Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2357
Peer-review started: November 11, 2020
First decision: December 21, 2020
Revised: January 2, 2021
Accepted: January 27, 2021
Article in press: January 27, 2021
Published online: April 6, 2021
Processing time: 138 Days and 16.3 Hours
Proliferative glomerulonephritis with monoclonal immunoglobulin G (IgG) deposits (PGNMID) is a newly recognized rare disease. The renal pathology is characterized by prominent manifestations of membranous hyperplasia, which are easy to misdiagnose. The clinical symptoms are severe. Massive proteinuria and hypoproteinemia are conspicuous, and most patients are accompanied by renal insufficiency and microscopic hematuria.
A 27-year-old woman was admitted to a hospital for macroscopic hematuria and proteinuria 4 years prior, and renal biopsy in the hospital suggested moderate-to-severe mesangial proliferating glomerulonephritis (MsPGN). She had taken a glucocorticoid, cyclophosphamide, mycophenolate mofetil, and other treatments and achieved brief partial remission. Recently, the patient visited our hospital due to massive proteinuria. Repeated renal biopsy and re-evaluation of the first biopsy obtained 4 years previously revealed monoclonal immunoglobulin deposition in the glomeruli. A bone marrow examination was performed to exclude hematologic malignancy, and a diagnosis of PGNMID was established. The patient showed remission after four cycles of a bortezomib + cyclophosphamide + dexamethasone scheme.
PGNMID is usually misdiagnosed as MsPGN or membranoproliferative glomerulonephritis. Although it often occurs in middle-aged and elderly individuals, it cannot be readily excluded in young people, even when serum immunofixation electrophoresis is negative. IgG subtype and light chain staining are necessary when this disease is highly suspected. An accurate diagnosis at the earliest stage may avoid the overuse of glucocorticoids and immunosuppressants.
Core Tip: Proliferative glomerulonephritis with monoclonal immunoglobulin G deposits (PGNMID) is a rare renal disease whose pathogenesis is not fully understood. Due to its complexity, this disease is easy to misdiagnose. We report the case of a young female patient with gross hematuria and foamy urine. Renal biopsy in another hospital suggested mesangial proliferating glomerulonephritis (MsPGN). Her condition did not improve significantly after treatment with glucocorticoids and immuno-suppressants. Recently, repeated renal biopsy at our hospital suggested PGNMID. This case suggests that although PGNMID often occurs in middle-aged and elderly individuals, it cannot be readily excluded in young people with the pathological type of membranoproliferative glomerulonephritis or MsPGN, even when immunofixation electrophoresis is negative. An accurate diagnosis at the earliest stage may minimize the use of glucocorticoids and immunosuppressants.
- Citation: Xu ZG, Li WL, Wang X, Zhang SY, Zhang YW, Wei X, Li CD, Zeng P, Luan SD. Proliferative glomerulonephritis with monoclonal immunoglobulin G deposits in a young woman: A case report. World J Clin Cases 2021; 9(10): 2357-2366
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2357.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2357
Proliferative glomerulonephritis with monoclonal immunoglobulin G (IgG) deposits (PGNMID), first reported by Nasr et al[1] in 2004, is a newly recognized disease characterized by glomerular monoclonal IgG deposition, and histopathological manifestations of renal biopsy show glomerular proliferative lesions. A total of 56.8% of PGNMID patients have been diagnosed with membranoproliferative glomerulo-nephritis (MPGN) based on light microscopy, with prominent manifestations of membranous hyperplasia[2]. Due to its complexity, the pathogenesis of PGNMID is not fully understood.
The disease is commonly observed in middle-aged and elderly people, among whom 65% are over 50 years old[2]. The incidence of PGNMID diagnosis by autologous kidney biopsy is approximately 0.07%-0.17%[2,3]. The main clinical manifestations of PGNMID include proteinuria, hematuria, and renal insufficiency.
We report a young female patient who was diagnosed with mesangial proliferating mesangial proliferating glomerulonephritis (MsPGN) due to proteinuria in another hospital, with poor therapeutic effects. She was recently admitted to our hospital, and repeated renal biopsy suggested PGNMID.
