-18].
The SD-OCT examination of patients with AION includes three parts of thickness images: macular retinal thickness map; mGCC layer thickness map (mGCC); and pRNFL image. AION are diseases of ganglion cell axons, and axon injury is the primary lesion. The swelling of the neuronal bodies is secondary to axonal lesions. In the case of severe axon injury, the axoplasmic flow is blocked. Over a period, the ganglion cell bodies degenerate leading to apoptosis, followed by atrophy and thinning, and finally the corresponding non-functional swelling axons shrink and thin consecutively. The three images are obtained simultaneously, and then the sequence of these causality injuries is analyzed and matched. In addition, high quality and high definition are required to reduce the error caused by the image.
The probabilistic analysis of the clinical significance of mGCC figure: RNFL (retinal nerve fiber thickness in macular area), GCL + (representing the thickness of the GCL and the IPL, GCL + IPL), GCL++ (representing the thickness of GCL and RNFL added together, which is the thickness of the three layers of the entire ganglion cell). Strikingly, GCL++ constitutes the addition of the three layers in an algebraic sum. Nonetheless, if GCL+ is slightly damaged (probability chart shows yellow as dominant) and RNFL shows swelling and thickening, then GCL++ is normal as swelling thickening and slight shrinking thinning counteract each other. Kupersmith et al[8] also reported RGC layer thinning within 1 mo of NAION. Acute optic nerve injury, such as NAION, where swelling obscures early demonstration of RNFL thinning, the GCL and the inner plexiform layer are severely unaffected, providing a reliable measure of retinal neuronal structure with three-dimensional segmentation. Thinning within 1 mo to 2 mo of onset is before the RNFL swelling subsides. Before 1 mo of NAION, the RGC body was lost and atrophy or the dendritic arborizations was reduced. After 1 mo, RNFL thickness measurements partially reflected the loss and thinning of affected axons. By 3 mo, most eyes showed thinning of RNFL, while the thinning of GCL + IPL essentially ceased. These data suggest that RNFL subclinical edema or swelling lasts for at least 3 mo. This suggests that GCL + IPL measurement is superior to RNFL thickness as a biomarker for early structural loss of NAION[8].
Scanning laser polarimetry (SLP) is based on the delay of irradiation of polarized light by intracellular organelles in parallel tissues in the axons of RGCs and has been used for RNFL measurements in other different optic neuropathy. The comparison of OCT and SLP suggests that the diagnostic validity of SLP may be superior to OCT in terms of RNFL measurement. SD-OCT and SLP with high sensitivity and specificity for RNFL retinal imaging may be an additional tool for the diagnosis optic neuropathy[19].
The SD-OCT examination is objective and non-injurious and can be widely used in the clinical examination. Also, it is more reliable than the visual field, which is also indispensable[20,21]. Because the change in the visual field occurs before the mGCC shrinks (Figure 1), both should be confirmed for accuracy and reliability. Figure 1 shows that in the early stage of the onset of the disease, mGCC was at the stage of swelling thickening or normal thickness high limit stage (before atrophy), and the visual field was changed. Thus, the visual field examination of this stage was critical and indispensable. However, during the middle and late stages of the disease, both visual field and mGCC were abnormal (mGCC appeared horizontal or circular, ring-shaped, or vertical line demarcation atrophy) with location-based diagnostic value. When mGCC showed bilateral atrophy of the midvertical line, it could objectively locate the lesion of optic chiasma. Interestingly, when mGCC displayed the atrophy of the midperpendicular line, the lesion could be located in the relevant area of the posterior optic pathway (optic bundle, visual radiation, and visual cortex). Furthermore, when the horizontal suture or similar circle occurred in mGCC, the atrophy of the annular central area indicated the location of the lesion in the anterior optic nerve segment of the optic disc-optic chiasma. The mGCC examination is a valuable method for clinical diagnosis and differential diagnosis of diseases, especially for patients who could not complete the visual field examination. The case in Figure 1 also showed that some of the ganglion cells in the swelling stage of mGCC have some functions, while some had no function, even if the vision and visual field were abnormal. Thus, if the cause of the disease was removed immediately, at least partial visual acuity and visual field might be restored.
The mGCC of AION showed horizontal atrophy. In addition, the visual field showed vascular and nerve tract defects or horizontal defect, which was associated with glaucoma with similarities in the changes in the visual field of the late normal intraocular pressure glaucoma and the patients with retinal artery occlusion at convalescent stage. Furthermore, the intraocular pressure level and intraocular pressure fluctuation necessitate further exploration to repeatedly measure the intraocular pressure curve for 24 h. In the recovery stage of retinal artery occlusion, atrophy of retinal inner layer must exist, that is, atrophy of retinal bipolar cell layer and GCL at the same time, while atrophy of GCL only exists in AION.
The altered mGCC thickness in patients with AION was examined by SD-OCT. It reflected the early mGCC swelling in the cases with AION (subnormal eye stage in the early stage of clinical onset), horizontal boundary atrophy thinning in the middle stage mGCC (metaphase segregation), and peripheral optic nerve fibers atrophied with the corresponding mGCC in the late stage (late clinical stable atrophy stage). The clinical three stages, except the early stage, coincided with the change in the visual field. Furthermore, visual field and mGCC examination exhibit specific and indispensable functions.
