Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5302
Peer-review started: February 25, 2021
First decision: April 13, 2021
Revised: April 26, 2021
Accepted: April 29, 2021
Article in press: April 29, 2021
Published online: July 6, 2021
Processing time: 118 Days and 15.9 Hours
Scoliosis is a complex three-dimensional deformity of spine and one of the common complications of collagen VI-related myopathy, caused by mutations in collagen type VI alpha 1 chain (COL6A1), COL6A2, and COL6A3 genes. The typical clinical presentations of collagen VI-related myopathy include weakness, hypoto
A 28-year-old female presented with scoliosis for 28 years without weakness, hypotonia, laxity of distal joints, and contracture of proximal joints. Computed tomography and magnetic resonance imaging revealed hemivertebra, butterfly vertebra, and the missing vertebral space. Patients underwent orthopedic surgery and paravertebral muscle biopsy. The Cobb angle dropped from 103.4° to 52.9°. However, the muscle biopsy showed neurogenic muscular atrophy with myogenic lesions, suggesting congenital muscular dystrophy. Gene analysis indicated that mutations in COL6A1 (c.1612-10G>A) and COL6A2 (c.115+10G>T, c.2749G>A). Immunohistochemistry staining for collagen VI displayed shallow and discontinuous. Eventually, the patient was diagnosed as collagen VI-related myopathy.
This newly found subtype of collagen VI-related myopathy has no typical manifestations; however, it is characterized by severe scoliosis and congenital vertebral deformity.
Core Tip: Here, we report a patient diagnosed with congenital scoliosis initially with deformity of the vertebral body. However, gene analyses indicated mutations in collagen type VI alpha 1 chain (COL6A1) (c.1612-10G>A) and COL6A2 (c.115+10G>T, c.2749G>A). Immunohistochemistry of collagen VI displayed shallow and discontinuous staining. This newly found subtype of collagen VI-related myopathy has no weakness, hypotonia, laxity of distal joints, and contractures of proximal joints and is characterized by severe scoliosis and congenital vertebral deformity, indicating that the underlying etiology of congenital scoliosis may be a special type of collagen VI-related myopathy.
- Citation: Li JY, Liu SZ, Zheng DF, Zhang YS, Yu M. Collagen VI-related myopathy with scoliosis alone: A case report and literature review . World J Clin Cases 2021; 9(19): 5302-5312
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5302.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5302
Characterized by lateral curvature and axial rotation, scoliosis is a complex three-dimensional deformity of the spine[1]. One of the causes is the abnormal development of the vertebral body, called congenital scoliosis[2]. This congenital malformation is mainly caused by asymmetric development of the spine during 4-6 wk of gestation[3]. The incidence rate of malformation in the live fetus is about 1/1000[4], which can be divided into segmental abnormalities, formation abnormalities, or composite abnormalities[5]. Some scholars believe that the occurrence of congenital scoliosis relate to genetic factors (e.g., T-box transcription factor 6 gene) and environmental factors (e.g., hypoxia)[6,7].
Besides the congenital scoliosis, scoliosis is one of the common complications of neuromuscular diseases, which can be caused by progressive muscle weakness of the paravertebral muscles[8]. It usually appears in the late stage of the disease and is associated with other clinical associated manifestations[8]. Among the neuromuscular diseases, collagen VI-related myopathy is one of the neuromuscular diseases that can lead to scoliosis including Ullrich congenital muscular dystrophy, Bethlem myopathy and various related diseases, caused by mutations in collagen type VI alpha 1 chain (COL6A1), COL6A2, and COL6A3 genes[9]. In a physiological situation, collagen VI (COL6) is widely found in the extracellular matrix and is important for the structure and function of skeletal muscle[10]. As a result, mutation of this gene causes signi
Here, we report a patient diagnosed with congenital scoliosis initially. Without limb weakness, hypotonia, laxity of distal joints, and contractures of proximal joints, she does not have any typical manifestations. After the spinal surgery, the patient was discharged, and her Cobb angle dropped from 103.4° to 52.9°. To determine the cause, intraoperative paravertebral muscle biopsy revealed muscular atrophy with myogenic lesions. Gene testing showed three mutations in COL6A1 and COL6A2. Immunohistochemistry staining for collagen VI displayed part of the muscle fiber muscle membrane that stained to be shallow and widened, with a few portions of muscle fiber staining to be discontinuous. Eventually, the patient was diagnosed with a new type of collagen VI-related myopathy confined to the paraspinal muscle.
A 28-year-old female presented with scoliosis for 28 years and was admitted to the hospital from March 2019 to August 2019.
The patient was a 28-year-old female who presented with scoliosis for 28 years from birth. An attempt was made to install the external fixation with braces to the patient, but the effect was poor. In recent 4 years, the patient suffered from lower back pain progressively.
The patient had a free previous medical history.
The patient denied any personal history of alcohol and cigarette consumption. Her family history has nothing notable.
Physical examination showed that the patient did not have obvious weakness, hypotonia, laxity, or contractures of the extremity joint. The mobility of wrist joints, metacarpophalangeal joints, distal interphalangeal joints and metatarsophalangeal joints was normal, and there was no abnormal skin mass. With the exception of superficial sensation abnormity tested by a safety pin, at the proximal lateral front of the left thigh, the rest parts sensation is normal, and the muscle strength of the limbs was classified as grade 5/5[11]. The physiological reflex of the patient was normal, except for the disappearance of abdominal wall reflex, and the pathological signs were negative. Sitting and standing time was not limited, but the patient’s walking ability is affected and can only walk 1000 m due to low back pain.
Her menstrual history was generally normal. The menstruation started from age 14. Its period was 5-7 d. Its cycle was 25-28 d. The last menstruation was January 24, 2019. The color was normal without blood clots and without dysmenorrhea.
The patient denied congenital scoliosis teratogenic factors such as hypoxia during pregnancy, vitamin A deficiency, diabetes, and preeclampsia. Family history of scoliosis was denied. Adam’s forward bend test was positive, suggesting that the patient had a high likelihood of scoliosis[12].
Her laboratory examinations have nothing notable.
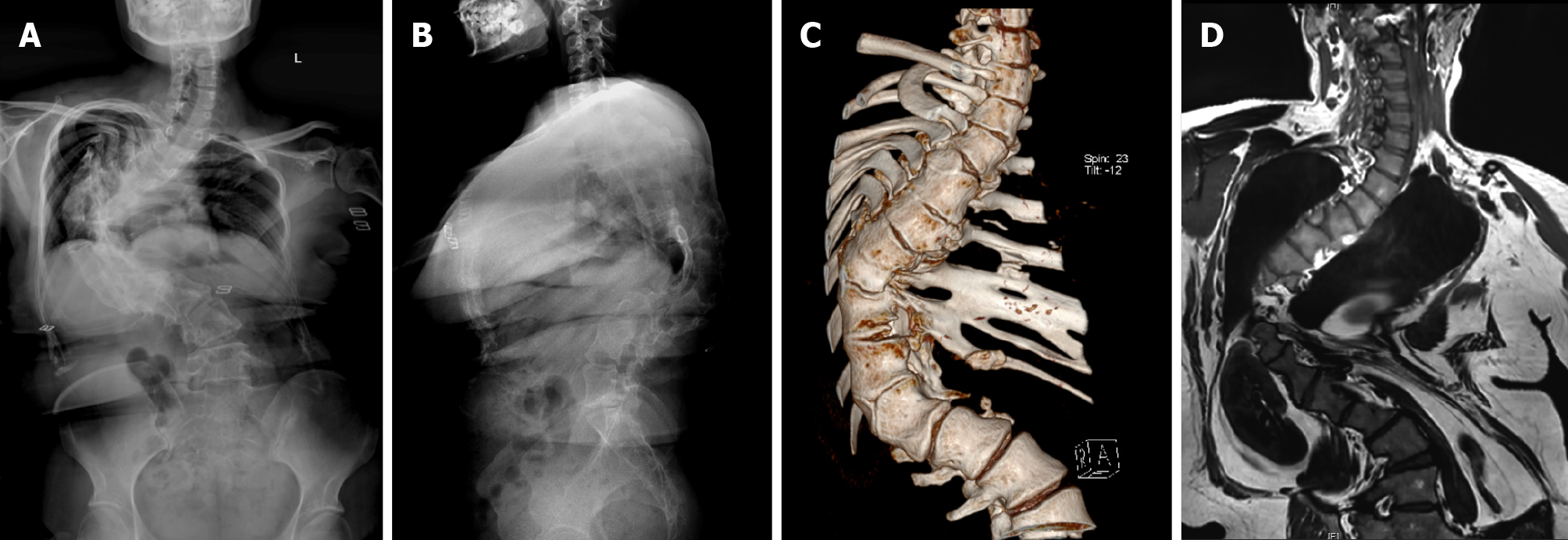
X-ray, computed tomography (CT), and magnetic resonance imaging (MRI) of the whole spine (Figure 1) showed that the Cobb angle was 103.4°, and the T4-6, T8, T12-L1 vertebral body was deformed. At T12-T1, the vertebral bodies fused, the intervertebral space disappeared, and the posterior margin kyphosis. The T11 vertebral body was hemivertebra. There was no obvious herniation of intervertebral disc and no obvious narrowing of vertebral canal. Based on the history, symptoms and signs, the patient was initially diagnosed as mixed congenital scoliosis initially.
The patient and her family members provided informed consent to publish case reports, photographs and carry intraoperative muscle biopsies and ‘whole exome sequencing (Slim version)’ at KangSo Medical Laboratory in Beijing, which was approved by the institutional review board of the authors’ affiliated institutions.
Histological examination: Biopsy from the multifidus of the concave and convex sides of the thoracic vertebra was performed. The patients and their families were informed that data from the case would be submitted for publication, and gave their consent.
The process of muscle biopsy was performed as described by Meier et al[13]. The biopsy tissue was covered with a semi-moist saline gauze pad and transferred immediately. After gently drying, a small portion of the tissue was separated for electron microscopy, and the other fresh tissue was embedded with tragacanth gum. Then the samples were placed in liquid nitrogen-precooled isopentane for 20 s and then stored in a -20° freezer. 5 μm thick tissue slices were cut in a low temperature slicer for hematoxylin and eosin staining, special staining: nicotinamide adenine dinucleotide + hydrogen (NADH), Modified Gomori trichrome (MGT) and immunohistochemistry staining: dystrophin-1, dystrophin-2, dystrophin-3, myosin, major histocompatibility complex I, cluster of differentiation 4 (CD4), CD8, CD163, and CD20.
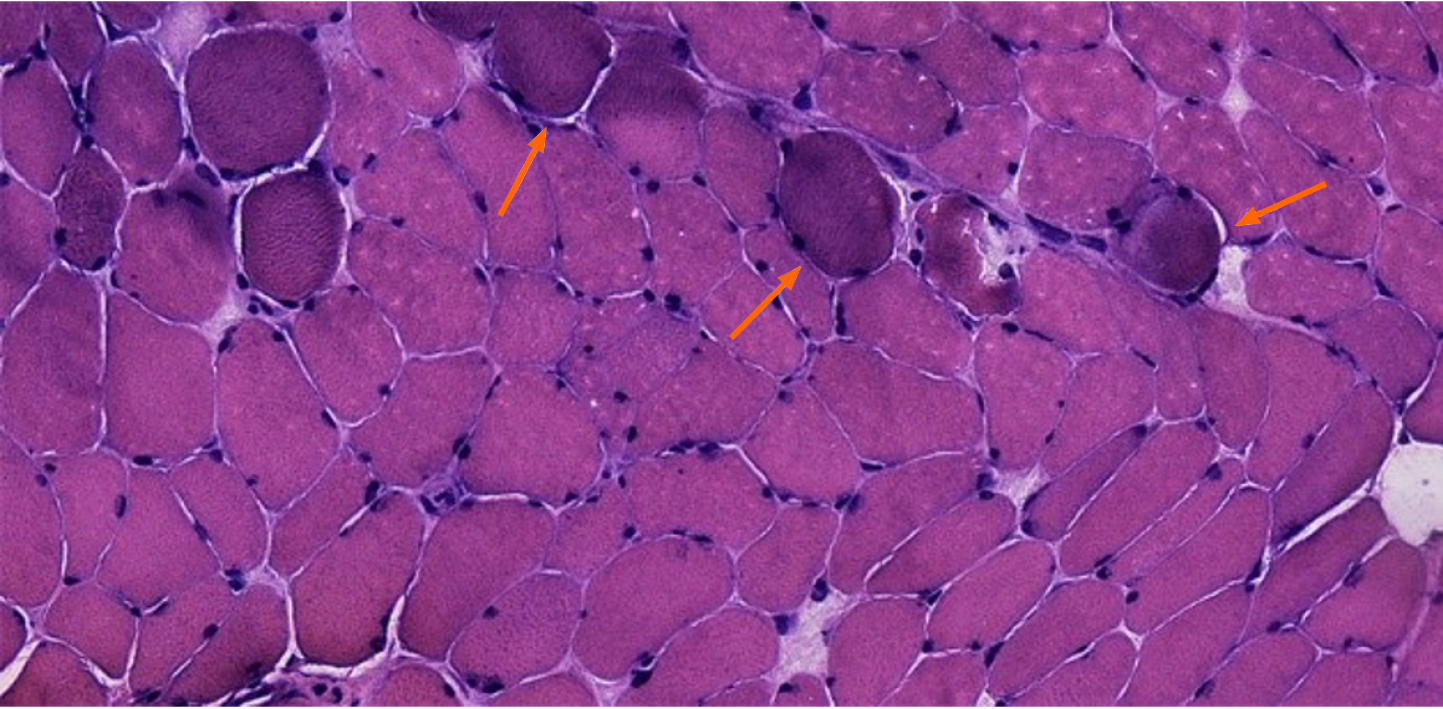
Optical microscope showed that the main part of the biopsy was skeletal muscle tissue, within a few light to medium volume reduced muscle fibers, muscle fiber gap widening, visible minority nuclear ingression, occasional crack, fibrous connective tissue hyperplasia, and a bunch of severe contraction inside muscle fibers, but no obvious degeneration and necrosis were observed. Numerous tendon-like structures formed by dense connective tissue can be seen around the muscle tract (Figure 2).
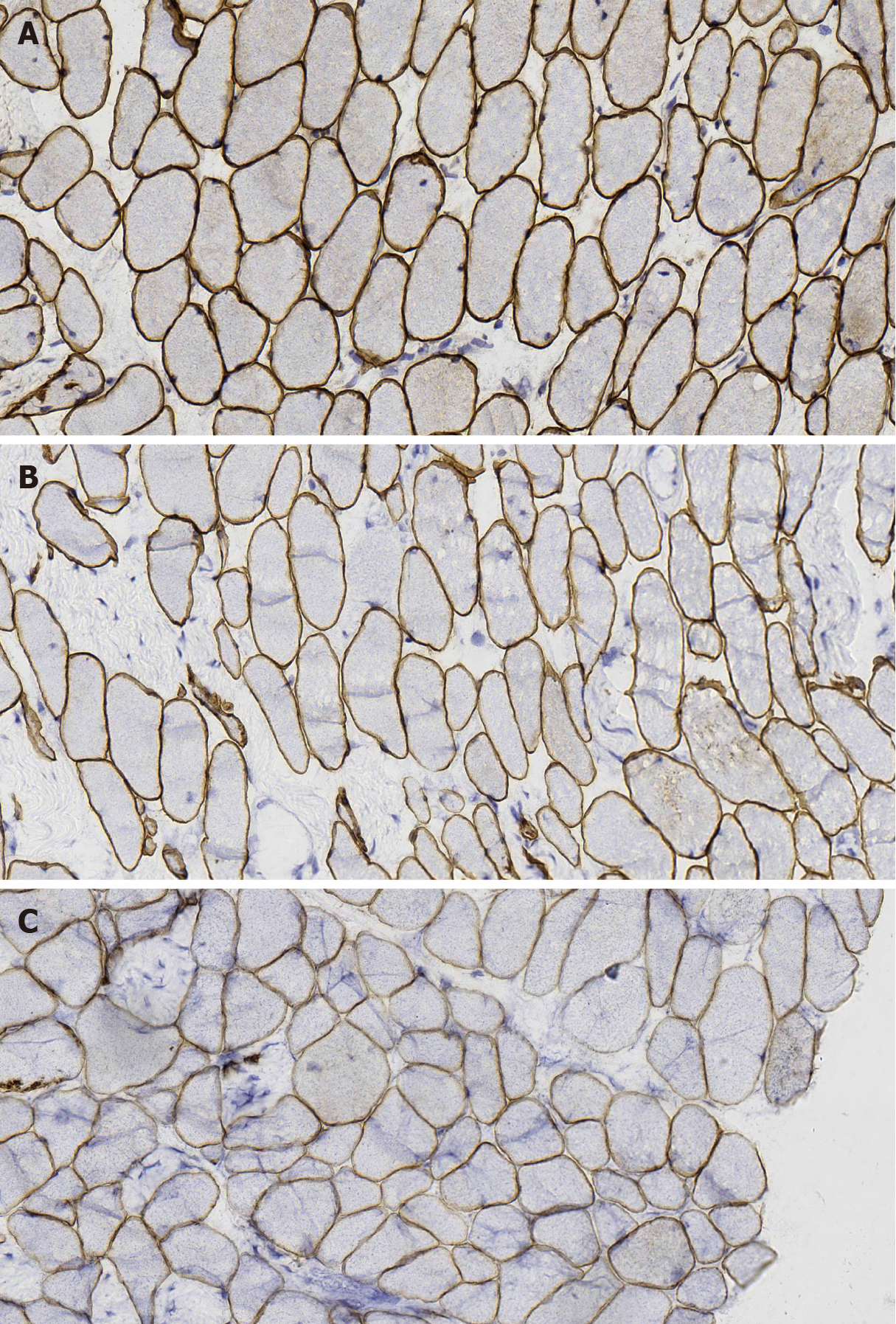
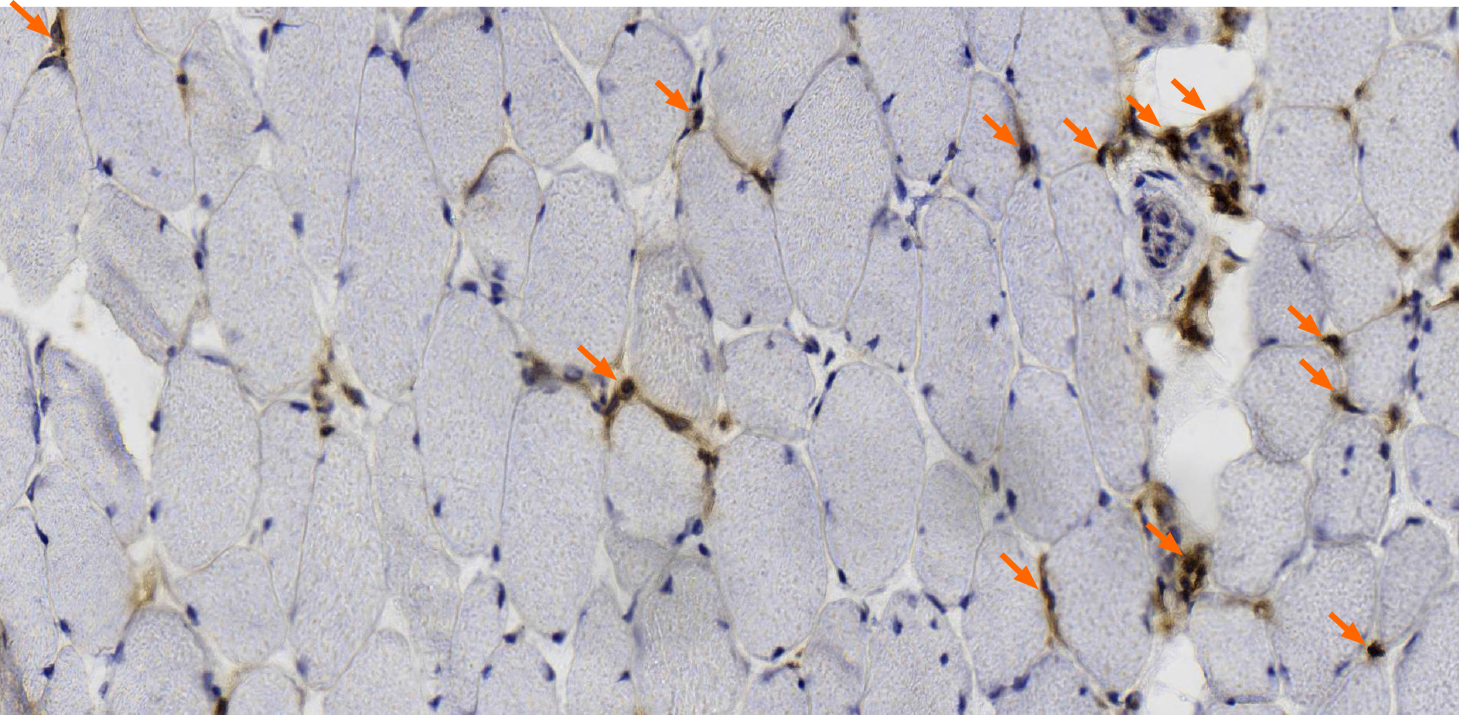
MGT staining showed a slight increase in reddening particles in some muscle fibers; NADH-tetrazolium reductase staining showed balanced distribution of the two types of fibers, and a small amount of muscle fibers were rotated. Immunohistochemical staining with dystrophin-1 antibody showed positive staining of muscle sarcolemma; dystrophin-2 and -3 antibody staining showed uneven staining of a few muscle sarcolemma (Figure 3). CD4- and CD8-positive cells and other inflammatory cells were occasionally infiltrated around the small vessels (Figure 4).
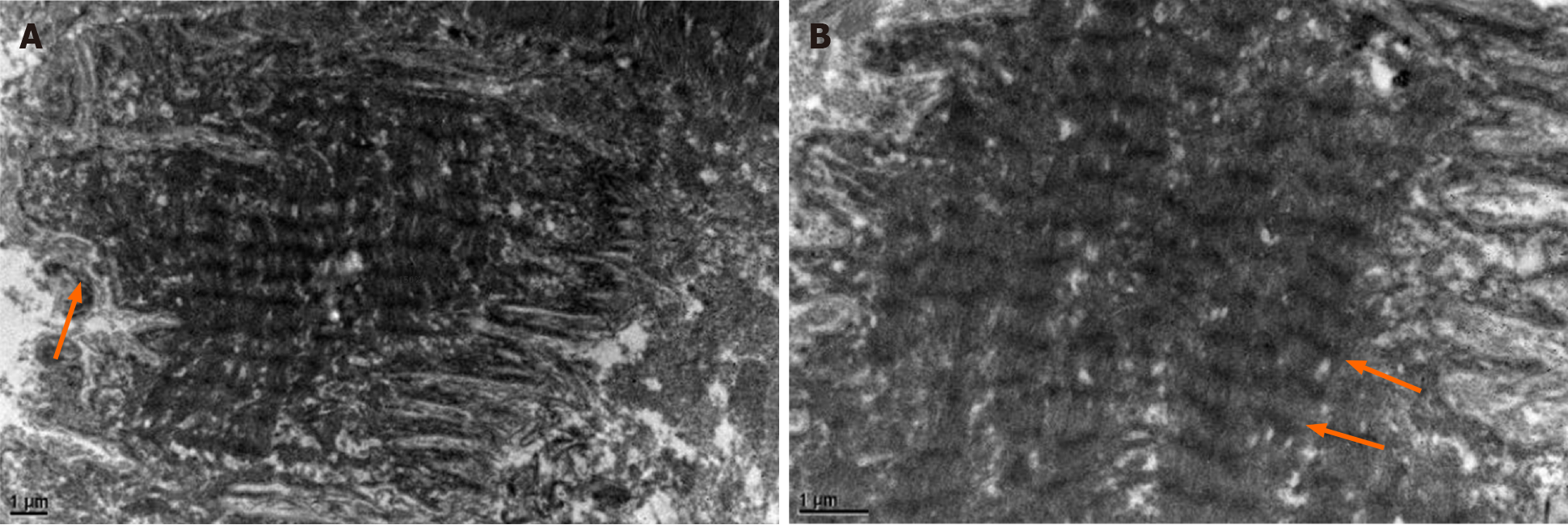
Electron microscopy showed that most of the muscle fibers were corrugated and the arrangement of extracellular matrix was slightly disordered. Individual myofibers and myoplasm coagulation with focal muscle mass formation. The inner myofibrils of some muscle fibers were arranged neatly, but the a-band structure was unclear (Figure 5).
Biopsy results suggested that neurogenic muscular atrophy with myogenic damage, congenital muscular dystrophy, and another myopathy were more likely (Table 1)[14]. To further investigate the reason for this presentation, patients agreed to have their genes tested for a clear diagnosis.
| Antibody | Result | Antibody | Result |
| Dystrophin | + | Dystrophin-2 | A few sarcolemma staining in different shades |
| Dystrophin-1 | + | Dystrophin-3 | A few sarcolemma staining in different shades |
| Dysferlin | + | HLA-DR | Vascular endothelium and inflammatory cells + |
| Lambda | - | MHC-1 | A few sarcolemma and myoplasm+ |
| CD20 | Very few + | CD4 | A few vessel wall and interstitial substance + |
| CD163 | - | CD8 | A few vessel wall and interstitial substance + |
| CD138 | Interstitial substance visible occasionally + |
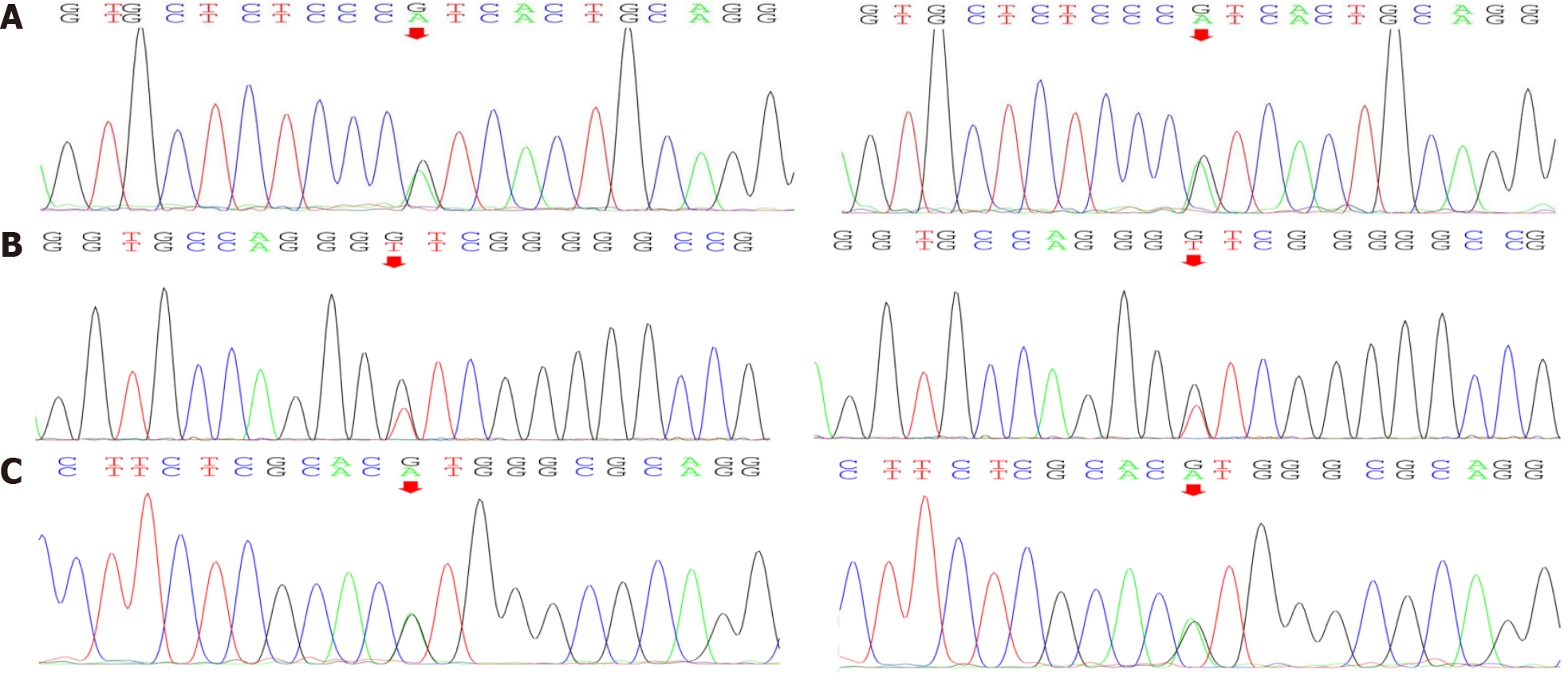
Gene testing: Gene testing resulted in the identification of four heterozygous variants of significance (included in the genetic package for hereditary myopathy) (Figure 6): one COL6A1 splicing variant (c.1612-10G>A), one COL6A2 splicing variant (c.115+10G>T), and one COL6A2 missense variant (c.2749G>A). Of those variants, COL6A2 missense variant (c.2749G>A) is considered as a disease-causing mutation by ‘Mutation Taster’, deleterious by ‘Sortig Intolerant from Tolerant’ and probably-damaging by ‘Polyphen2’ (Table 2). Therefore, the patient was diagnosed with collagen VI-related myopathy with scoliosis alone from the gene.
| Gene | Locus | Variant | Consequence | Location | Type | Prediction |
| COL6A1 | NM_001848 | C.1612-10G>A | - | Intron24 | Heterozygote | - |
| COL6A2 | NM_001849 | C.115+10G>T | - | Intron2 | Heterozygote | - |
| COL6A2 | NM_001849 | C.2749G>A | P. Val917Met | Exon28 | Heterozygote | Disease causing |
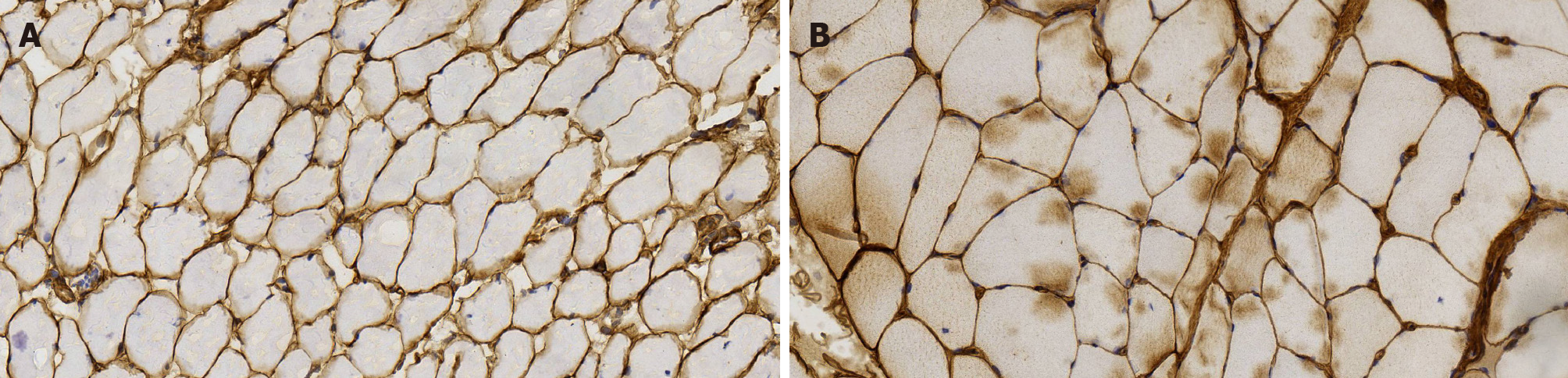
COL6 staining: Based on the results of gene examination, we carried out COL6 immunohistochemical staining of the patient (with scoliosis) (Figure 7A), which indicated the low expression of COL6, compared with another inflammatory myopa
Collagen VI-related myopathy diagnosis was finally affirmed at the gene and protein levels.
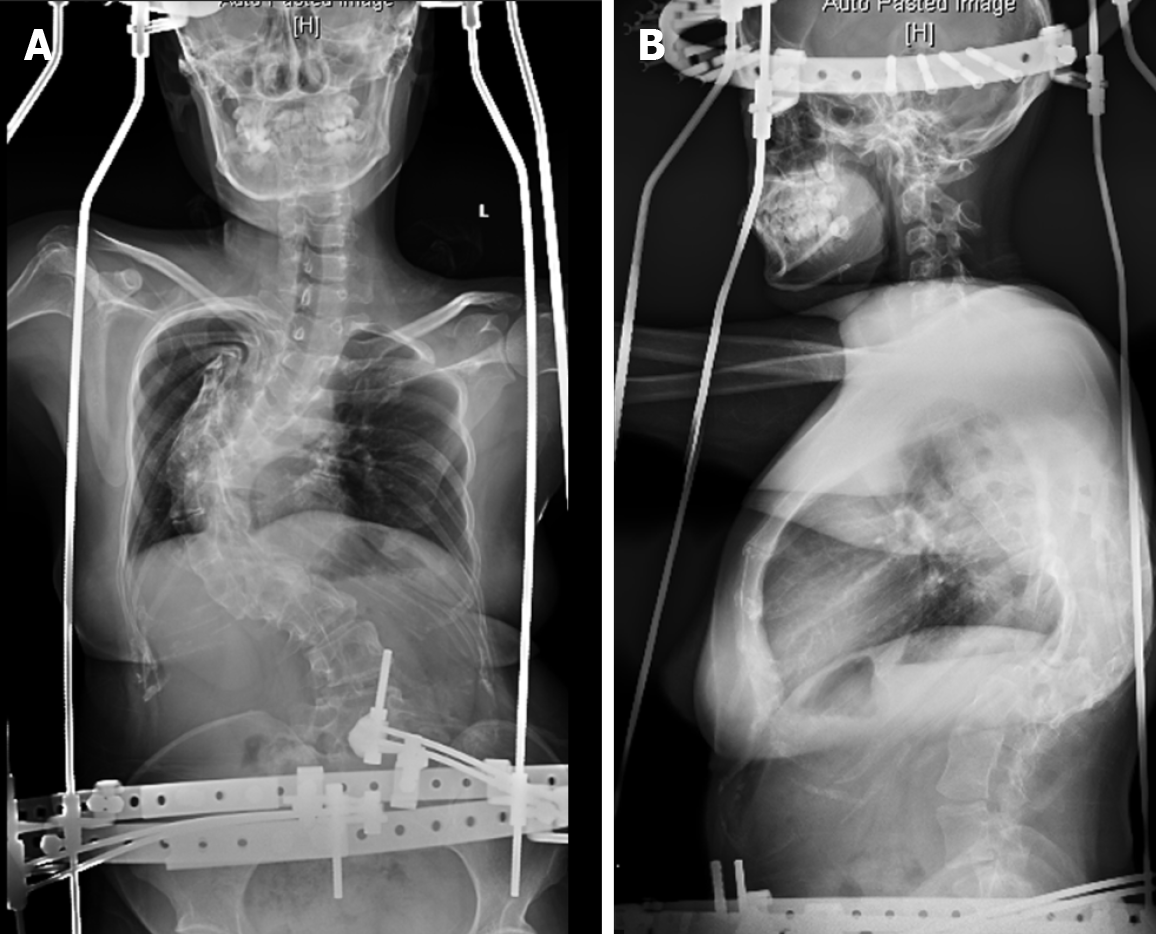
Because the patient had been born with scoliosis and had a large Cobb angle, we chose the preoperative ‘halo-socket distraction’ for the patient to enhance spinal flexibility and reduce the risk of nerve injury during surgery, the patient underwent ‘halo-pelvic distraction’ on March 16, 2019 with 13 cm distraction in 4 wk[15] (Figure 8). On May 10, 2019, the patient underwent an operation to place posterior T2-L4 pedicle screw internal fixation, T9 semi-vertebral resection, T10 total vertebral resection, intervertebral and interarticular bone grafting.
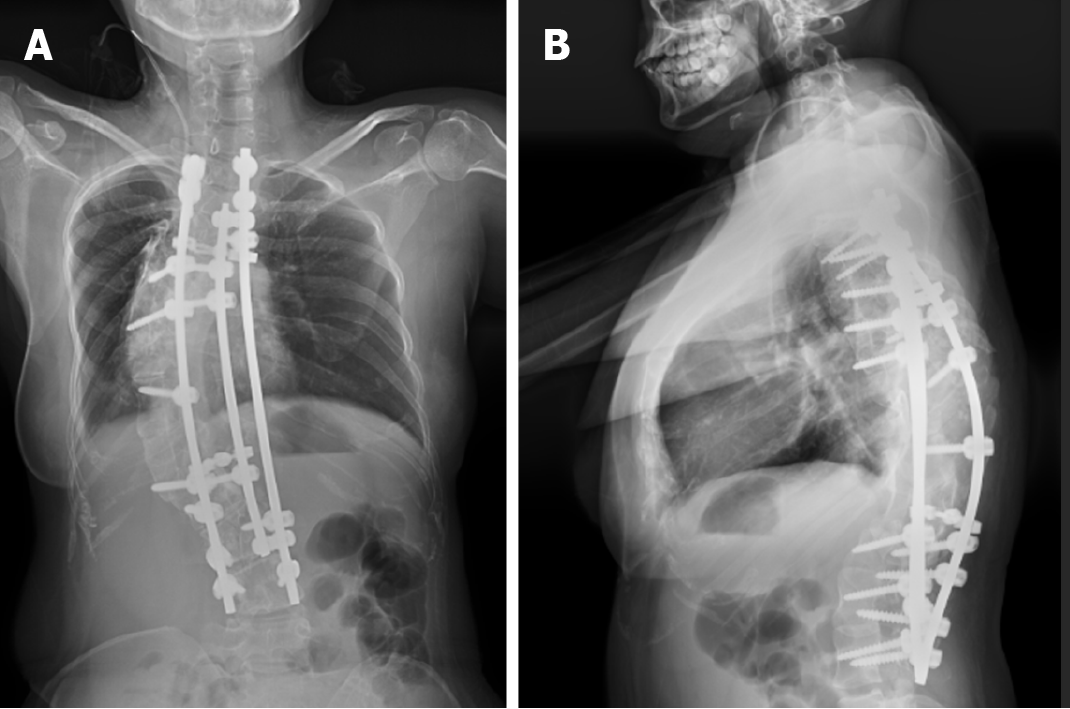
The operation was successful and the patient discharged. X-ray, CT, and MRI of the whole spine (Figure 9) showed that the postoperative Cobb angle improvement to 52.9°.