A 27-year-old Chinese woman with hematuria and albuminuria for over 4 years was admitted to our nephrology department on December 15, 2019
After 1 mo of gross hematuria and frothy urine, the patient visited her local hospital for treatment in June 2015. Laboratory examinations showed the following: 24 h urine protein (UP) 3.09 g/d; serum albumin, 24.6 g/L; serum creatinine (Scr), 120 μmoL/L; immunofixation electrophoresis (IFE), serum protein electrophoresis (SPEP), and autoimmune markers, including anti-neutrophil cytoplasmic antibody, antinuclear antibody, anti-double stranded DNA, and anti-glomerular basement membrane antibody, that were negative. Histological examination of the renal biopsy at that time revealed moderate-to-severe MsPGN with crescent formation. The patient was given methylprednisolone injections (500 mg/d, 3 d in total, then 40 mg/d). A few days later, she was discharged, stopped taking the drugs on her own and then went to a private clinic in Hong Kong for further treatment. She had taken prednisone and cyclophosphamide (CTX) for 3 mo; CTX was then replaced by mycophenolate mofetil (MMF) (the doses are unknown). This treatment was applied for 2 years, after which MMF was substituted with tacrolimus. The dosages were gradually reduced and then withdrawn on August 28, 2018, as prescribed. During this time, her 24 h UP fluctuated between 2 and 3 g/d, and her Scr level was in the 80 to 110 μmoL/L range. The nephritic syndrome relapsed in January 2019, and the previous specialist treated her with prednisone (500 mg/d, 3 d in total) and CTX (100 mg/d). CTX was replaced by MMF (1 g/d) 1 mo later because of severe hair loss and irregular menstruation. Prednisone was reduced gradually and then withdrawn completely in October 2019. The patient underwent a checkup on December 15, 2019, and her laboratory data were as follows: Scr, 92 μmoL/L; serum albumin, 38.3 g/L; UP, 3+; urine occult blood, 3+.
The patient had no diagnosed history of metabolic disease, coronary heart disease, or liver disease.
Slight pitting edema was noted in both lower extremities. Other physical features included a mild anemic appearance. No swelling of surface lymph nodes was found.
On admission, laboratory data revealed the following values: Hemoglobin (Hb), 71 g/L; Scr, 94 μmoL/L; serum uric acid, 528 μmoL/L; serum albumin, 24.6 g/L; globulin, 19.7 g/L; calcium, 1.97 mmoL/L; complement C3, 0.84 g/L; C4 0.19 g/L; UP, 2+; urine occult blood, 2+; 24 h UP, 1.1 g/d. Liver function was normal. We detected no anti-DNA, anti-glomerular basement membrane, or anti-neutrophil cytoplasmic antibodies and no hepatitis B virus, hepatitis C virus (HCV), or human immunodeficiency virus. IFE and SPEP did not show any paraproteins, although the urine free light chain ratio (κ/λ) was as high as 6.7974. Computed tomography of the chest, abdomen, and pelvis detected no enlarged lymph nodes.
No obvious abnormality.