Presently the changes of macular ganglion cell complex (mGCC) thickness were assessed for neuro-ophthalmology and mGCC atrophic injury caused by chiasma opticum, visual radiation, and visual cortical diseases. This study aimed to explore the mGCC injury at different stages in anterior ischemic optic neuropathy (AION) and the clinical significance.
The pathogenesis, clinical manifestations, and clinical treatments of AION are yet elusive. The spectral domain optical coherence tomography examination is objective and non-injurious and can be widely used in the clinical examination of the AION.
Through study, the mGCC injury was different at the stages in AION. The most severe ganglion cell layer + inner plexus layer thinning occurred early when potential neuroprotective or protective therapy must be provided before 3 wk to reduce retinal ganglion cells loss.
Ganglion cell layer plus inner plexiform layer is acutely unaffected in the early inflammatory edema stage of the AION and provides a reliable method to measure the structure of retinal neurons using optical coherence tomography 3D segmentation.
The “subnormal eye” was put forward as a clinical phenomenon often observed by the authors during mGCC examination. The onset time of AION was defined as early stage (within 3 wk of onset), middle stage (course of disease of 3 wk to 2 mo), and late stage (course of disease > 2 mo).
The ganglion cell layer + inner plexus layer segmentation measurement of the spectral domain optical coherence tomography was superior to RNFL thickness as a biomarker for early structural loss in nerve ophthalmology.
The mGCC analysis can be widely used in the study of nerve ophthalmology.
Thank you to our scientific research team, Professor Gu W, Professor Liang J, Doctor An N, Doctor Zhang X, my family, Ms. Bi DG and my lovely daughter Anne.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hollo G S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, Crainiceanu CM, Durbin MK, Oakley JD, Meyer SA, Frohman EM, Calabresi PA. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 2. | Ho JK, Stanford MP, Shariati MA, Dalal R, Liao YJ. Optical coherence tomography study of experimental anterior ischemic optic neuropathy and histologic confirmation. Invest Ophthalmol Vis Sci. 2013;54:5981-5988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Cho JW, Sung KR, Lee S, Yun SC, Kang SY, Choi J, Na JH, Lee Y, Kook MS. Relationship between visual field sensitivity and macular ganglion cell complex thickness as measured by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:6401-6407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Kardon RH. Role of the macular optical coherence tomography scan in neuro-ophthalmology. J Neuroophthalmol. 2011;31:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Shon K, Sung KR. Assessment of macular ganglion cell loss patterns in neurologic lesions that mimic glaucoma. Korean J Ophthalmol. 2014;28:314-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Kupersmith MJ, Garvin MK, Wang JK, Durbin M, Kardon R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler. 2016;22:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Gonul S, Koktekir BE, Bakbak B, Gedik S. Comparison of the ganglion cell complex and retinal nerve fibre layer measurements using Fourier domain optical coherence tomography to detect ganglion cell loss in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2013;97:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Kupersmith MJ, Garvin MK, Wang JK, Durbin M, Kardon R. Retinal Ganglion Cell Layer Thinning Within One Month of Presentation for Non-Arteritic Anterior Ischemic Optic Neuropathy. Invest Ophthalmol Vis Sci. 2016;57:3588-3593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Koh VT, Tham YC, Cheung CY, Wong WL, Baskaran M, Saw SM, Wong TY, Aung T. Determinants of ganglion cell-inner plexiform layer thickness measured by high-definition optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:5853-5859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC, Calabresi PA, Maguire MG, Cutter GR, Balcer LJ. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 461] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 11. | Na JH, Kook MS, Lee Y, Baek S. Structure-function relationship of the macular visual field sensitivity and the ganglion cell complex thickness in glaucoma. Invest Ophthalmol Vis Sci. 2012;53:5044-5051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB, Wilson JA, Maguire MG, Galetta SL, Frohman E, Calabresi PA, Schuman JS, Balcer LJ. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 13. | Aggarwal D, Tan O, Huang D, Sadun AA. Patterns of ganglion cell complex and nerve fiber layer loss in nonarteritic ischemic optic neuropathy by Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:4539-4545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 674] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 15. | Raza AS, Cho J, de Moraes CG, Wang M, Zhang X, Kardon RH, Liebmann JM, Ritch R, Hood DC. Retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch Ophthalmol. 2011;129:1529-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Takagi ST, Kita Y, Yagi F, Tomita G. Macular retinal ganglion cell complex damage in the apparently normal visual field of glaucomatous eyes with hemifield defects. J Glaucoma. 2012;21:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Mwanza JC, Durbin MK, Budenz DL, Sayyad FE, Chang RT, Neelakantan A, Godfrey DG, Carter R, Crandall AS. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012;119:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 18. | Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591-1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 353] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 19. | Garas A, Simó M, Holló G. Nerve fiber layer and macular thinning measured with different imaging methods during the course of acute optic neuritis. Eur J Ophthalmol. 2011;21:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Rebolleda G, Sánchez-Sánchez C, González-López JJ, Contreras I, Muñoz-Negrete FJ. Papillomacular bundle and inner retinal thicknesses correlate with visual acuity in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2015;56:682-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Kupersmith MJ, Kardon R, Durbin M, Horne M, Shulman J. Scanning laser polarimetry reveals status of RNFL integrity in eyes with optic nerve head swelling by OCT. Invest Ophthalmol Vis Sci. 2012;53:1962-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |