Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4627
Peer-review started: March 2, 2021
First decision: April 4, 2021
Revised: April 6, 2021
Accepted: April 26, 2021
Article in press: April 26, 2021
Published online: June 26, 2021
Processing time: 101 Days and 0.7 Hours
The main clinical manifestation of Alzheimer’s disease (AD) is memory loss, which can be accompanied by neuropsychiatric symptoms at different stages of the disease. Amygdala is closely related to emotion and memory.
To evaluate the diagnostic value of amygdala on structural magnetic resonance imaging (sMRI) for AD.
In this study, 22 patients with AD and 26 controls were enrolled. Their amygdala volumes were measured by sMRI and analyzed using an automatic analysis software.
The bilateral amygdala volumes of AD patients were significantly lower than those of the controls and were positively correlated with the hippocampal volumes. Receiver operating characteristic curve analyses showed that the sensitivity of the left and right amygdala volumes in diagnosing AD was 80.8% and 88.5%, respectively. Subgroup analyses showed that amygdala atrophy was more serious in AD patients with neuropsychiatric symptoms, which mainly included irritability (22.73%), sleep difficulties (22.73%), apathy (18.18%), and hallucination (13.64%).
Amygdala volumes measured by sMRI can be used to diagnose AD, and amygdala atrophy is more serious in patients with neuropsychiatric symptoms.
Core Tip: Amygdala volume measured by structural magnetic resonance imaging can be used for the diagnosis of Alzheimer’s disease, and the degree of amygdala atrophy is more severe in patients with neuropsychiatric symptoms.
- Citation: Wang DW, Ding SL, Bian XL, Zhou SY, Yang H, Wang P. Diagnostic value of amygdala volume on structural magnetic resonance imaging in Alzheimer’s disease. World J Clin Cases 2021; 9(18): 4627-4636
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4627.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4627
Amygdala, a part of the medial temporal lobe, is closely related to emotion and memory, and is one of the key brain regions in the pathogenesis of Alzheimer’s disease (AD)[1,2]. Studies have shown that the dysfunction of amygdala plays an important role in the emotional disorder of AD[3,4]. The pathological changes of AD brain such as senile plaque formation, nerve fiber tangle and neuron loss can appear in the amygdala[5-8]. Clinical studies have shown that injury of the amygdala can lead to agitation, irritability, anxiety, and apathy[9-11]. Patients with anxiety disorder, autism, phobia, and post-traumatic stress disorder can have dysfunction of amygdala[12,13]. Medial temporal lobe is the earliest site of brain atrophy in AD[14]. There have been a lot of studies on MRI to evaluate the degree of atrophy of medial temporal lobe, but fewstudies on amygdala[15,16].
In this study, the amygdala volumes were measured by structural magnetic resonance imaging (sMRI) both in AD patients and controls to evaluate the diagnostic value of amygdala. The correlation between amygdala atrophy and clinical features of AD was also analyzed.
Twenty-two patients with AD and twenty-six controls were recruited from the Second Hospital of Shandong University (Shandong, China) between May 2017 and July 2019. The average age of the AD group was 73.9 ± 7.5 years, and the ratio of male to female was 1:1. Among the 22 patients, 4 cases were mild AD, 10 cases were moderate AD, and 8 cases were severe AD (Table 1). The inclusion and exclusion criteria were shown in our published article[17]. The study was approved by the Medical Research Ethics Committee of the Second Hospital of Shandong University and informed consent was obtained from all participants.
All subjects received a cranial MRI scan and underwent neuropsychological tests including the Chinese version of the Mini Mental State examination[18], the Clinical Dementia Rating (CDR) scale (0: No dementia; 1: Mild dementia; 2: Moderate dementia; and 3: Severe dementia)[19], the Hamilton depression scale, and neuropsychiatric inventory (NPI)[20,21]. The NPI scale was used to evaluate the mental and behavioral symptoms of all AD patients and the severity of the symptoms was graded. The scale scores included the total scores of patients and psychological distress scores of caregivers. The former was obtained by multiplying the frequency of mental symptoms by the severity[20,21].
All subjects received three-dimensional brain volume sequence brain MRI scans. T1-weighted image were analyzed using a set of automated analysis tools, and the steps were described in detail in our previous article[17]. The bilateral amygdala volumes were measured both in AD patients and controls.
Categorical variable data are expressed as a frequency and percentage. Numerical data are presented as the mean ± SD or median (interquartile range). Spearman’s rank or Pearson correlation coefficient was used for the correlation analyses. Quantitative values were compared using the Student’s t-test or Mann-Whitney U test. One-way analysis of variance or the Kruskal-Wallis test was used to compare the data among three groups. The χ2 test was used to assess differences in categorical data. Diagnostic values were evaluated by receiver operating characteristic (ROC) curves and the area under the curve (AUC). P < 0.05 was considered for a statistically significant difference. Statistical analyses were performed using SPSS Statistics (version 24.0) and GraphPad Prism version 5.0 software.