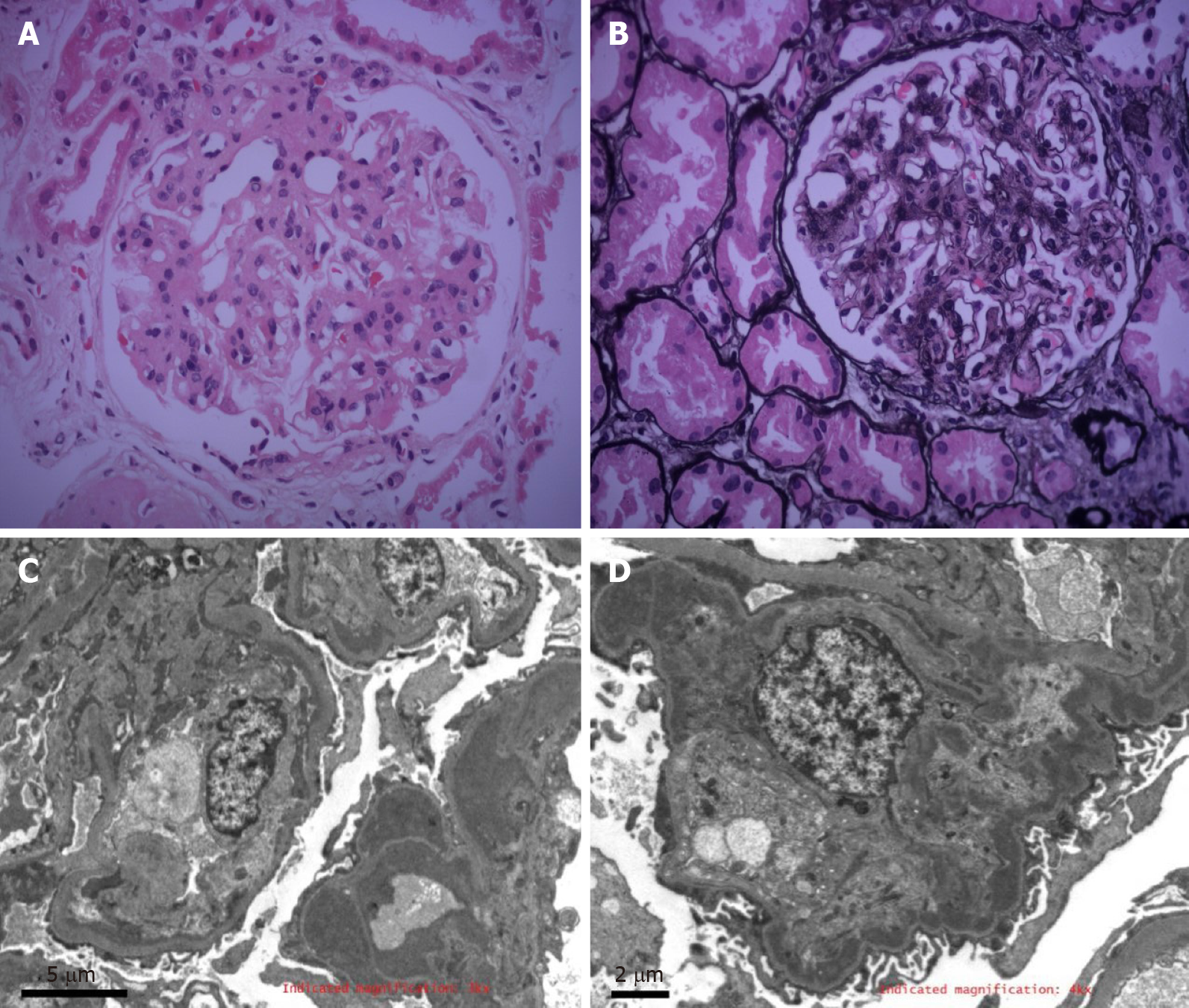
The histological examination of the renal biopsy showed 27 glomeruli, 11 of which were globally sclerotic and 3 of which were segmentally sclerotic; no crescent was found. Marked mesangial cells and matrix proliferation were observed in the remaining glomeruli, with glomerular lobulation and focal endothelial hyperplasia. Segmental basement membrane thickening, mesangial interposition, and tram track signs were visible. Endocapillary proliferative changes were noted by electron microscopy. The glomerular capillary loops were compressed, and the lumen was stenotic. Segmental mesangial matrix interposition was found. Furthermore, electron-dense deposits were observed in the subendothelial and mesangial areas; at the ultrastructural level, the deposits were not fibrillary or microtubular structures. Part of the interstitial region was infiltrated by inflammatory cells (Figure 1).
