Published online Jan 16, 2021. doi: 10.12998/wjcc.v9.i2.308
Peer-review started: July 6, 2020
First decision: September 12, 2020
Revised: September 28, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: January 16, 2021
Processing time: 185 Days and 19.5 Hours
Bile acids (BAs) are classically known to play a vital role in the metabolism of lipids and in absorption. It is now well established that BAs act as signaling molecules, activating different receptors (such as farnesoid X receptor, vitamin D receptor, Takeda G-protein-coupled receptor 5, sphingosine-1-phosphate, muscarinic receptors, and big potassium channels) and participating in the regulation of energy homeostasis and lipid and glucose metabolism. In addition, increased BAs can impair cardiovascular function in liver cirrhosis. Approximately 50% of patients with cirrhosis develop cirrhotic cardiomyopathy. Exposure to high concentrations of hydrophobic BAs has been shown to be related to adverse effects with respect to vascular tension, endothelial function, arrhythmias, coronary atherosclerotic heart disease, and heart failure. The BAs in the serum BA pool have relevant through their hydrophobicity, and the lipophilic BAs are more harmful to the heart. Interestingly, ursodeoxycholic acid is a hydrophilic BA, and it is used as a therapeutic drug to reverse and protect the harmful cardiac effects caused by hydrophobic elevated BAs. In order to elucidate the mechanism of BAs and cardiovascular function, abundant experiments have been conducted in vitro and in vivo. The aim of this review was to explore the mechanism of BAs in the cardiovascular system.
Core Tip: In the literature, there are some reviews on the relationship between bile acids (BAs) and the cardiovascular system. However, this is the first review to use molecular and cellular mechanisms of related pathways to explore the possible mechanism of BAs in the pathogenesis of cardiovascular disease and to classify the role of BAs in heart and other organs using a tabular form. The goal was to provide readers a more comprehensive, deeper, and clearer understanding of the function of BAs.
- Citation: Zhang R, Ma WQ, Fu MJ, Li J, Hu CH, Chen Y, Zhou MM, Gao ZJ, He YL. Overview of bile acid signaling in the cardiovascular system. World J Clin Cases 2021; 9(2): 308-320
- URL: https://www.wjgnet.com/2307-8960/full/v9/i2/308.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i2.308
Bile acids (BAs) comprise the primary catabolic pathway of cholesterol metabolism in the body, consisting of steroid cores and side-chains that can bind with either taurine or glycine groups[1]. BAs are also the primary lipid component of bile, because their side chains can combine with carboxylic acid or sulfonic acid group, granting these molecules water-soluble and lipid-soluble amphiphilic properties. BAs are divided into the free and combined types according to their structure. Cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA) constitute the main free BAs. The 24-carboxyl groups of the above free BAs combine with glycine or taurine to form combined BAs, which increase water solubility[2]. The hydrophobicity of BAs depends on the location and number of hydroxyl groups in the structure of the ring, which is related to cytotoxicity that can be reduced by hydroxylation of the BAs[3-5].
There are several mechanisms for the cytotoxicity of hydrophobic BAs. For example, BAs facilitate the production of reactive oxygen species (ROS) that oxidize and modify lipids, protein, and nucleic acids, eventually leading to apoptosis of the hepatocyte[6]. In addition, hydrophobic BAs can activate liver Kupffer cells to produce ROS, which may further insult liver cells[7]. In addition, mitochondria also play a role in the toxicity of BAs[8]. The order of BA hydrophobicity is LCA > DCA > CDCA > CA > UDCA[9] (Table 1). Hydrophilic BAs antagonize the cytotoxicity of hydrophobic BAs, and this antagonism correlates with their hydrophilicity[10]. BAs are also divided into primary and secondary BAs according to their sources. Primary BAs are directly synthesized from cholesterol in liver cells and are stored in the gallbladder. When stimulated by food digestion, primary BAs are secreted into the intestine. Secondary BAs are produced from primary BAs by intestinal bacteria, which are then reabsorbed by the brush border cells of the small intestine and transported back to the liver through the hepatic portal vein circulation. In normal physiological conditions, approximately 95% of BAs are reabsorbed.
| Hydrophobicity | Types of bile acids |
| Low | UDCA |
| ↓ | CA |
| CDCA | |
| DCA | |
| High | LCA |
BA flow occurs continuously between the intestines and the liver and is called “enterohepatic circulation”, which is a critical regulatory mechanism of the rate of BA metabolism, maintaining the balance of BAs and cholesterol in the body and preventing the formation of cholesterol stones[11].
The farnesoid X receptor (FXR) was the first identified receptor of BA signaling, discovered in 1999[12]. The G-protein-coupled receptor specific for BAs, the Takeda G-protein-coupled receptor 5 (TGR5), was subsequently identified[13]. Moreover, there are other types of receptors reportedly involved in regulating BA signaling, such as the muscarinic (M) receptors, sphingosine-1-phosphate (S1P), and large conductance voltage-and Ca2+-activated potassium (K+) [big potassium (BK)] channels. This section primary summarizes research on BAs as signaling molecules (Table 2)[14-28].