In this case, with the exception of scoliosis and limited walking ability due to low back pain, the patient had no other pathological symptoms and signs in physical examina
Collagen VI-related myopathy is caused by the mutation of COL6A1, COL6A2 and COL6A3, which leads to the structural disorders of the α1 α2 and α3 subunits, then affects the formation of tetramers and the following assembly process, resulting in collagen VI fibers abnormity[16]. Since collagen VI fiber is closely related to the functional structure of skeletal muscle, mutation of this gene can cause muscle damage[10]. According to the different lesion site, the disease can be presented as a continuous spectrum of diseases from light to heavy[17]. The relatively mild Bethlem myopathy is characterized by muscular weakness, hypotonia, laxity of distal joints, contractures of proximal joints and bone deformity[18]. Besides the symptoms described above, relatively severe UCMD also manifests a ‘sandpaper’ like rash, congenital hip joint dislocation, torticular neck, scoliosis, high arched palate, and prominent heel[19]. In addition, the disease also includes limb girdle muscular dystrophy, muscle sclerosis myopathy[17].
With scoliosis as the chief complaint, the patient was diagnosed with collagen VI-related myopathy by muscle biopsy and gene testing. Considering the diversity of clinical phenotypes of this disease[20], this patient might be a special subtype, called “Collagen VI-related myopathy with scoliosis alone”. In this subtype, scoliosis is the main clinical manifestation, accompanied with spinal congenital malformations such as hemivertebra and paraspinal muscle atrophy and lesions, without limb contracture or hyperextension. However, our report is only an isolated case, and further cases and studies are needed if this subtype is to be included in the disease spectrum.
According to the medical history, the patient has no exposure to teratogenic factors during pregnancy. Therefore, we assume that the congenital abnormality of the vertebral may relate to the gene mutation of COL6A1 and COL6A2. So far, no major susceptibility genes for sporadic congenital spinal malformations in humans have been identified[21]. In zebrafish model, COL8A1 gene mutation leads to abnormal spinal cord folding in embryos, which leads to spinal deformity, suggesting that extracellular matrix is closely related to notochord normal differentiation[22]. In fact, as a part of the extracellular matrix, various types of collagen are involved in the formation of the spine[23-26]. During the segmentation period, COL15 is mainly expressed in the notochord, and its protein products are only deposited in the basement membrane around the notochord[23]. The expressions of COL2A1 and COL18A1 are also regula
In this case scoliosis was severe and muscle strength was normal, implying the patient lesion was confined to the paravertebral muscle and did not affect the muscles in other parts of body. This location-specific muscle injury confirmed the poor correlation between genotype and phenotype of collagen VI-related myopathy, similarly to the reports of Kim et al[27] and Okada et al[20]. Also, it suggests that there might be some undetected regulators in collagen VI-related myopathy[27], such as copy number variation, non-coding region variation and pathogenic variation of other genes[28]. These regulators may be missed in PCR-based gene detection, resulting in poor correlation between genotypes and phenotypes[29]. About 25% of the clinically diagnosed gene mutation negative collagen-related myopathy could find a highly repetitive intron mutation in COL6A1 by RNA sequencing[30]. In addition, the comparative genomic hybridization (CGH) technique based on oligonucleotide arrays is also used in studies to detect copy number variations collagen VI-related myopathy and is considered as a useful complementary diagnostic test[29]. This suggests that RNA sequencing and CGH techniques can be used for further research, which may reveal the intrinsic molecular regulatory mechanism of clinical manifestations of collagen VI-related myopathy in the future.
In addition, this case provides new options for the etiology of scoliosis. Scoliosis without myopathy-related signs might be homologous with collagen VI-related myopathy and could even be classified as a new subtype. Researchers believe that the pathogenesis of scoliosis is related to the deep paravertebral muscle activity[31], and find unbalanced bilateral muscle function in scoliosis[32]. Since then, a large number of researchers have found significant changes in the number, types and sizes of muscle fibers in paraspinal muscle biopsies of scoliosis patients, as well as the muscle lesions such as fat infiltration, atrophic necrosis, fibrosis of bundle membrane and endo
In this study, we present a report of collagen VI-related myopathy with scoliosis alone. Caused by heterozygous mutation in COL6A1 and COL6A2, the lesion of this subtype is limited to the paravertebral muscle and the development of vertebral body, suggesting patient might be one of the unidentified subtypes of collagen VI-related myopathy with scoliosis alone. On the other side, for patients with scoliosis, the underlying pathogenesis might be a special type of myopathy or neuromuscular disease, which can be further studied by paraspinal muscle MRI, myopathy-related kinases, electromyography and paraspinal muscle biopsy, providing an important entry point for scoliosis etiology.
| 1. | Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 369] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Shakil H, Iqbal ZA, Al-Ghadir AH. Scoliosis: review of types of curves, etiological theories and conservative treatment. J Back Musculoskelet Rehabil. 2014;27:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Pahys JM, Guille JT. What's New in Congenital Scoliosis? J Pediatr Orthop. 2018;38:e172-e179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Giampietro PF, Blank RD, Raggio CL, Merchant S, Jacobsen FS, Faciszewski T, Shukla SK, Greenlee AR, Reynolds C, Schowalter DB. Congenital and idiopathic scoliosis: clinical and genetic aspects. Clin Med Res. 2003;1:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | McMaster MJ, Ohtsuka K. The natural history of congenital scoliosis. A study of two hundred and fifty-one patients. J Bone Joint Surg Am. 1982;64:1128-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 270] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Fei Q, Wu Z, Wang H, Zhou X, Wang N, Ding Y, Wang Y, Qiu G. The association analysis of TBX6 polymorphism with susceptibility to congenital scoliosis in a Chinese Han population. Spine (Phila Pa 1976). 2010;35:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Sparrow DB, Chapman G, Smith AJ, Mattar MZ, Major JA, O'Reilly VC, Saga Y, Zackai EH, Dormans JP, Alman BA, McGregor L, Kageyama R, Kusumi K, Dunwoodie SL. A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell. 2012;149:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Nagai T, Tsuchiya Y, Maruyama A, Takemitsu M, Nonaka I. Scoliosis associated with central core disease. Brain Dev. 1994;16:150-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Lampe AK, Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005;42:673-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | Kanagawa M, Toda T. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J Hum Genet. 2006;51:915-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Florence JM, Pandya S, King WM, Robison JD, Baty J, Miller JP, Schierbecker J, Signore LC. Intrarater reliability of manual muscle test (Medical Research Council scale) grades in Duchenne's muscular dystrophy. Phys Ther. 1992;72:115-22; discussion 122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 231] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Côté P, Kreitz BG, Cassidy JD, Dzus AK, Martel J. A study of the diagnostic accuracy and reliability of the Scoliometer and Adam's forward bend test. Spine (Phila Pa 1976). 1998;23:796-802; discussion 803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 141] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Meier MP, Klein MP, Krebs D, Grob D, Müntener M. Fiber transformations in multifidus muscle of young patients with idiopathic scoliosis. Spine (Phila Pa 1976). 1997;22:2357-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Bönnemann CG. The collagen VI-related myopathies Ullrich congenital muscular dystrophy and Bethlem myopathy. Handb Clin Neurol. 2011;101:81-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | O'Brien JP, Yau AC, Smith TK, Hodgson AR. Halo pelvic traction. A preliminary report on a method of external skeletal fixation for correcting deformities and maintaining fixation of the spine. J Bone Joint Surg Br. 1971;53:217-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Yurchenco PD, Birk DE, Mecham RP. Extracellular Matrix Assembly and Structure. In: A volume in Biology of Extracellular Matrix. Academic Press, 2013. |
| 17. | Yonekawa T, Nishino I. Ullrich congenital muscular dystrophy: clinicopathological features, natural history and pathomechanism(s). J Neurol Neurosurg Psychiatry. 2015;86:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Bertini E, Pepe G. Collagen type VI and related disorders: Bethlem myopathy and Ullrich scleroatonic muscular dystrophy. Eur J Paediatr Neurol. 2002;6:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Ullrich O. Kongenitale, atonisch-sklerotische Muskeldystrophie, ein weiterer Typus der heredodegenerativen Erkrankungen des neuromuskulären Systems. Zeitschrift für die gesamte Neurologie und Psychiatrie. 1930;126:171-201. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Okada M, Kawahara G, Noguchi S, Sugie K, Murayama K, Nonaka I, Hayashi YK, Nishino I. Primary collagen VI deficiency is the second most common congenital muscular dystrophy in Japan. Neurology. 2007;69:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Ghebranious N, Blank RD, Raggio CL, Staubli J, McPherson E, Ivacic L, Rasmussen K, Jacobsen FS, Faciszewski T, Burmester JK, Pauli RM, Boachie-Adjei O, Glurich I, Giampietro PF. A missense T (Brachyury) mutation contributes to vertebral malformations. J Bone Miner Res. 2008;23:1576-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Gray RS, Wilm TP, Smith J, Bagnat M, Dale RM, Topczewski J, Johnson SL, Solnica-Krezel L. Loss of col8a1a function during zebrafish embryogenesis results in congenital vertebral malformations. Dev Biol. 2014;386:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Pagnon-Minot A, Malbouyres M, Haftek-Terreau Z, Kim HR, Sasaki T, Thisse C, Thisse B, Ingham PW, Ruggiero F, Le Guellec D. Collagen XV, a novel factor in zebrafish notochord differentiation and muscle development. Dev Biol. 2008;316:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Haftek Z, Morvan-Dubois G, Thisse B, Thisse C, Garrone R, Le Guellec D. Sequence and embryonic expression of collagen XVIII NC1 domain (endostatin) in the zebrafish. Gene Expr Patterns. 2003;3:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Al Kaissi A, Ryabykh S, Pavlova OM, Ochirova P, Kenis V, Chehida FB, Ganger R, Grill F, Kircher SG. The Managment of cervical spine abnormalities in children with spondyloepiphyseal dysplasia congenita: Observational study. Medicine (Baltimore). 2019;98:e13780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Ueda Y, Oda Y, Kawashima A, Tsuchiya H, Tomita K, Nakanishi I. Collagenous and basement membrane proteins of chordoma: immunohistochemical analysis. Histopathology. 1992;21:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Kim SY, Kim WJ, Kim H, Choi SA, Lee JS, Cho A, Jang SS, Lim BC, Kim KJ, Kim JI, Hahn SH, Chae JH. Collagen VI-related myopathy: Expanding the clinical and genetic spectrum. Muscle Nerve. 2018;58:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Fan Y, Liu A, Wei C, Yang H, Chang X, Wang S, Yuan Y, Bonnemann C, Wu Q, Wu X, Xiong H. Genetic and clinical findings in a Chinese cohort of patients with collagen VI-related myopathies. Clin Genet. 2018;93:1159-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Bovolenta M, Neri M, Martoni E, Urciuolo A, Sabatelli P, Fabris M, Grumati P, Mercuri E, Bertini E, Merlini L, Bonaldo P, Ferlini A, Gualandi F. Identification of a deep intronic mutation in the COL6A2 gene by a novel custom oligonucleotide CGH array designed to explore allelic and genetic heterogeneity in collagen VI-related myopathies. BMC Med Genet. 2010;11:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Cummings BB, Marshall JL, Tukiainen T, Lek M, Donkervoort S, Foley AR, Bolduc V, Waddell LB, Sandaradura SA, O'Grady GL, Estrella E, Reddy HM, Zhao F, Weisburd B, Karczewski KJ, O'Donnell-Luria AH, Birnbaum D, Sarkozy A, Hu Y, Gonorazky H, Claeys K, Joshi H, Bournazos A, Oates EC, Ghaoui R, Davis MR, Laing NG, Topf A; Genotype-Tissue Expression Consortium; Kang PB, Beggs AH, North KN, Straub V, Dowling JJ, Muntoni F, Clarke NF, Cooper ST, Bönnemann CG, MacArthur DG. Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 540] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 31. | Saatok T, Dahlberg E, Bylund P, Eriksson E, Gustafsson JA. Steroid hormone receptors, protein, and DNA in erector spinae muscle from scoliotic patients. Clin Orthop Relat Res. 1984;197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 32. | Guo X, Chau WW, Hui-Chan CW, Cheung CS, Tsang WW, Cheng JC. Balance control in adolescents with idiopathic scoliosis and disturbed somatosensory function. Spine (Phila Pa 1976). 2006;31:E437-E440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Wajchenberg M, Martins DE, Luciano RP, Puertas EB, Del Curto D, Schmidt B, Oliveira ABS, Faloppa F. Histochemical analysis of paraspinal rotator muscles from patients with adolescent idiopathic scoliosis: a cross-sectional study. Medicine (Baltimore). 2015;94:e598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Luciano Rde P, Puertas EB, Martins DE, Faloppa F, Del Curto D, Rodrigues LM, Schmidt B, de Oliveira AS, Wajchenberg M. Adolescent idiopathic scoliosis without limb weakness: a differential diagnosis of core myopathy? BMC Musculoskelet Disord. 2015;16:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Mannion AF, Meier M, Grob D, Müntener M. Paraspinal muscle fibre type alterations associated with scoliosis: an old problem revisited with new evidence. Eur Spine J. 1998;7:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Dubowitz V. Neuromuscular disorders in childhood. Old dogmas, new concepts. Arch Dis Child. 1975;50:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li X, Naem A, Sillevis R S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yuan YY