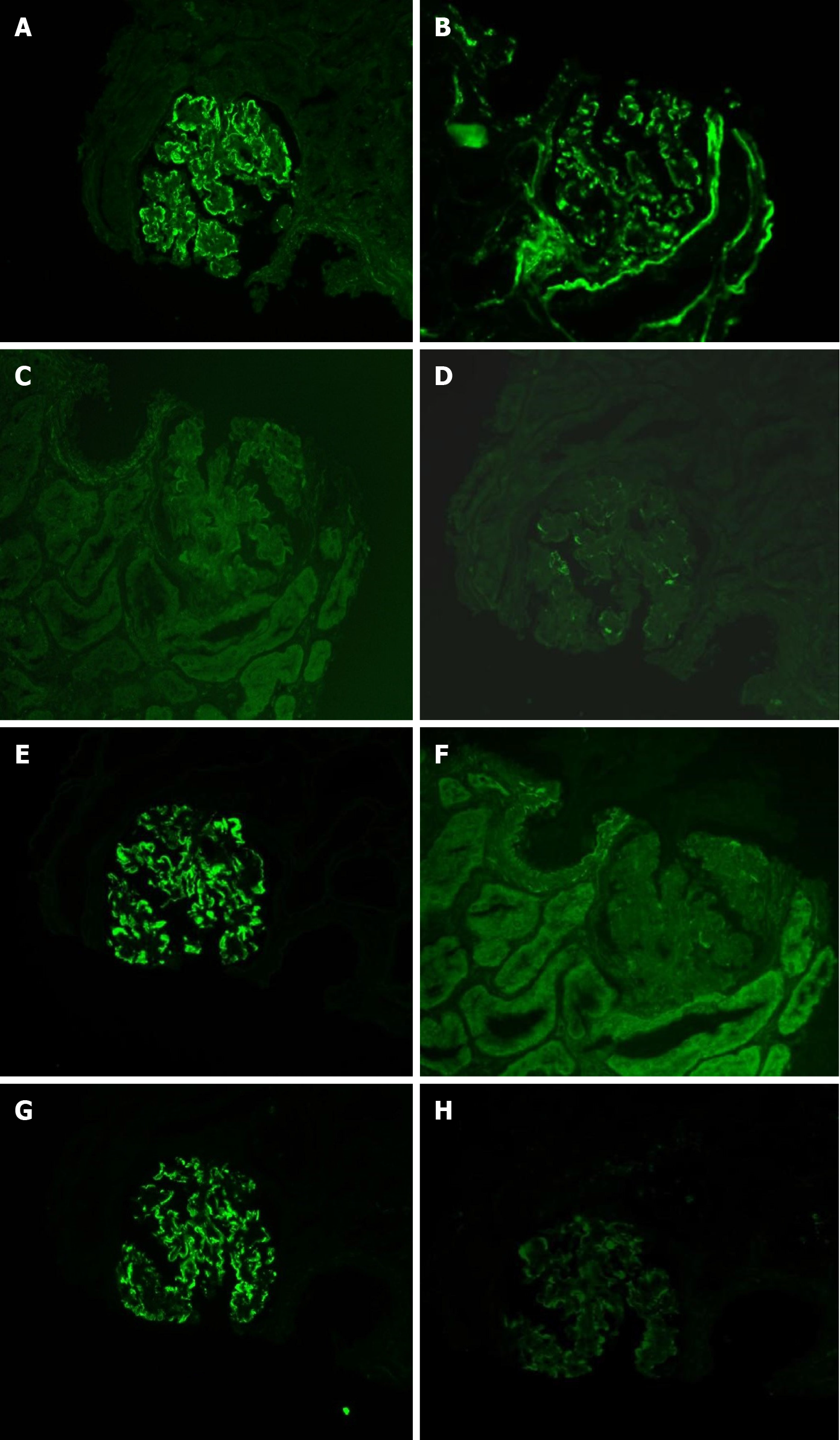
Immunohistology revealed positive petaloid deposition of IgG (3+), C3 (2+), IgM (1+), and C1q (+/-) along the capillary loops; IgA and fibrinogen (Fib) were negative. According to additional tests, IgG3 (3+), Kappa (3+), Lambda (1+), IgG1, IgG2, IgG4, phospholipase A2 receptor, thrombospondin type-1 domain-containing 7A, ascorbic acid, HCV, hepatitis B surface antigen, hepatitis B e antigen, hepatitis B core antigen, and Congo red and oxide Congo red staining were positive (Figure 2).