| Receptor | Tissue | Cardiovascular tissue | Ligand | Regulatory mechanism | Direct action | Ref. |
| FXR | Liver, kidney, gastrointestinal | Arteries, cardiomyocytes | CDCA, LCA, DCA | Regulates BSEP expression and participates in the process of liver injury, activates I-BABP expression to mediate cholesterol secretion, inhibits vascular inflammation, decreases lipogenesis synthesis, increases lipoprotein clearance to prevent atherosclerosis, ameliorates post-MI cardiac dysfunction and remodeling | No | [14-16] |
| PXR,VDR | Liver | t-Tubulescardiomyocytes | LCA | Down-regulates CYP7A1 expression to eliminate BA toxicity, maintains lipid metabolism, regulates intracellular calcium flow and contractile forces, maintains the normal operation of cardiomyocytes | No | [17-19] |
| M | Nervous, intestinal, gastrointestinal | Aorta, cardiomyocytes | TC, LCT, TCA | Stimulates acetylcholine-induced inositol phosphorylation and MAP kinase phosphorylation, inhibits cAMP, amplitude, reduces CM contraction | Yes | [20,21] |
| S1P | Liver, nervous, immune | Endothelial smooth muscle, cardiomyocytes | TCA, UDCA | Inhibits formation of cAMP and antagonizes adrenergic receptor-mediated contractile force, protects heart from ischemia/reperfusion injury, involved in remodeling and differentiation of cardiac fibroblasts | No | [22,23] |
| TGR5 | Liver, glands, fat, muscle, enteric, immune | Aortic endothelial | CA, DCA, CDCA, TLCA, TCDCA | Inhibits the secretion of TNF induced by LPS, downregulates GSK3 and upregulates PKB associated with cardiac hypertrophy, metabolic transformation of energy in cardiomyocytes | Yes | [24,25] |
| BK channel | Liver, brain | Endothelial, cardiomyocytes | LCA | Cause vasodilation in liver and cerebral artery, plays role in diabetes, through mitochondrial BK channels confer cardioprotection | Yes | [26-28] |
The FXR was identified as a BA receptor in 1999 and is highly expressed in the liver, kidney, and gastrointestinal tract[29]. Since then, many studies on BA receptors have been performed, and CDCA is the most potent endogenous ligand of FXR. CDCA binding to FXR causes a conformational change of FXR, facilitating formation with the retinoic acid X receptor in the cytoplasm. The latter enters the nucleus to recognize target gene promoter regions and regulates the transcription of target genes by FXR response elements[30]. For the LCA-induced cholestasis model, it was reported that CDCA activity down-regulated the bile salt export pump expression. This resulted in increased BA concentration and decreased liver bile secretion, which precipitated liver injury[31]. CDCA can also activate the expression of FXR in the intestinal tract, thereby activating intestinal acid-binding protein expression to mediate cholesterol secretion[32]. FXR is also expressed in the cardiovascular system, including in the coronary arteries, aorta, atherosclerotic arteries, and cardiomyocytes[33]. It was reported that suppression of the proliferator-activated receptor-γ co-activator 1α gene, which is a key regulator of fatty acid metabolism, caused development of cardiac dysfunction in FXR and SHP double-knockout mice (a model of cirrhosis)[34]. This suggested that BAs may reduce plasma triglyceride levels and prevent signs of atherosclerosis[35]. FXR ligands also inhibit the inflammatory response of rat aortic smooth muscle cells induced by interleukin-1β. The putative mechanism of this effect includes activating the ligand resistance of FXR binding nuclear factor-kappa B (NF-κB) to resist this pro-inflammatory pathway. This suggested that FXR agonists have anti-atherosclerosis potential[36,37]. Liu et al[38] and Yang et al[39] reported that increased tumor necrosis factor alpha (TNFα) and NF-κB and decreased cardiac function were observed in bile duct ligation (BDL) animal models, and anti-TNFα antibody therapy significantly improved this cardiac dysfunction. These studies suggested that there are relationships among bacterial translocation, increased activity of the endocannabinoid TNFα, NF-κB, and cardiac dysfunction. BAs (primarily DCA) have antibacterial properties because they can destroy the integrity of bacteria, which can affect the composition of the intestinal microbiota[40]. Pu et al[41] found in cultured cardiomyocytes that activation of FXR through mitochondrial death signal transduction causes significant apoptosis, which was verified in a myocardial ischemia/reperfusion injury mouse model, demons-trating that FXR signaling might be involved in the growth and apoptosis of cardiomyocytes. These studies suggest that BAs play diverse roles by activating distinct receptors in different tissues, including different BAs that activate FXR receptors in the heart tissue to exert anti-atherosclerosis or pro-atherosclerosis functions.
After FXR was identified as a nuclear BA receptor, two other receptors, the pregnane X receptor (PXR) and the vitamin D receptor (VDR), were found[42]. FXR, PXR, and VDR play essential roles in eliminating BA-induced toxicity by down-regulating cholesterol 7α hydroxylase (CYP7A1) expression[43], which is a rate-limiting enzyme for BA synthesis. In an animal study, activation of PXR was shown to regulate energy and lipid metabolism, thereby preventing obesity and insulin resistance caused by a high-fat diet. Thus, this study demonstrated the essential role of PXR in maintaining lipid metabolism[44]. VDR is expressed in almost every tissue in the human body and is classified as an endocrine nuclear receptor[45]. The VDR receptor can be activated by LCA, its natural ligands, and 1α, 25-dihydroxy-vitamin D3 [1α, 25 (OH)2-D3]. Two ligands were found to activate the VDR signaling pathway through extracellular signal-regulated kinase 1/2, leading to phosphorylation of VDR and translocation into the nucleus. VDR can inhibit CYP7A1 transcription, thereby protecting hepatocyte cells from further damage due to cholestatic liver injury[46]. It localizes the cardiomyocyte t-tubules. According to previous studies, the t-tube is the ideal location for regulating intracellular calcium flow and contractile forces. The inflow rate of calcium through calcium channels primarily determines the speed and pressure of myocardial contraction[47]. Loss of VDR selectivity in cardiomyocytes leads to enlargement, hypertrophy, and systolic and diastolic dysfunction of cardiomy-ocytes[48]. Vitamin D supplementation improves left ventricular structure and function in heart failure (HF) patients[49], illustrating that expression of VDR is vital for maintaining the normal function of cardiomyocytes. Nuclear receptors are transcription factors and cannot explain the immediate changes in myocardial function caused by BA stimulation. However, as the duration of action of BAs varies, their possible effects on the heart vary.
BAs also interact with membrane receptors to activate a cascade of intracellular effectors. BAs interact with three membrane G-protein-coupled receptors, the M receptors, TGR5, and S1P receptor. M receptors are widely expressed in the gastrointestinal tract, intestinal smooth muscle, and central nervous system and can be divided into M1, M2, M3, M4, and M5 receptors. Among these, there are two classes based on conjugation with different G proteins that stimulate phosphoinositide hydrolysis (M1, M3, and M5) or inhibit adenylate cyclase (M2 and M4)[50]. The ligand for M3 receptors is the BA lithocholyltaurine[51], which can stimulate acetylcholine-induced inositol phosphorylation and mitogen-activated kinase phosphorylation[52]. Taurocholate binds to the M2 receptor, inhibiting cyclic AMP (cAMP), affecting transient calcium amplitude, and reducing myocardial cell contraction[53]. The role of the remaining muscarinic receptors in the heart is unclear due to the lack of identified corresponding ligands.