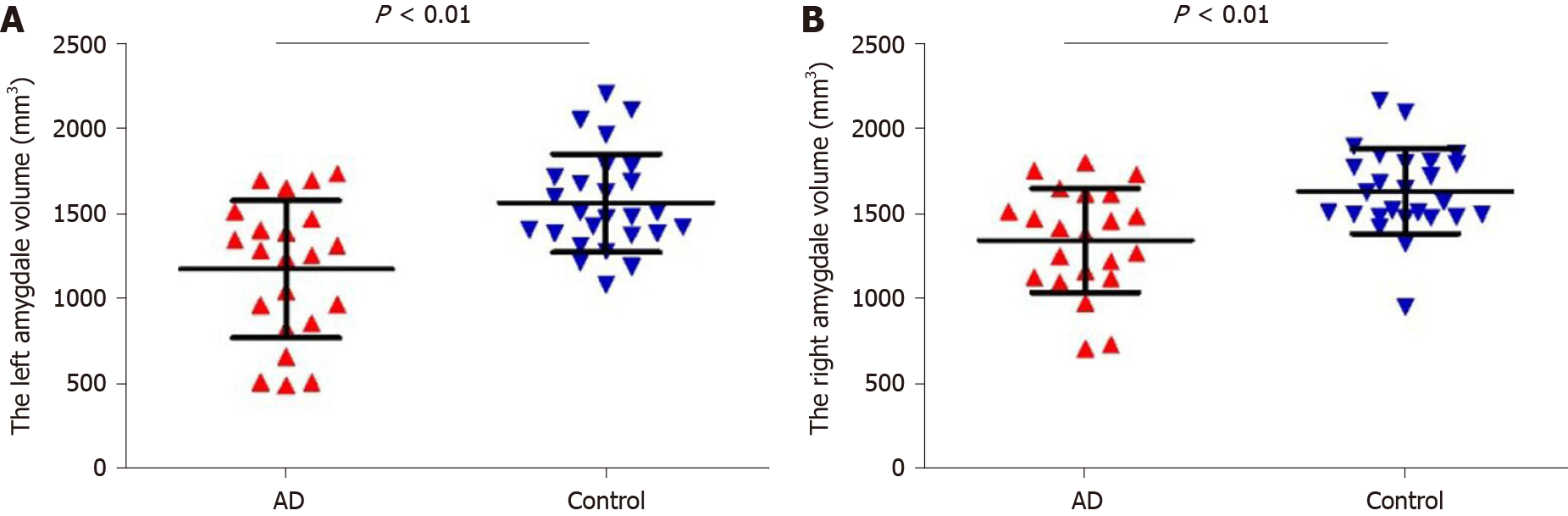
The left amygdala volume of the AD group was 1170.75 ± 402.63 mm3, and that of the control group was 1559.65 ± 289.02 mm3. The right amygdala volume of the AD group was 1342.01 ± 307.68 mm3, and that of the control group was 1629.97 ± 250.24 mm3. Student’s t-test showed that the bilateral amygdala volume in the AD group was significantly smaller than that in the control group (Figure 1, P < 0.01).
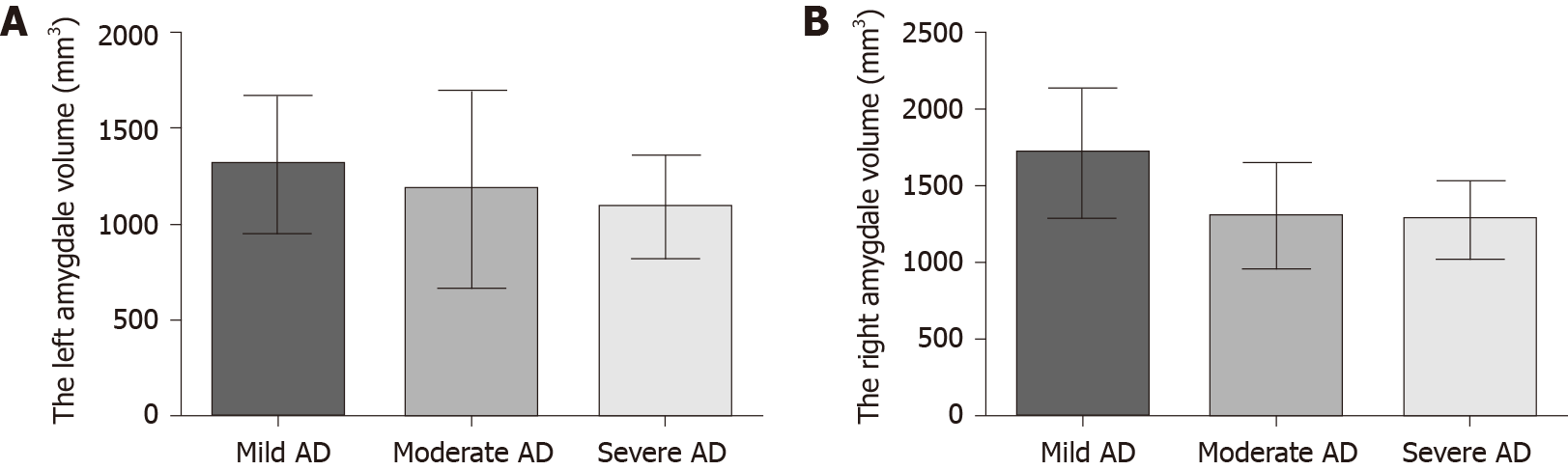
The severity of dementia was graded by CDR score (4 mild AD, 10 moderate AD, 8 severe AD). As shown in Figure 2, the bilateral amygdala volume became smaller with the aggravation of dementia, but there was no significant statistical difference among the three subgroups (Figure 2, P > 0.05).