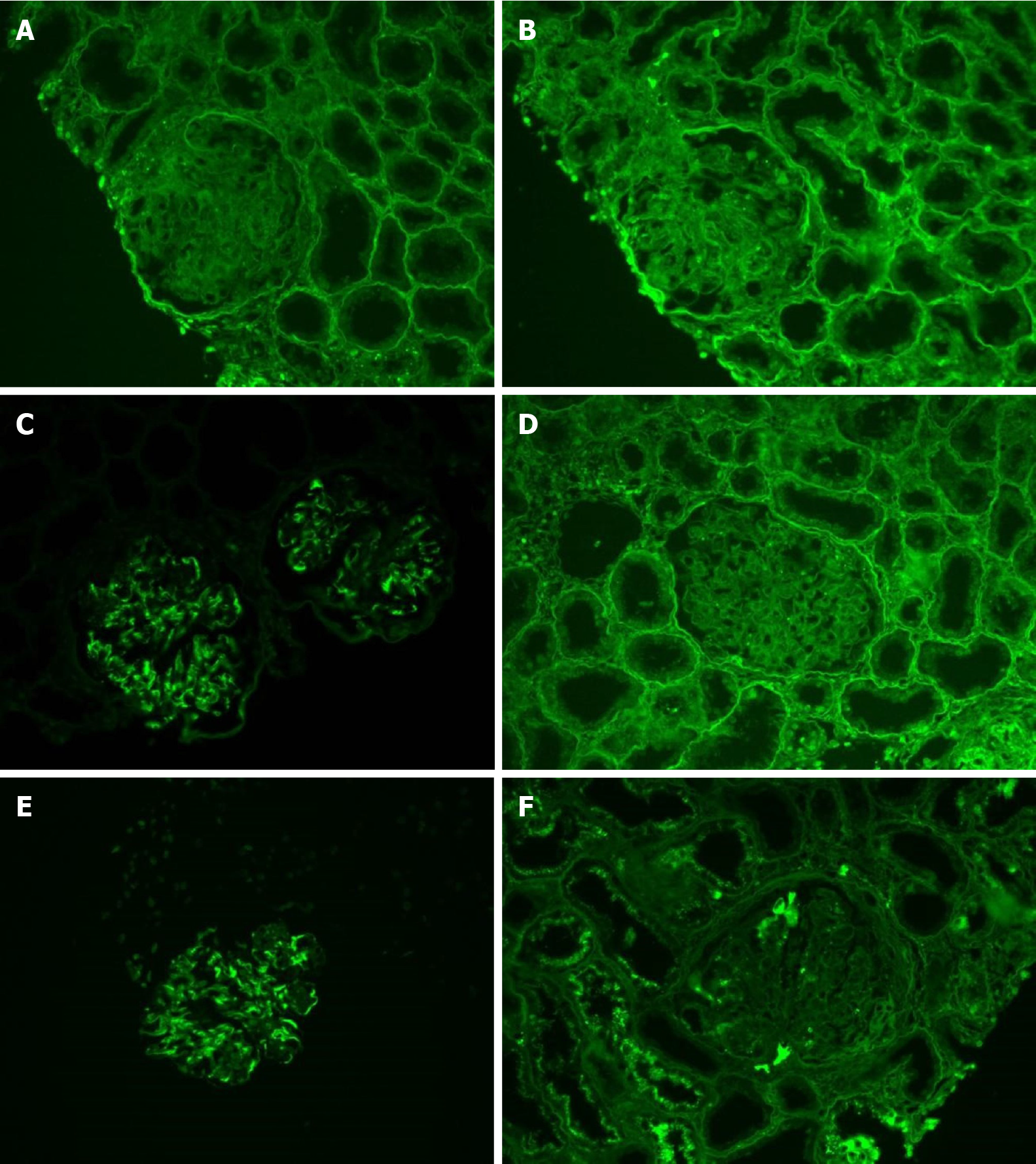
The clinicians who previously treated this patient kindly provided the glass slides of her first renal biopsy specimens. To determine the nature of the deposits, cryosections were freshly prepared for light chain and IgG subclass staining, and subsequent immunofluorescence analyses of these samples confirmed monoclonal deposition of IgG3κ in the glomeruli (Figure 3).
Bone marrow smears showed hyperplasia of the granulocyte series, erythron series, and megakaryocytic series. Biopsy revealed active bone marrow proliferation, without any obvious mutant cells. A bone marrow flow cytometry immunofluorescence assay indicated no immunophenotypic abnormal evidence of multiple myeloma, acute leukemia, plasma cells, non-Hodgkin's lymphoma, or high-risk myelodysplastic syndrome. Multiple myeloma-associated gene mutation analysis, karyotype analysis of bone marrow chromosomes, and fluorescence in situ hybridization, including Vysis TP53/CEP17, cytocell RB1(13q14), Vysis IGH, and cytocell CKS1B/CDKN2C(P18), were all negative.