S1P is the most effective substrate for sphingolipids and is produced by phospho-rylation of sphingolipids catalyzed by sphingolipid kinase. SIP determines cell fate through pro-apoptotic or survival signals. There are five subtypes of S1P receptor, which are S1P1, S1P2, S1P3, S1P4, and S1P5. Highly expressed S1P1 and S1P2 are detected in hepatocytes and activate extracellular signal-regulated kinase 1/2 and protein kinase B (PKB). S1P1, S1P2, and S1P3 receptors are primarily located in the heart, whereas S1P4 and S1P5 are limited to the nervous and immune systems[54]. Taurocholic acid induces S1P2 receptor expression and promotes cholangiocarcinoma growth[55]. In cardiomyocytes, the S1P1 receptor is the foremost expressed subtype, and its activation inhibits the formation of cAMP and antagonizes adrenergic receptor-mediated contractile force. Low levels of the S1P3 receptor mediate the bradycardia effect of S1P agonists. Studies have shown that S1P2 and S1P3 receptors play essential roles in heart protection from in vivo-mediated ischemia/reperfusion injury in mice using knockout mice. S1P receptors are also involved in proliferation, remodeling, and cardiac fibroblasts’ differentiation. Furthermore, S1P receptors are found in smooth muscle cells and endothelial cells, which could mediate peripheral vascular tension and responses of the endothelium. Despite these findings, the role of the regulatory system in the cardiovascular system remains unclear[56].
TGR5 expression is detected in different cell types, such as fat cells, endocrine glands, muscle, immune cells, and the enteric nervous system. TGR5 has been reported to inhibit the response of rabbit alveolar macrophages to BAs (DCA, CDCA, and LCA), subsequently inhibiting the secretion of TNFα induced by lipopolysaccharide (LPS)[57]. TGR5 also protects the liver by inhibiting expression of cytokines induced by LPS in Kupffer cells[58]. LPS-induced inhibition of mitophagy increases oxidative stress and promotes inflammation in hepatic stellate cells during the process of acute liver failure[59]. In recent years, TGR5 mRNA has been identified in human, rabbit, cow, and mouse heart tissues[60]. The effects of mouse cardiac-specific TGR5 activated by taurodeoxycholic acid include LCA down-regulation of glycogen synthase kinase-3 and up-regulation of PKB, which are known to be associated with cardiac hypertrophy[61]. TGR5 is also expressed in aortic endothelial cells that play an anti-atherosclerotic role through producing nitric oxide (NO) in a dose-dependent manner, inhibiting NF-κB activity, and regulating monocyte adhesion and the inflammatory response[62]. BA activation of TGR5 is also involved in the metabolic transformation of energy in cardiomyocytes. However, the mechanism of TGR5’s action on cardiomyocytes remains to be clarified.
Studies have shown that in addition to known BA receptors (FXR, LXR, VDR, PXR, TGR5, M, and S1P), BAs can also activate nonclassical receptor reactions, such as large-conductance voltage-and Ca2+-activated potassium (K+) (BK) channels[63]. It has been speculated that systemic vasodilation in hepatobiliary disease partly causes vascular smooth muscle cells’ relaxation through the activation of BKCa. LCA induced a 30% increase in cerebral artery vasodilation in an endothelium-independent manner. This effect was eliminated in a BK β-1 subunit knockout mouse model, demonstrating that the role of this potassium channel subunit in diabetes is essential[64]. In another study, BAs were shown to activate the BK pathway in cirrhosis patients and to increase the risk of developing cirrhotic cardiomyopathy. Meanwhile, taurine conjugated hydrophobic BAs activate BK channels, which can expand outward potassium currents, reduce the duration of action potentials, and exert negative inotropic effects[65]. Since this receptor primarily mediates ion changes, it is speculated to play a primary role in the cardiac conduction related functions.
Studies have shown that BAs can regulate vascular tension. Increased BAs in the liver portal vein of rats with BDL were observed to decrease norepinephrine-induced vasoconstriction. These findings show that BAs are vasodilators. According to previous studies, the primary driver of cardiovascular disease is endothelial dysfunction, which leads to an imbalance in the synthesis and release of harmful and protective mediators, among which NO is the most important[66,67]. FXR is identified in vascular smooth muscle cells and endothelium, and, as a transcription factor, it can regulate vascular relaxation and contraction by altering the term of vasoactive molecules or other receptors. Studies have shown that activated FXR induces vasodilation in endothelial cells by down-regulating endothelin-1 (IL-1), up-regulating endothelial NO synthase (eNOS), modulating angiotensin II receptor expression, and inhibiting inflammation and migration in vascular smooth muscle cells[68]. It was found that CDCA activation leads to decreased IL-1 mRNA expression in a concentration-dependent manner. As is known, IL-1 is the most effective vasoconstrictor, and its BA-inhibited expression may be an essential factor in systemic vasodilation in cirrhotic patients[69]. The same team proposed the presence of FXR response elements in the promoter region of eNOS. Their activation led to up-regulation of eNOS and subsequent increases in the production of vasodilated NO[70]. S1P receptor 2 (S1PR2) is another BA-sensitive receptor found in vascular smooth muscle cells that is involved in NO signaling. Nevertheless, it works through inhibiting the synthase of inducible NO, thus reducing a part of NO levels in vascular injury[71-73].
In contrast, long-term stimulation of FXR weakens NO-dependent vasodilation due to increased cGMP passivation in smooth muscle cells. These observations suggest that short-term and long-term FXR stimulation has differential effects on NO production and sensitivity[74]. Thus, time should be taken into account in the study of BA receptor-related effects. Pak et al[75] found that BA increases can cause vasodilation, and speculated that this might occur by inhibiting calcium from passing through membrane channels. This effect has no relationship with blockers or endothelial stripping. However, it is strongly affected by the type of BAs, and hydrophobic and lipophilic BAs are more likely to induce vasodilation. The authors speculate on the mechanism by which BAs achieve this effect and conclude that they must directly interact with cell membrane components, emphasizing the role of BA components rather than merely the concept that increasing concentration is essential in the cardiovascular function.
Recent clinical studies have found that fasting total bile acid (TBA) serum levels are closely related to the severity of coronary heart disease (CHD), serving as an indicator of the seriousness of the severity CHD[76]. According to research, the fasting levels of BA concentration inhibit atherosclerosis[77]. Animal models are resistant to developing atherosclerosis because they can excrete excess cholesterol by secreting large amounts of BAs into the intestines[78]. We hypothesize that patients with coronary atherosc-lerotic disease might have impaired BA secretion and excretion, resulting in high serum cholesterol levels that promote progression of atherosclerotic lesions. Clinical studies have shown that fecal BA content in CHD patients is indeed significantly lower than that of non-CHD control groups[79]. BAs and their synthetic derivatives exert anti-atherogenic effects by activating the FXR receptor in some animal models. Oral administration of CDCA derivatives to apolipoprotein E-deficient mice reduced aortic plaque formation by 95% and reduced aortic expression of inflammatory factors, including IL-6, IL-1, etc.[80]. In mammalian models, oral administration of BAs or their synthetic derivatives reduce serum triglycerides and total cholesterol levels, and inhibit the formation of atherosclerosis in a dose-dependent manner. These findings suggest that oral administration of BAs or their synthetic derivatives may represent a method for treating atherosclerotic lesions[81].
Interestingly, Fxr-/- mice showed pro-atherogenic lipid characteristics even when fed a high-fat/high-cholesterol diet but did not show enhanced atherosclerosis. Studies have demonstrated that CDCA or FXR ligand activated by FXR can reduce the activity of cardiomyocytes by triggering apoptosis of cardiomyocytes, promoting myocardial ischemia/reperfusion injury. These conflicting observations suggest that further in vivo studies are needed to determine the effect of the BA-FXR interaction on atheros-clerosis.
Through previous studies, we know that the influence of BAs on cardiac function can be divided into indirect and direct effects. Direct effects require interaction between BAs and cardiomyocytes to affect the conduction and contraction of the myocardium. These effects may be receptor-dependent or independent. The cardiotoxicity of BAs was observed when high doses of BAs intravenously injected into animal specimens caused severe bradycardia. Further studies confirmed the dose-dependent negative time-varying effect of BAs on cardiomyocytes[82]. Binash et al[65] reported that in vitro sodium taurocholate slightly increased the outward potassium current, reduced the calcium current, and slowed the inward sodium current, ultimately reducing the duration of the action potential. Voltage clamp experiments in mice demonstrated that BAs decrease slow inward Na+ and Ca2+ currents and increase outward K+ currents. Of note, BAs can alter the function of heart muscle cells as pacemakers. In a partial in vivo study, investigators found the plasma nonursodeoxycholic BA ratio was significantly increased in the atrial fibrillation group. Data analysis showed that the serum ursodeoxycholic BA concentration and nonursodeoxycholic BA ratio were independent predictors of atrial fibrillation[83]. BAs can affect the exchange of sodium and calcium on the myocardial cell membrane as polar amphiphilic molecules, leading to depolarization of the resting potential and inducing posterior depolarization of cells. Subsequent depolarization and triggering are one of the initiating mechanisms of HF.
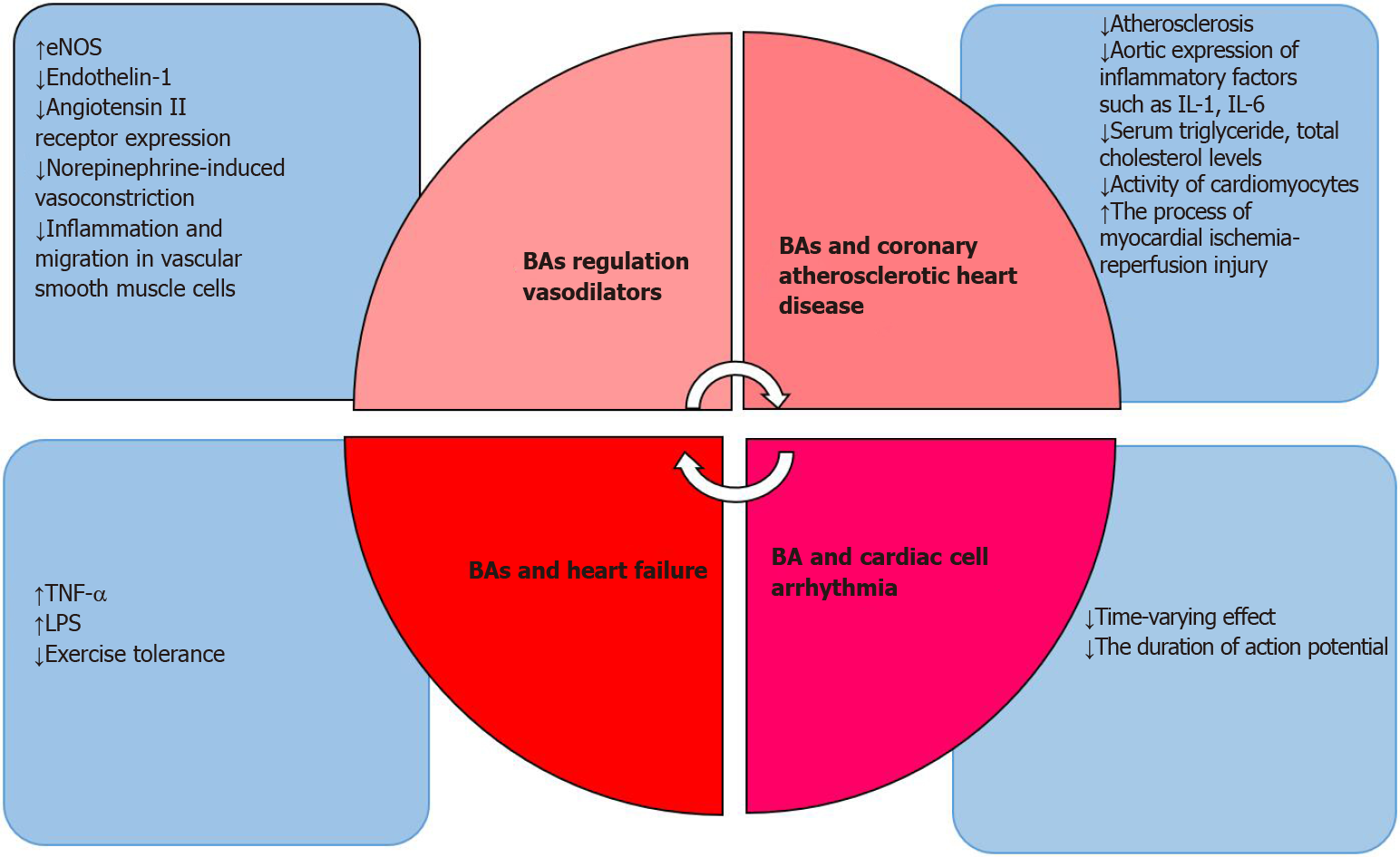
As the relationship between BAs and the heart continues to evolve, BAs have been shown to also play a role in HF[84]. Vascular endothelial dysfunction is one of the critical manifestations of chronic HF. Injury to endothelial function will activate endothelium-dependent injury pathways, eventually leading to decreased exercise tolerance and affecting the quality of life in HF patients. One of the most important factors is TNF-α[85]. LPS, which exists in the cell walls of Gram-negative bacteria in the gut, can enter the circulation through swollen intestinal mucosa due to decreased intestinal mucosal barrier function during the development of chronic HF[86]. Secondary BAs are produced from primary BAs under the action of intestinal microorganisms, indicating that the intestinal flora is related to the severity of HF[87]. In a recently published cross-sectional study, serum primary BA levels in patients with chronic HF decrease revealed specific secondary BA level increases and an increased ratio of secondary BAs to primary BAs. Therefore, the relationship between BAs and cardiomyocytes involves a complex regulatory system of multiple factors and multiple systems (organ, tissue, cellular, molecular, and endocrine) that interact with each other (as shown in Figure 1).
Cirrhosis is often accompanied by cardiac dysfunction, which has aroused interest in the study of the relationship between abnormal BA metabolism and cardiac pathology. The relative risk of cardiovascular disease after primary sclerosing cholangitis onset was 3.34 and after primary biliary cholangitis (PBC) was 2.2[88,89]. Significant prolongation of the corrected QT (QTc) interval was found in PBC patients, which may lead to ventricular arrhythmia and increase the risk of sudden death[90,91]. Furthermore, studies have shown that patients with PBC have significantly reduced heart rate variability and stress-response sensitivity[92].
Intrahepatic cholestasis of pregnancy (ICP) is a condition in which the mother’s TBA concentration is higher than the normal range. ICP can cause accumulation of BAs in fetal serum, inducing fetal heart problems[93]. Studies in patients with mild and severe ICP have shown that TBA concentration is associated with ventricular arrhythmia[94].
Various causes of cholestatic disease eventually lead to cirrhosis[95]. It is estimated that about half of all cirrhosis cases result in cirrhotic cardiomyopathy (CC), which is characterized by systolic or diastolic dysfunction and can lead to morphological changes in the heart[96]. A prolonged QT interval is the most common feature of CC. The mechanism is unclear, but most studies have shown that it is determined by a combination of factors[97]. Studies also found that the severity of cirrhosis is positively correlated with prolonged QTc septum, and it is more common in alcoholic cirrhosis[98]. The mouse BDL model revealed that elevated BAs increase the production of NO by mediating intracellular Ca2+ signaling and induce apoptosis of cardiomyocytes, leading to CC. It showed that NO can also promote apoptosis and inhibit autophagy in hepatocellular carcinoma. Ma et al[99] showed that reduced flow of the myocardial membrane in BDL rats, resulting in adrenergic dysfunction and the inability to produce cAMP, ultimately resulted in decreased contractility of the myocardium. However, most of the data obtained from the modeling of BDL has involved rodents, so the mechanism of application in the human body remains to be further confirmed.
UDCA is a highly hydrophilic BA that was initially used to treat chronic liver disease because it can dissolve cholesterol and reduce cholesterol absorption[100]. In animal experiments, increased BA concentrations often lead to arrhythmia and decreased cardiac function, while UDCA does not. UDCA has both apoptotic and anti-apoptotic effects, suggesting that it plays distinct roles depending on the cell type[101]. It protects the myocardium by counteracting more hydrophobic BAs, which is thought to be mediated by protein kinase C and intracellular calcium (Ca2+)[102]. Lee et al[103] found that UDCA protects myocardial damage by enhancing the recovery of systolic cardiac function during ischemia-reperfusion and reducing the release of lactate dehydrogenase during ischemia-reperfusion. In addition, the protective effect of UDCA on myocardial reperfusion injury in rats is thought to occur by inhibiting the mitochondrial permeability transition pore, which is dependent on the phosphoinositide 3 kinase/PKB pathway[104]. In patients with HF, UDCA therapy has been shown to improve endothelial-dependent and nondependent vasodilation, thereby maintaining impaired NO production of arterial blood flow[105]. In another clinical study, patients with chronic HF received 4 wk UDCA 500 mg twice daily, resulting in improved ischemic blood flow. Furthermore, levels of gamma-glutamyltransferase, aspartate transaminase, and tumor necrosis factor receptor 1 (TNFR1) were reduced and liver function improved after treatment compared to before treatment[106]. UDCA also protects fetal arrhythmia through BA-induced function[107]. Although numerous studies on UDCA have shown that its use can improve myocardial injury, the mechanisms are not well understood. To further confirm the importance of UDCA in humans, more patients are needed for more comprehensive studies.
Based on the above discussion, we know that BA signaling plays a vital role through receptor-dependent (FXR, VDR, TGR5, S1P, M) and channel-mediated (BK channel) mechanisms in different cell types. Particularly, for the cardiac system, most studies have shown that BA signaling affects heart function, and cardiac dysfunction in liver diseases, such as cirrhosis and ICP, is common. Interestingly, UDCA was found to play a protective role in dysrhythmia. Future work should be devoted to deciphering the complex interactions between BAs and their receptors to provide a pharmaco-logical basis for the clinical treatment of related diseases.
The authors thank the Xi’an Jiaotong University School of Medicine for the financial support and facilities provided.
| 1. | Zhuang S, Li Q, Cai L, Wang C, Lei X. Chemoproteomic Profiling of Bile Acid Interacting Proteins. ACS Cent Sci. 2017;3:501-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Di Ciaula A, Garruti G, Lunardi Baccetto R, Molina-Molina E, Bonfrate L, Wang DQ, Portincasa P. Bile Acid Physiology. Ann Hepatol. 2017;16:s4-s14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 391] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 3. | Chen J, Zhao KN, Chen C. The role of CYP3A4 in the biotransformation of bile acids and therapeutic implication for cholestasis. Ann Transl Med. 2014;2:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 4. | Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev. 2004;36:703-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Chen J, Raymond K. Nuclear receptors, bile-acid detoxification, and cholestasis. Lancet. 2006;367:454-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Sokol RJ, Straka MS, Dahl R, Devereaux MW, Yerushalmi B, Gumpricht E, Elkins N, Everson G. Role of oxidant stress in the permeability transition induced in rat hepatic mitochondria by hydrophobic bile acids. Pediatr Res. 2001;49:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu C, Chen Y, Cai W, Wu J. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget. 2016;7:83951-83963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Palmeira CM, Rolo AP. Mitochondrially-mediated toxicity of bile acids. Toxicology. 2004;203:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 469] [Cited by in RCA: 540] [Article Influence: 31.8] [Reference Citation Analysis (13)] |
| 10. | Danchenko E, Petermann H, Chirkin A, Dargel R. Effect of bile acids on the proliferative activity and apoptosis of rat hepatocytes. Exp Toxicol Pathol. 2001;53:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1372] [Cited by in RCA: 1541] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 12. | Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 2015;5:135-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 13. | Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 365] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 14. | Shaik FB, Prasad DV, Narala VR. Role of farnesoid X receptor in inflammation and resolution. Inflamm Res. 2015;64:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Mencarelli A, Fiorucci S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J Cell Mol Med. 2010;14:79-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Xia Y, Zhang F, Zhao S, Li Y, Chen X, Gao E, Xu X, Xiong Z, Zhang X, Zhang J, Zhao H, Wang W, Wang H, Guo Y, Liu Y, Li C, Wang S, Zhang L, Yan W, Tao L. Adiponectin determines farnesoid X receptor agonism-mediated cardioprotection against post-infarction remodelling and dysfunction. Cardiovasc Res. 2018;114:1335-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Pike JW, Meyer MB, Lee SM, Onal M, Benkusky NA. The vitamin D receptor: contemporary genomic approaches reveal new basic and translational insights. J Clin Invest. 2017;127:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 18. | Zhang J, Huang W, Qatanani M, Evans RM, Moore DD. The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem. 2004;279:49517-49522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Glenn DJ, Cardema MC, Gardner DG. Amplification of lipotoxic cardiomyopathy in the VDR gene knockout mouse. J Steroid Biochem Mol Biol. 2016;164:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Ibrahim E, Diakonov I, Arunthavarajah D, Swift T, Goodwin M, McIlvride S, Nikolova V, Williamson C, Gorelik J. Bile acids and their respective conjugates elicit different responses in neonatal cardiomyocytes: role of Gi protein, muscarinic receptors and TGR5. Sci Rep. 2018;8:7110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Takagi N, Miyake-Takagi K, Takagi K, Tamura H, Takeo S. Altered extracellular signal-regulated kinase signal transduction by the muscarinic acetylcholine and metabotropic glutamate receptors after cerebral ischemia. J Biol Chem. 2002;277:6382-6390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Donati C, Meacci E, Nuti F, Becciolini L, Farnararo M, Bruni P. Sphingosine 1-phosphate regulates myogenic differentiation: a major role for S1P2 receptor. FASEB J. 2005;19:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochim Biophys Acta. 2008;1781:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Keitel V, Häussinger D. Role of TGR5 (GPBAR1) in Liver Disease. Semin Liver Dis. 2018;38:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Eblimit Z, Thevananther S, Karpen SJ, Taegtmeyer H, Moore DD, Adorini L, Penny DJ, Desai MS. TGR5 activation induces cytoprotective changes in the heart and improves myocardial adaptability to physiologic, inotropic, and pressure-induced stress in mice. Cardiovasc Ther. 2018;36:e12462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Bukiya AN, McMillan JE, Fedinec AL, Patil SA, Miller DD, Leffler CW, Parrill AL, Dopico AM. Cerebrovascular dilation via selective targeting of the cholane steroid-recognition site in the BK channel β1-subunit by a novel nonsteroidal agent. Mol Pharmacol. 2013;83:1030-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Zhu Y, Ye P, Chen SL, Zhang DM. Functional regulation of large conductance Ca2+-activated K+ channels in vascular diseases. Metabolism. 2018;83:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Bentzen BH, Olesen SP, Rønn LC, Grunnet M. BK channel activators and their therapeutic perspectives. Front Physiol. 2014;5:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1717] [Cited by in RCA: 1793] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 30. | Laffitte BA, Kast HR, Nguyen CM, Zavacki AM, Moore DD, Edwards PA. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem. 2000;275:10638-10647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Yu J, Lo JL, Huang L, Zhao A, Metzger E, Adams A, Meinke PT, Wright SD, Cui J. Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem. 2002;277:31441-31447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Tu H, Okamoto AY, Shan B. FXR, a bile acid receptor and biological sensor. Trends Cardiovasc Med. 2000;10:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci U S A. 2004;101:3668-3673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | Desai MS, Mathur B, Eblimit Z, Vasquez H, Taegtmeyer H, Karpen SJ, Penny DJ, Moore DD, Anakk S. Bile acid excess induces cardiomyopathy and metabolic dysfunctions in the heart. Hepatology. 2017;65:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 35. | Hageman J, Herrema H, Groen AK, Kuipers F. A role of the bile salt receptor FXR in atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Li YT, Swales KE, Thomas GJ, Warner TD, Bishop-Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27:2606-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 37. | Ghosh S, Dass JFP. Study of pathway cross-talk interactions with NF-κB leading to its activation via ubiquitination or phosphorylation: A brief review. Gene. 2016;584:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 38. | Liu H, Lee SS. Nuclear factor-kappaB inhibition improves myocardial contractility in rats with cirrhotic cardiomyopathy. Liver Int. 2008;28:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Yang YY, Liu H, Nam SW, Kunos G, Lee SS. Mechanisms of TNFalpha-induced cardiac dysfunction in cholestatic bile duct-ligated mice: interaction between TNFalpha and endocannabinoids. J Hepatol. 2010;53:298-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | De Fabiani E, Mitro N, Gilardi F, Galmozzi A, Caruso D, Crestani M. When food meets man: the contribution of epigenetics to health. Nutrients. 2010;2:551-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Pu J, Yuan A, Shan P, Gao E, Wang X, Wang Y, Lau WB, Koch W, Ma XL, He B. Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J. 2013;34:1834-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 42. | Adachi R, Shulman AI, Yamamoto K, Shimomura I, Yamada S, Mangelsdorf DJ, Makishima M. Structural determinants for vitamin D receptor response to endocrine and xenobiotic signals. Mol Endocrinol. 2004;18:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281:19081-19091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Ma Y, Liu D. Activation of pregnane X receptor by pregnenolone 16 α-carbonitrile prevents high-fat diet-induced obesity in AKR/J mice. PLoS One. 2012;7:e38734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1214] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 46. | Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol Endocrinol. 2010;24:1151-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 49. | Rodriguez AJ, Mousa A, Ebeling PR, Scott D, de Courten B. Effects of vitamin D supplementation on inflammatory markers in heart failure: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2018;8:1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 50. | Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 487] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 51. | Cheng K, Khurana S, Chen Y, Kennedy RH, Zimniak P, Raufman JP. Lithocholylcholine, a bile acid/acetylcholine hybrid, is a muscarinic receptor antagonist. J Pharmacol Exp Ther. 2002;303:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Raufman JP, Chen Y, Cheng K, Compadre C, Compadre L, Zimniak P. Selective interaction of bile acids with muscarinic receptors: a case of molecular mimicry. Eur J Pharmacol. 2002;457:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Sheikh Abdul Kadir SH, Miragoli M, Abu-Hayyeh S, Moshkov AV, Xie Q, Keitel V, Nikolaev VO, Williamson C, Gorelik J. Bile acid-induced arrhythmia is mediated by muscarinic M2 receptors in neonatal rat cardiomyocytes. PLoS One. 2010;5:e9689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Serriere-Lanneau V, Teixeira-Clerc F, Li L, Schippers M, de Wries W, Julien B, Tran-Van-Nhieu J, Manin S, Poelstra K, Chun J, Carpentier S, Levade T, Mallat A, Lotersztajn S. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. 2007;21:2005-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Liu R, Li X, Qiang X, Luo L, Hylemon PB, Jiang Z, Zhang L, Zhou H. Taurocholate Induces Cyclooxygenase-2 Expression via the Sphingosine 1-phosphate Receptor 2 in a Human Cholangiocarcinoma Cell Line. J Biol Chem. 2015;290:30988-31002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Means CK, Brown JH. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res. 2009;82:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 57. | Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435-9440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1271] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 58. | Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 59. | Zhang X, Jin L, Tian Z, Wang J, Yang Y, Liu J, Chen Y, Hu C, Chen T, Zhao Y, He Y. Nitric oxide inhibits autophagy and promotes apoptosis in hepatocellular carcinoma. Cancer Sci. 2019;110:1054-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1641] [Cited by in RCA: 1746] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 61. | Desai MS, Shabier Z, Taylor M, Lam F, Thevananther S, Kosters A, Karpen SJ. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology. 2010;51:2097-2107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 62. | Kida T, Tsubosaka Y, Hori M, Ozaki H, Murata T. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1663-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 63. | Dopico AM, Walsh JV Jr, Singer JJ. Natural bile acids and synthetic analogues modulate large conductance Ca2+-activated K+ (BKCa) channel activity in smooth muscle cells. J Gen Physiol. 2002;119:251-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol. 2007;72:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Binah O, Rubinstein I, Bomzon A, Better OS. Effects of bile acids on ventricular muscle contraction and electrophysiological properties: studies in rat papillary muscle and isolated ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1987;335:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Fiorucci S, Zampella A, Cirino G, Bucci M, Distrutti E. Decoding the vasoregulatory activities of bile acid-activated receptors in systemic and portal circulation: role of gaseous mediators. Am J Physiol Heart Circ Physiol. 2017;312:H21-H32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Guizoni DM, Vettorazzi JF, Carneiro EM, Davel AP. Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide. 2020;94:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Zhang Q, He F, Kuruba R, Gao X, Wilson A, Li J, Billiar TR, Pitt BR, Xie W, Li S. FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc Res. 2008;77:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S, Pitt B, Xie W, Li S. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res. 2006;98:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Li J, Wilson A, Kuruba R, Zhang Q, Gao X, He F, Zhang LM, Pitt BR, Xie W, Li S. FXR-mediated regulation of eNOS expression in vascular endothelial cells. Cardiovasc Res. 2008;77: 169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Nakajima T, Okuda Y, Chisaki K, Shin WS, Iwasawa K, Morita T, Matsumoto A, Suzuki JI, Suzuki S, Yamada N, Toyo-Oka T, Nagai R, Omata M. Bile acids increase intracellular Ca(2+) concentration and nitric oxide production in vascular endothelial cells. Br J Pharmacol. 2000;130:1457-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Khurana S, Raina H, Pappas V, Raufman JP, Pallone TL. Effects of deoxycholylglycine, a conjugated secondary bile acid, on myogenic tone and agonist-induced contraction in rat resistance arteries. PLoS One. 2012;7:e32006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Machida T, Matamura R, Iizuka K, Hirafuji M. Cellular function and signaling pathways of vascular smooth muscle cells modulated by sphingosine 1-phosphate. J Pharmacol Sci. 2016;132:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Kida T, Murata T, Hori M, Ozaki H. Chronic stimulation of farnesoid X receptor impairs nitric oxide sensitivity of vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H195-H201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Pak JM, Adeagbo AS, Triggle CR, Shaffer EA, Lee SS. Mechanism of bile salt vasoactivity: dependence on calcium channels in vascular smooth muscle. Br J Pharmacol. 