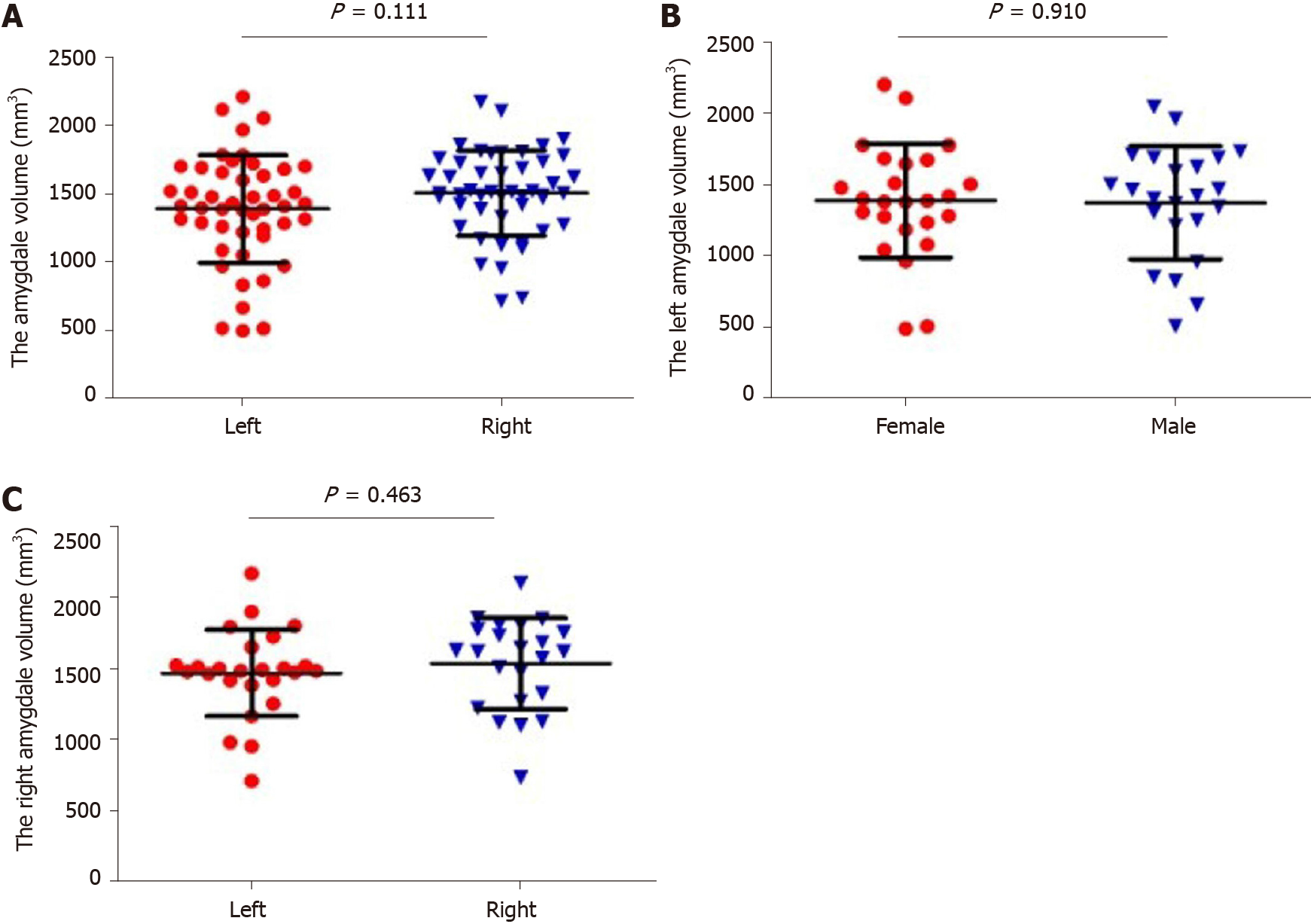
Student’s t-test was used to compare the bilateral volume of the amygdala. There was no significant difference between the left and right amygdala (Figure 3A, P > 0.05). Twenty-five women and twenty-three men were recruited in this study. Student’s t-test was used to observe the difference in amygdala volume between different genders. As shown in Figure 3B and C, there was no significant difference in amygdala volume between the female and male subjects (P > 0.05).
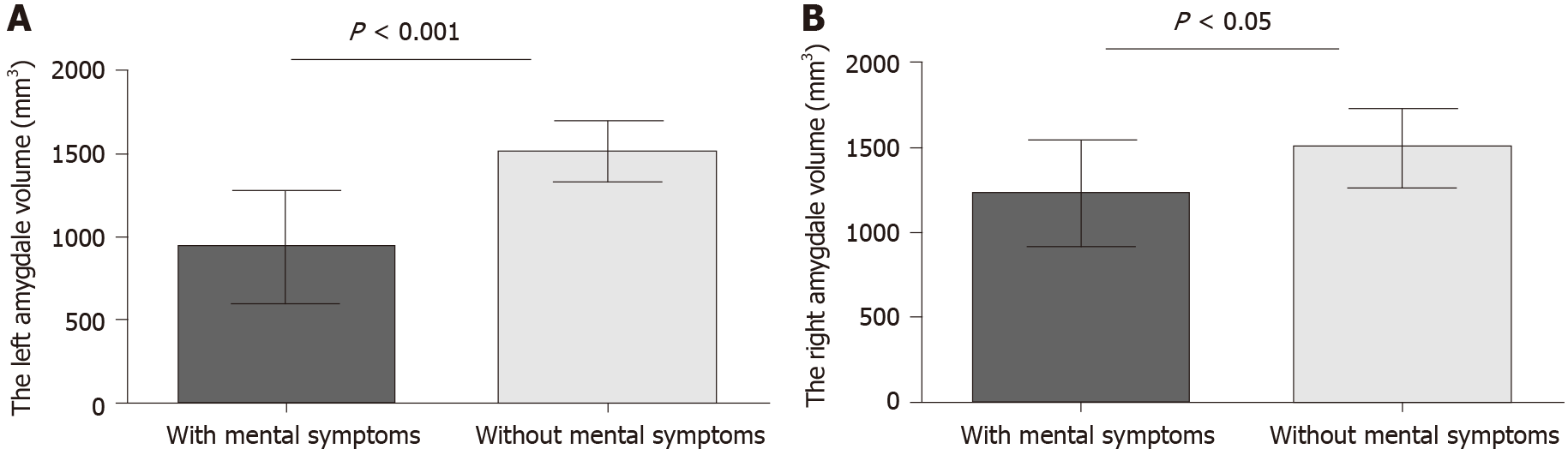
NPI can be used to evaluate the mental symptoms of dementia patients and the psychological distress of caregivers. All AD patients were divided into two groups according to mental symptoms. Thirteen patients had mental symptoms according to NPI scores (Table 2). The volumes of the bilateral amygdala were significantly different between the two groups, and the atrophy of AD patients with mental symptoms was more serious (Figure 4A, P = 0.0002; Figure 4B, P = 0.042).
| Case | NPI score, points | Item score, points | Psychological distress scores of caregivers, points |
| 1 | 27 | Hallucination 6, irritability 12, sleep difficulties 9 | 10 |
| 2 | 24 | Hallucination 12, sleep difficulties 12 | 6 |
| 3 | 14 | Agitation 6, irritable 8 | 6 |
| 4 | 20 | Disinhibition 12, sleep difficulties 8 | 9 |
| 5 | 20 | Anxiety 8, indifference 12 | 6 |
| 6 | 24 | Agitation 12, irritable 12 | 8 |
| 7 | 12 | Hallucination 12 | 5 |
| 8 | 21 | Indifference 12, irritable 9 | 7 |
| 9 | 32 | Delusion 12, aggressive 8, sleep difficulties 12 | 13 |
| 10 | 12 | Depression 12 | 4 |
| 11 | 20 | Delusion 12, irritable 8 | 9 |
| 12 | 18 | Indifference 12, sleep difficulties 6 | 8 |
| 13 | 12 | Indifference 12 | 4 |
As shown in Table 3, 59.1% (13/22) of the AD patients had at least one kind of neuropsychiatric symptom, the most common of which was irritability (22.73%), sleep difficulties (22.73%), apathy (18.18%), and hallucination (13.64%). For mild AD (n = 4), one patient (25%) had mental symptoms, manifested as irritability and agitation. Sixty percent (6/10) of moderate AD patients showed abnormal mental behavior, of which irritability and delusion were the most common. Seventy-five percent (6/8) of severe patients had neuropsychiatric symptom, and irritability and delusion were the most common.
| Symptoms | Total cases, n = 22 | Mild AD, n = 4 | Moderate AD, n = 10 | Severe AD, n = 8 |
| Irritability | 5 (22.73) | 1 (25) | 2 (20) | 2 (25) |
| Sleep difficulties | 5 (22.73) | 0 | 4 (40) | 1 (12.5) |
| Indifference | 4 (18.18) | 0 | 3 (30) | 1 (12.5) |
| Hallucination | 3 (13.64) | 0 | 2 (20) | 1 (12.5) |
| Agitation | 2 (9.09) | 1 (25) | 1 (10) | 0 |
| Delusion | 2 (9.09) | 0 | 0 | 2 (25) |
| Disinhibition | 1 (4.55) | 0 | 0 | 1 (12.5) |
| Anxiety | 1 (4.55) | 0 | 1 (10) | 0 |
| Aggressive | 1 (4.55) | 0 | 0 | 1 (12.5) |
| Depression | 1 (4.55) | 0 | 0 | 1 (12.5) |
| At least one of the above symptoms | 13 (59.1) | 1 (25) | 6 (60) | 6 (75) |
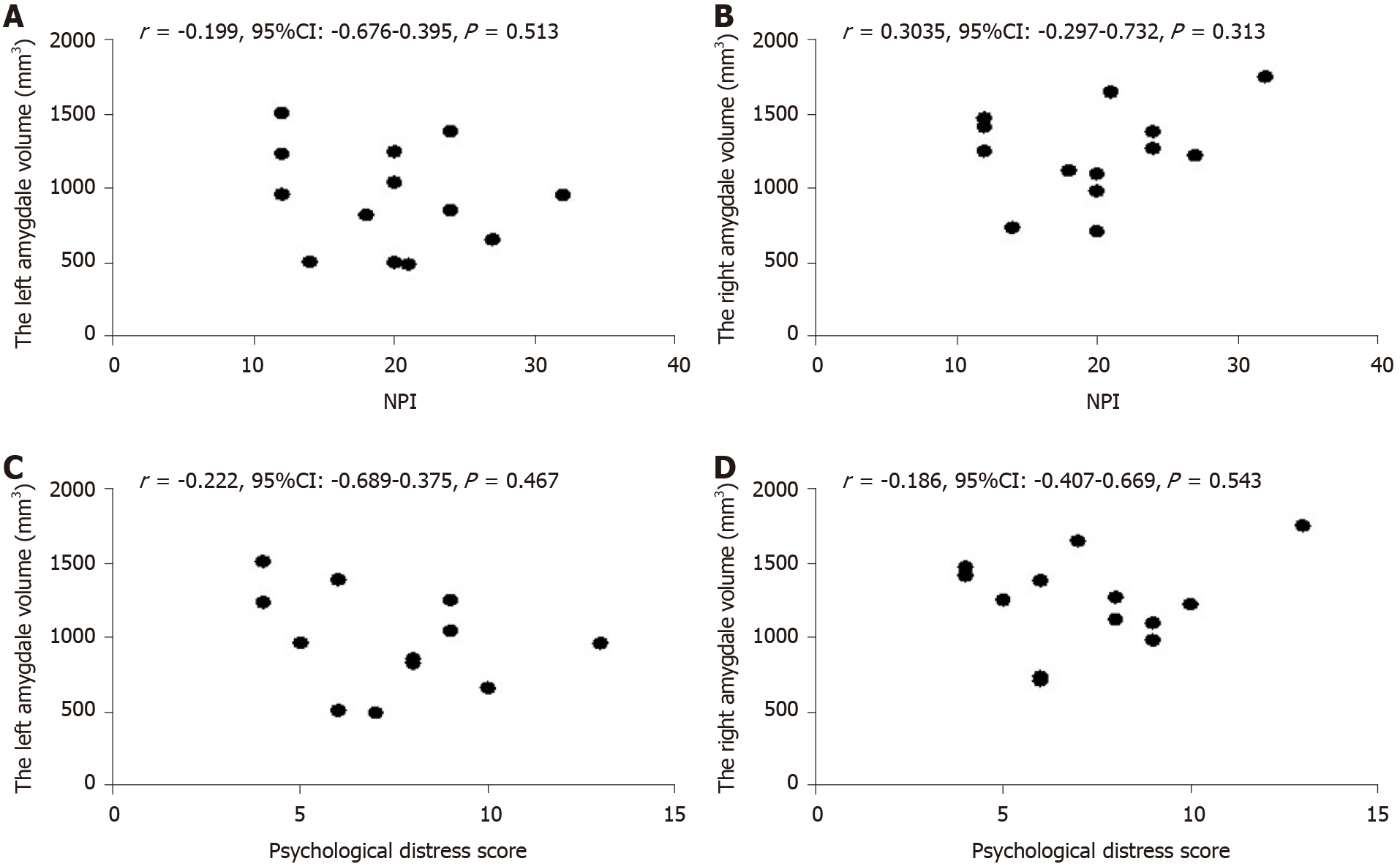
Pearson correlation analysis showed that there was no significant correlation between NPI scores and amygdale volumes (Figure 5A and B, P > 0.05). In addition, there was no correlation between the psychological distress scores of caregivers and amygdala volumes (Figure 5C and D, P > 0.05).
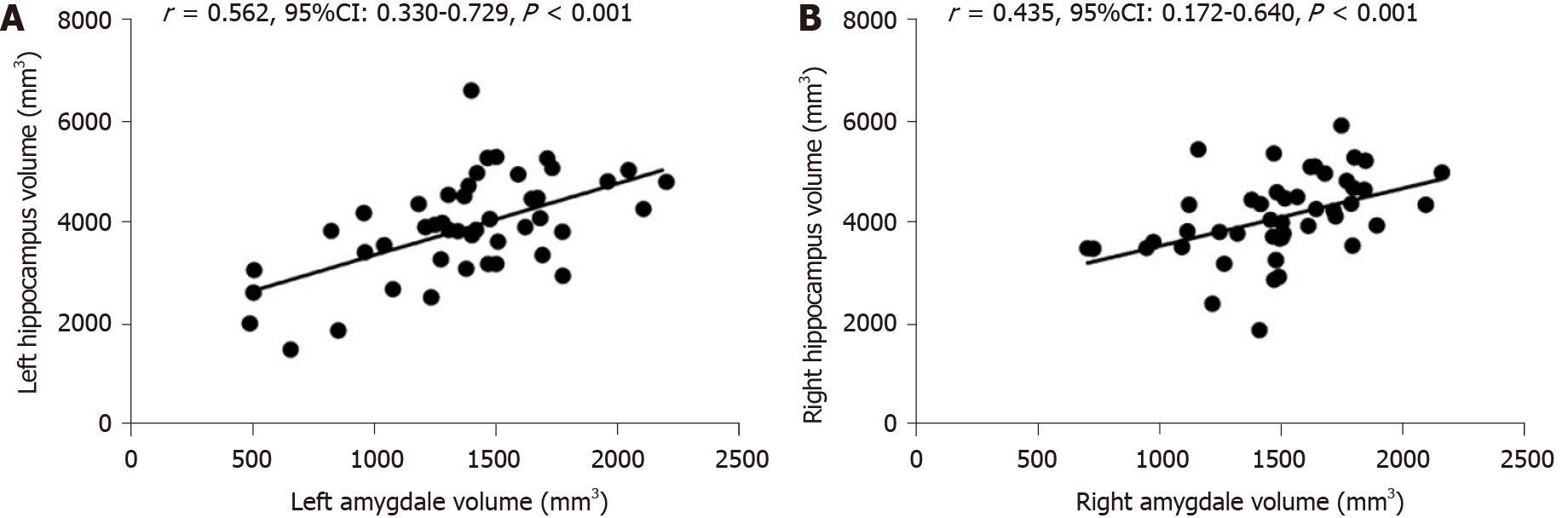
Hippocampal atrophy is a characteristic imaging manifestation of AD, the amygdala attaches to its end, both of them are involved in the pathogenesis of AD. Our previous study measured the volume of hippocampus in all subjects[17]. To further evaluate the relationship between the two brain regions, we analyzed the correlation between the volume of the amygdala and hippocampus.
Pearson correlation analysis showed that there was a significant correlation between left amygdala and hippocampal volumes (Figure 6A, r = 0.562, 95%CI: 0.330-0.729, P < 0.001). As shown in Figure 6B, there was a significant correlation between the right amygdala volume and hippocampal volume (r = 0.435, 95%CI: 0.172-0.640, P < 0.001).
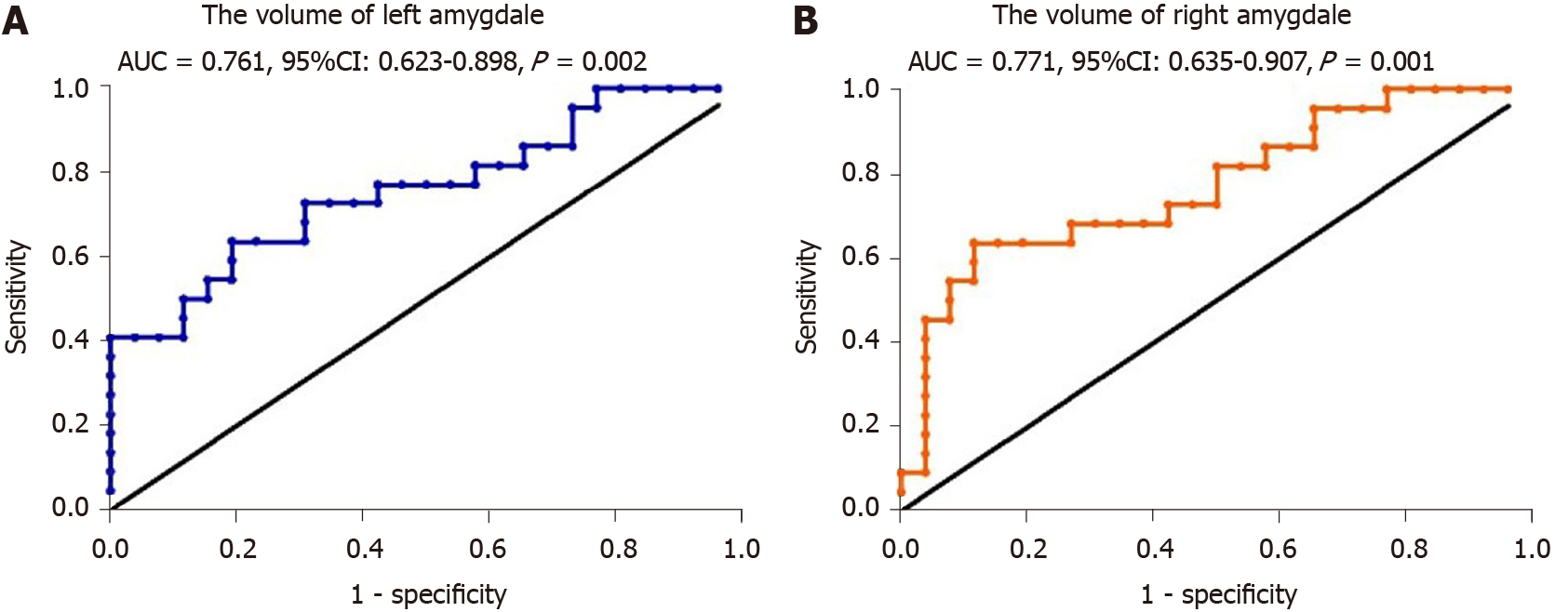
To evaluate the potential of amygdala volume serving as a novel biomarker for AD, ROC curves were plotted. The AUC was 0.761 for the left amygdala volume, the sensitivity was 80.8%, the specificity was 63.6%, and the cutoff point was 1358 (Figure 7A, P = 0.002). The AUC was 0.771 for the right amygdala volume, the sensitivity was 88.5%, the specificity was 63.6% and the cutoff point was 1472 (Figure 7B, P = 0.001).
In this study, the amygdala volumes were measured by sMRI and analyzed using a set of automated analysis tools to evaluate the diagnostic value of amygdala for AD. The results showed that the bilateral amygdala volumes of AD patients were significantly smaller than those of the controls, and were positively correlated with the hippocampal volumes, consistent with previous reports[22,23].
Poulin et al[16] found that amygdala atrophy was obvious in early AD and was related to the severity of dementia. Yue et al[15] reported that volume of the right amygdala and hippocampus in patients with subjective cognitive decline. However, none of the previous studies evaluated the diagnostic value of amygdala, and there were few studies on the correlation between amygdala atrophy and clinical characteristics. Different from previous studies, we found that the sensitivity of the left and right amygdala volumes in diagnosing AD were 80.8% and 88.5%, respectively, and the cut-off value of the amygdala volume for diagnosis of AD was obtained. Amygdala volume measured by sMRI may be used as a potential imaging marker for the diagnosis of AD. One previous study showed that the degree of amygdala atrophy is correlated with the severity of the dementia[16,24]. We found that the volume of bilateral amygdala became smaller with the aggravation of dementia through subgroup analysis, but there was no significant statistical difference. The reason may be that the sample size of this study was too small.
The atrophy of the amygdala was more serious in AD patients with mental symptoms in this study, consistent with previous studies[16]. About 80%-90% of patients with AD experience at least one kind of psychobehavioral symptoms, such as anxiety, agitation, irritability, aggression, disinhibition, depression, apathy, which can reduce the quality of life of patients and increase the burden of care[25-28]. The amygdala is attached to the terminal part of the hippocampus and is associated with multiple brain regions to regulate multiple stages of memory formation[1,2]. Some misfolded proteins such as Tau, Aβ, α-synuclein, and TAR DNA-binding protein 43 are present in the amygdala in neurodegenerative diseases[29]. The decreased functional connectivity among the amygdala, inferior frontal gyrus, and medial prefrontal cortex plays an important role in the development of depression in AD patients[30,31]. Previous research has shown that dysfunction of the amygdala plays an important role in the emotional disorder of AD[6-8]. The injury of amygdala can lead to agitation, irritability, anxiety, and apathy[9,10]. Other studies have indicated that patients with anxiety disorder, autism, phobia, and post-traumatic stress disorder had dysfunction of amygdala[12,13].
NPI was used to evaluate the severity of mental symptoms of dementia patients and the psychological distress of caregivers in this study. Tanaka et al[32] reported a high frequency of apathy, depression, anxiety, and irritability in AD patients. Different from previous studies, we found that 59.1% of AD patients had neuropsychiatric symptoms, the most common of which were irritability, sleep difficulties, apathy, and hallucination. The reason may be that the patients recruited in this study were mostly mild to moderate dementia, leading to a low proportion of mental symptoms. This study also showed that the more serious dementia, the higher the proportion of patients with mental symptoms. This is consistent with previous studies[26].
There were some limitations in this study. Amygdala atrophy has also been seen in other neurodegenerative diseases, such as dementia with Lewy bodies and frontotemporal dementia[33,34]. It is necessary to compare the amygdala volume in other types of dementia in future in order to improve the specificity of amygdala in the diagnosis of AD.
Anyway, the amygdala volumes measured by sMRI can be used for diagnosing of AD and amygdala atrophy was more serious in those with neuropsychiatric symptoms.
Amygdala is closely related to emotion and memory. The dysfunction of amygdala plays an important role in the emotional disorder of Alzheimer’s disease (AD). There are few studies evaluating the degree of atrophy of amygdala measured by structural magnetic resonance imaging (sMRI).
The purpose of this study is to evaluate the diagnostic value of amygdala on sMRI for AD.
In this study, the amygdala volumes were measured by sMRI both in AD patients and controls to evaluate the diagnostic value of amygdala. The correlation between amygdala atrophy and clinical features of AD was also analyzed.
Twenty-two AD patients and twenty-six controls were enrolled in this study. Their amygdala volumes were measured and analyzed using a set of image analysis software. All subjects received a cranial MRI scan and underwent neuropsychological tests. The mental and behavioral symptoms of all AD patients and the severity of the symptoms was graded.
The bilateral amygdala volumes of AD patients were significantly lower than those of the controls. The sensitivity of the left and right amygdala volumes in diagnosing AD was 80.8% and 88.5%, respectively. The amygdala atrophy was more serious in AD patients with neuropsychiatric symptoms including irritability, sleep difficulties, apathy and hallucination.
In this study, we found that the bilateral amygdala volumes of AD patients were significantly smaller than those of the controls, and were positively correlated with the hippocampal volumes, consistent with previous reports. In addition, the atrophy of the amygdala was more serious in those with neuropsychiatric symptoms. This study confirmed that the amygdala is involved in the mental symptoms of AD.
It is necessary to compare the amygdala volume in other types of dementia in the future, in order to improve the specificity of amygdala for the diagnosis of AD.
| 1. | LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1022] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 2. | Hermans EJ, Battaglia FP, Atsak P, de Voogd LD, Fernández G, Roozendaal B. How the amygdala affects emotional memory by altering brain network properties. Neurobiol Learn Mem. 2014;112:2-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 889] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 4. | Hamann S. Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci. 2001;5:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 578] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Herzog AG, Kemper TL. Amygdaloid changes in aging and dementia. Arch Neurol. 1980;37:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 187] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Tsuchiya K, Kosaka K. Neuropathological study of the amygdala in presenile Alzheimer's disease. J Neurol Sci. 1990;100:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Scott SA, DeKosky ST, Scheff SW. Volumetric atrophy of the amygdala in Alzheimer's disease: quantitative serial reconstruction. Neurology. 1991;41:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Scott SA, DeKosky ST, Sparks DL, Knox CA, Scheff SW. Amygdala cell loss and atrophy in Alzheimer's disease. Ann Neurol. 1992;32:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 708] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 10. | Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer's disease. Biol Psychiatry. 2007;62:1388-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Kile SJ, Ellis WG, Olichney JM, Farias S, DeCarli C. Alzheimer abnormalities of the amygdala with Klüver-Bucy syndrome symptoms: an amygdaloid variant of Alzheimer disease. Arch Neurol. 2009;66:125-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev. 2015;57:271-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Nikolenko VN, Oganesyan MV, Rizaeva NA, Kudryashova VA, Nikitina AT, Pavliv MP, Shchedrina MA, Giller DB, Buligin KV, Sinelnikov MY. Amygdala: Neuroanatomical and Morphophysiological Features in Terms of Neurological and Neurodegenerative Diseases. Brain Sci. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12014] [Cited by in RCA: 11600] [Article Influence: 773.3] [Reference Citation Analysis (0)] |
| 15. | Yue L, Wang T, Wang J, Li G, Li X, Li W, Hu M, Xiao S. Asymmetry of Hippocampus and Amygdala Defect in Subjective Cognitive Decline Among the Community Dwelling Chinese. Front Psychiatry. 2018;9:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC; Alzheimer's Disease Neuroimaging Initiative. Amygdala atrophy is prominent in early Alzheimer's disease and relates to symptom severity. Psychiatry Res. 2011;194:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 17. | Wang D, Wang P, Bian X, Xu S, Zhou Q, Zhang Y, Ding M, Han M, Huang L, Bi J, Jia Y, Xie Z. Elevated plasma levels of exosomal BACE1AS combined with the volume and thickness of the right entorhinal cortex may serve as a biomarker for the detection of Alzheimer's disease. Mol Med Rep. 2020;22:227-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Yang Z, Holt HK, Fan JH, Ma L, Liu Y, Chen W, Como P, Zhang L, Qiao YL. Optimal Cutoff Scores for Alzheimer's Disease Using the Chinese Version of Mini-Mental State Examination Among Chinese Population Living in Rural Areas. Am J Alzheimers Dis Other Demen. 2016;31:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412-2414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5789] [Cited by in RCA: 7465] [Article Influence: 226.2] [Reference Citation Analysis (0)] |
| 20. | Tafazzoli A, Kansal A, Lockwood P, Petrie C, Barsdorf A. The Economic Impact of New Therapeutic Interventions on Neuropsychiatric Inventory (NPI) Symptom Scores in Patients with Alzheimer Disease. Dement Geriatr Cogn Dis Extra. 2018;8:158-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Hachimori A. [Neuropsychiatric Inventory (NPI)]. Nihon Rinsho. 2011;69 Suppl 8:439-442. [PubMed] |
| 22. | Lehtovirta M, Laakso MP, Soininen H, Helisalmi S, Mannermaa A, Helkala EL, Partanen K, Ryynänen M, Vainio P, Hartikainen P. Volumes of hippocampus, amygdala and frontal lobe in Alzheimer patients with different apolipoprotein E genotypes. Neuroscience. 1995;67:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Cavedo E, Boccardi M, Ganzola R, Canu E, Beltramello A, Caltagirone C, Thompson PM, Frisoni GB. Local amygdala structural differences with 3T MRI in patients with Alzheimer disease. Neurology. 2011;76:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | LeDoux J. The amygdala. Curr Biol. 2007;17:R868-R874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 986] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 25. | Mao Y, Fisher DW, Yang S, Keszycki RM, Dong H. Protein-protein interactions underlying the behavioral and psychological symptoms of dementia (BPSD) and Alzheimer's disease. PLoS One. 2020;15:e0226021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | van der Linde RM, Dening T, Matthews FE, Brayne C. Grouping of behavioural and psychological symptoms of dementia. Int J Geriatr Psychiatry. 2014;29:562-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Kales HC, Lyketsos CG, Miller EM, Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer's disease: an international Delphi consensus. Int Psychogeriatr. 2019;31:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 28. | Baquero M, Martín N. Depressive symptoms in neurodegenerative diseases. World J Clin Cases. 2015;3:682-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (9)] |
| 29. | Nelson PT, Abner EL, Patel E, Anderson S, Wilcock DM, Kryscio RJ, Van Eldik LJ, Jicha GA, Gal Z, Nelson RS, Nelson BG, Gal J, Azam MT, Fardo DW, Cykowski MD. The Amygdala as a Locus of Pathologic Misfolding in Neurodegenerative Diseases. J Neuropathol Exp Neurol. 2018;77:2-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 914] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 31. | Hu X, Song X, Yuan Y, Li E, Liu J, Liu W, Liu Y. Abnormal functional connectivity of the amygdala is associated with depression in Parkinson's disease. Mov Disord. 2015;30:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Tanaka H, Hashimoto M, Fukuhara R, Ishikawa T, Yatabe Y, Kaneda K, Yuuki S, Honda K, Matsuzaki S, Tsuyuguchi A, Hatada Y, Ikeda M. Relationship between dementia severity and behavioural and psychological symptoms in early-onset Alzheimer's disease. Psychogeriatrics. 2015;15:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Horínek D, Varjassyová A, Hort J. Magnetic resonance analysis of amygdalar volume in Alzheimer's disease. Curr Opin Psychiatry. 2007;20:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Barber R, Ballard C, McKeith IG, Gholkar A, O'Brien JT. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology. 2000;54:1304-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Clinical Neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rakhya P S-Editor: Wang JL L-Editor: Filipodia P-Editor: Liu JH