The patient was diagnosed with PGNMID in accordance with the monoclonal pattern of IgG3κ deposition found in both the first and second renal biopsy specimens.
We corrected the previous diagnosis result to PGNMID and immediately initiated four cycles of a bortezomib (B) + cyclophosphamide (C) + dexamethasone (D) (BCD) scheme within 5 mo. The specific formula was as follows: B (1.3 mg/m2) days 1, 4, 8, and 11 + C (0.3 g) days 1-4 + D (20 mg) days 1, 2, 4, 5, 8, 9, 11, and 12.
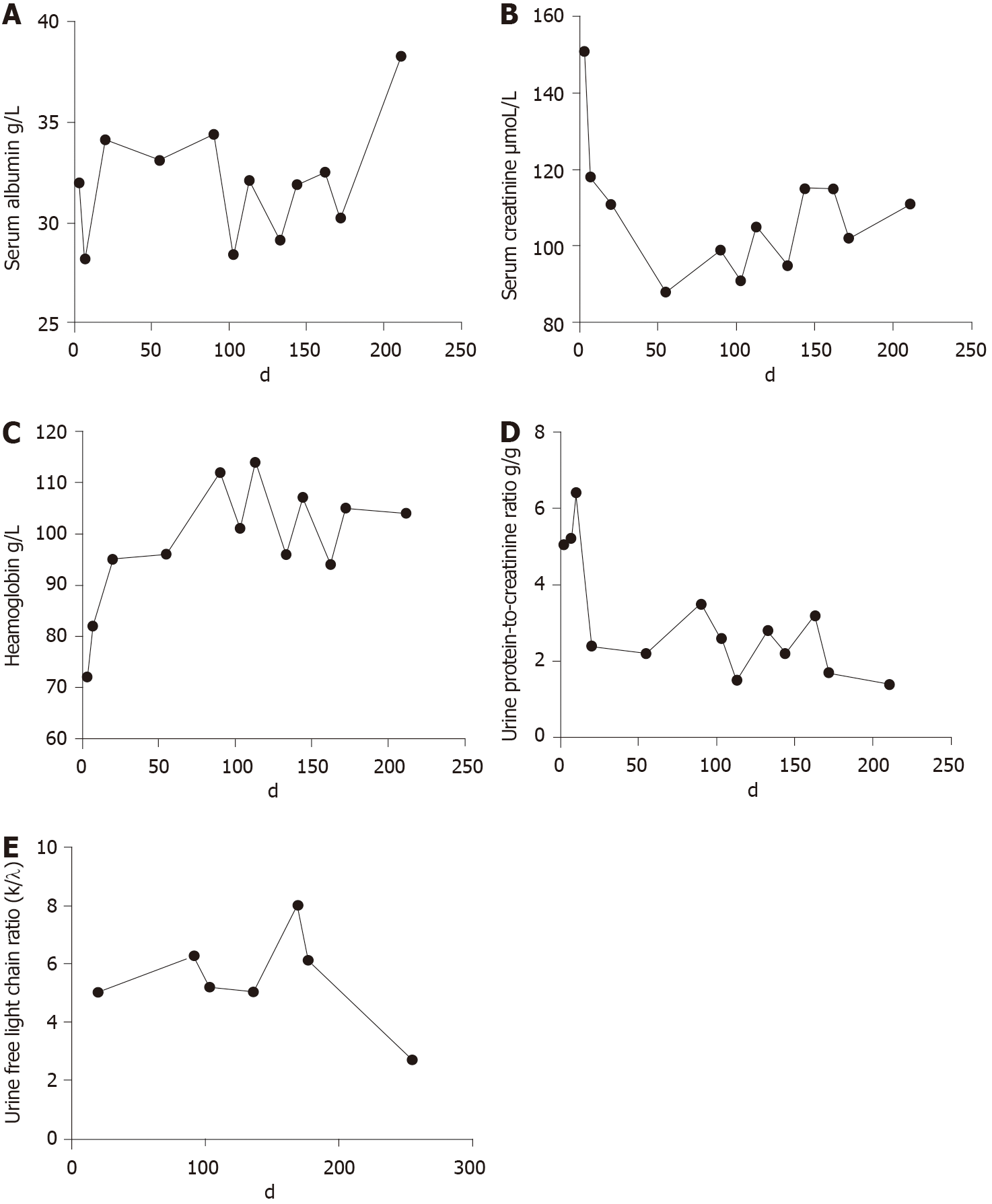
The patient was followed for over 200 d. No specific discomfort was reported during the period. Her condition improved after BCD treatment. At the last follow-up, her urine protein-to-creatinine ratio was 1.4 g/g, Scr was stable at 111 μmoL/L, complement was normal, and the urine free light chain ratio decreased from 5.0217 to 2.6894. In addition, her Hb was stable at 112 g/L, and the serum albumin level increased to 38.3 g/L (Figure 4).