1994;112:1209-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Li W, Shu S, Cheng L, Hao X, Wang L, Wu Y, Yuan Z, Zhou J. Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis. 2020;292:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 77. | Pols TW. TGR5 in inflammation and cardiovascular disease. Biochem Soc Trans. 2014;42:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Charach G, Rabinovich A, Argov O, Weintraub M, Rabinovich P. The role of bile Acid excretion in atherosclerotic coronary artery disease. Int J Vasc Med. 2012;2012:949672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Gylling H, Hallikainen M, Rajaratnam RA, Simonen P, Pihlajamäki J, Laakso M, Miettinen TA. The metabolism of plant sterols is disturbed in postmenopausal women with coronary artery disease. Metabolism. 2009;58:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J Lipid Res. 2005;46:2595-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 81. | Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 107] [Reference Citation Analysis (0)] |
| 82. | Joubert P. An in vivo investigation of the negative chronotropic effect of cholic acid in the rat. Clin Exp Pharmacol Physiol. 1978;5:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Rainer PP, Primessnig U, Harenkamp S, Doleschal B, Wallner M, Fauler G, Stojakovic T, Wachter R, Yates A, Groschner K, Trauner M, Pieske BM, von Lewinski D. Bile acids induce arrhythmias in human atrial myocardium--implications for altered serum bile acid composition in patients with atrial fibrillation. Heart. 2013;99:1685-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 84. | Mayerhofer CCK, Ueland T, Broch K, Vincent RP, Cross GF, Dahl CP, Aukrust P, Gullestad L, Hov JR, Trøseid M. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. J Card Fail. 2017;23:666-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 85. | Schumacher SM, Naga Prasad SV. Tumor Necrosis Factor-α in Heart Failure: an Updated Review. Curr Cardiol Rep. 2018;20:117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 86. | Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, Verri M, Dioguardi F. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016;4:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 326] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 87. | Kitai T, Tang WHW. Gut microbiota in cardiovascular disease and heart failure. Clin Sci (Lond). 2018;132:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Ludvigsson JF, Bergquist A, Montgomery SM, Bahmanyar S. Risk of diabetes and cardiovascular disease in patients with primary sclerosing cholangitis. J Hepatol. 2014;60:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Czul F, Peyton A, Levy C. Primary biliary cirrhosis: therapeutic advances. Clin Liver Dis. 2013;17:229-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Kempler P, Váradi A, Kádar E, Szalay F. Autonomic and peripheral neuropathy in primary biliary cirrhosis: evidence of small sensory fibre damage and prolongation of the QT interval. J Hepatol. 1994;21:1150-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Bogaard K, van der Steen MS, Tan HL, Tukkie R. Short-coupled variant of torsade de pointes. Neth Heart J. 2008;16:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Newton JL, Elliott C, Frith J, Ghazala C, Pairman J, Jones DE. Functional capacity is significantly impaired in primary biliary cirrhosis and is related to orthostatic symptoms. Eur J Gastroenterol Hepatol. 2011;23:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Geenes V, Lövgren-Sandblom A, Benthin L, Lawrance D, Chambers J, Gurung V, Thornton J, Chappell L, Khan E, Dixon P, Marschall HU, Williamson C. The reversed feto-maternal bile acid gradient in intrahepatic cholestasis of pregnancy is corrected by ursodeoxycholic acid. PLoS One. 2014;9:e83828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 94. | Kirbas O, Biberoglu EH, Kirbas A, Daglar K, Kurmus O, Danisman N, Biberoglu K. Evaluation of ventricular repolarization in pregnant women with intrahepatic cholestasis. Int J Cardiol. 2015;189:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Woolbright BL. Inflammation: Cause or consequence of chronic cholestatic liver injury. Food Chem Toxicol. 2020;137:111133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Ruiz-del-Árbol L, Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol. 2015;21:11502-11521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 97. | Zambruni A, Trevisani F, Caraceni P, Bernardi M. Cardiac electrophysiological abnormalities in patients with cirrhosis. J Hepatol. 2006;44:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 98. | Mozos I, Costea C, Serban C, Susan L. Factors associated with a prolonged QT interval in liver cirrhosis patients. J Electrocardiol. 2011;44:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Ma Z, Lee SS, Meddings JB. Effects of altered cardiac membrane fluidity on beta-adrenergic receptor signalling in rats with cirrhotic cardiomyopathy. J Hepatol. 1997;26:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Ambros-Rudolph CM, Glatz M, Trauner M, Kerl H, Müllegger RR. The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe. Arch Dermatol. 2007;143:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 385] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 102. | Murakami M, Une N, Nishizawa M, Suzuki S, Ito H, Horiuchi T. Incretin secretion stimulated by ursodeoxycholic acid in healthy subjects. Springerplus. 2013;2:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Lee WY, Han SH, Cho TS, Yoo YH, Lee SM. Effect of ursodeoxycholic acid on ischemia/reperfusion injury in isolated rat heart. Arch Pharm Res. 1999;22:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | Rajesh KG, Suzuki R, Maeda H, Yamamoto M, Yutong X, Sasaguri S. Hydrophilic bile salt ursodeoxycholic acid protects myocardium against reperfusion injury in a PI3K/Akt dependent pathway. J Mol Cell Cardiol. 2005;39:766-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Sinisalo J, Vanhanen H, Pajunen P, Vapaatalo H, Nieminen MS. Ursodeoxycholic acid and endothelial-dependent, nitric oxide-independent vasodilatation of forearm resistance arteries in patients with coronary heart disease. Br J Clin Pharmacol. 1999;47:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | von Haehling S, Schefold JC, Jankowska EA, Springer J, Vazir A, Kalra PR, Sandek A, Fauler G, Stojakovic T, Trauner M, Ponikowski P, Volk HD, Doehner W, Coats AJ, Poole-Wilson PA, Anker SD. Ursodeoxycholic acid in patients with chronic heart failure: a double-blind, randomized, placebo-controlled, crossover trial. J Am Coll Cardiol. 2012;59:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 107. | Miragoli M, Kadir SH, Sheppard MN, Salvarani N, Virta M, Wells S, Lab MJ, Nikolaev VO, Moshkov A, Hague WM, Rohr S, Williamson C, Gorelik J. A protective antiarrhythmic role of ursodeoxycholic acid in an in vitro rat model of the cholestatic fetal heart. Hepatology. 2011;54:1282-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Enosawa S, Park YM S-Editor: Huang P L-Editor: Filipodia P-Editor: Liu JH