As illustrated in our patient, PGNMID is an important phenotype of monoclonal gammopathy of renal significance. It has a dual nature of blood and kidney disease. Because of the complexity of its pathogenesis, the exact causes of PGNMID are still not fully understood. It is believed that the disease is caused by the deposition of intact immunoglobulins produced by clonally proliferating plasma cells or B cells in the glomeruli[4]. Preud’homme et al[5] reported that the clustering of hydrophobic amino acids in the complementarity determining region 1 in monoclonal immunoglobulin (MIg) creates a hydrophobic zone that might promote interactions favoring light chain aggregation and tissue precipitation[5]. For a definitive diagnosis, a complete examination including serum and urine immunofixation, protein electrophoresis, free light chain assay, and complete renal pathology is necessary. In 2009, Nasr et al[2] retrospectively identified 37 patients; according to IFE, 7 patients had a monoclonal spike (M-spike) in both serum and urine, and 4 patients had an M-spike detectable in the serum only, but no patient had an M-spike detectable in the urine only. To our knowledge, this is the first report of a PGNMID patient who had monoclonal protein in the urine only. The mechanism needs to be studied further.
There is no effective method to inhibit the deposition of MIg in tissues or directly remove the MIg deposited thus far. Some cases had achieved clinical complete recovery or partial recovery for the treatment of abnormal cloned cells[2]. Given that more than 50% of the glomeruli were sclerotic, we think that the PGNMID is irreversible in this case.
The treatment options mainly refer to the clinical experience in the therapies of hematologic malignancies such as multiple myeloma and amyloidosis. Andréau et al[6] believed that BCD scheme is a common regimen for the treatment of monoclonal gammopathies. Cell proliferation and cell cycle progression can be inhibited by dexamethasone in B lymphocytes[6]. Similarly, the activation/proliferation sequence and the differentiation phase of the B cell maturation sequence are suppressed by cyclophosphamide[7]. Bortezomib is a proteasome inhibitor that is regarded as a first-line drug for the treatment of plasma cell disease. It induces apoptosis of monoclonal plasma cells and inhibits renal fibrosis[8]. It is important to note that bortezomib may also induce acute interstitial nephritis[9]. Therefore, the renal function should be followed closely during medication.
The detection rate of circulating MIg in PGNMID patients is low. Nasr et al[2] reported that MIg can be detected in only 30% of patients and that abnormal plasma cells usually comprise less than 10% of bone marrow. Bhutani et al[10] found that 60% of kidney-related monoclonal diseases originate from plasma cell clones. Therefore, in the absence of circulating or bone marrow monoclonal evidence, bortezomib-based treatment is acceptable. However, due to the limited number of cases, no randomized clinical trial has been published. Previously, Noto et al[11] treated an elderly PGNMID case with dual therapy bortezomib and dexamethasone and achieved a satisfactory result. There is no doubt that BD scheme and BCD scheme[12] are both treatment options for PGNMID. In view of the fact that our case was a young female who was better able to withstand the side effects of the drugs, we chose the BCD scheme in order to stable her renal function as much as possible. After BCD chemotherapy, remission was achieved during follow-up, suggesting that this scheme may be an appropriate option for PGNMID. Nonetheless, further evidence-based research is still needed.
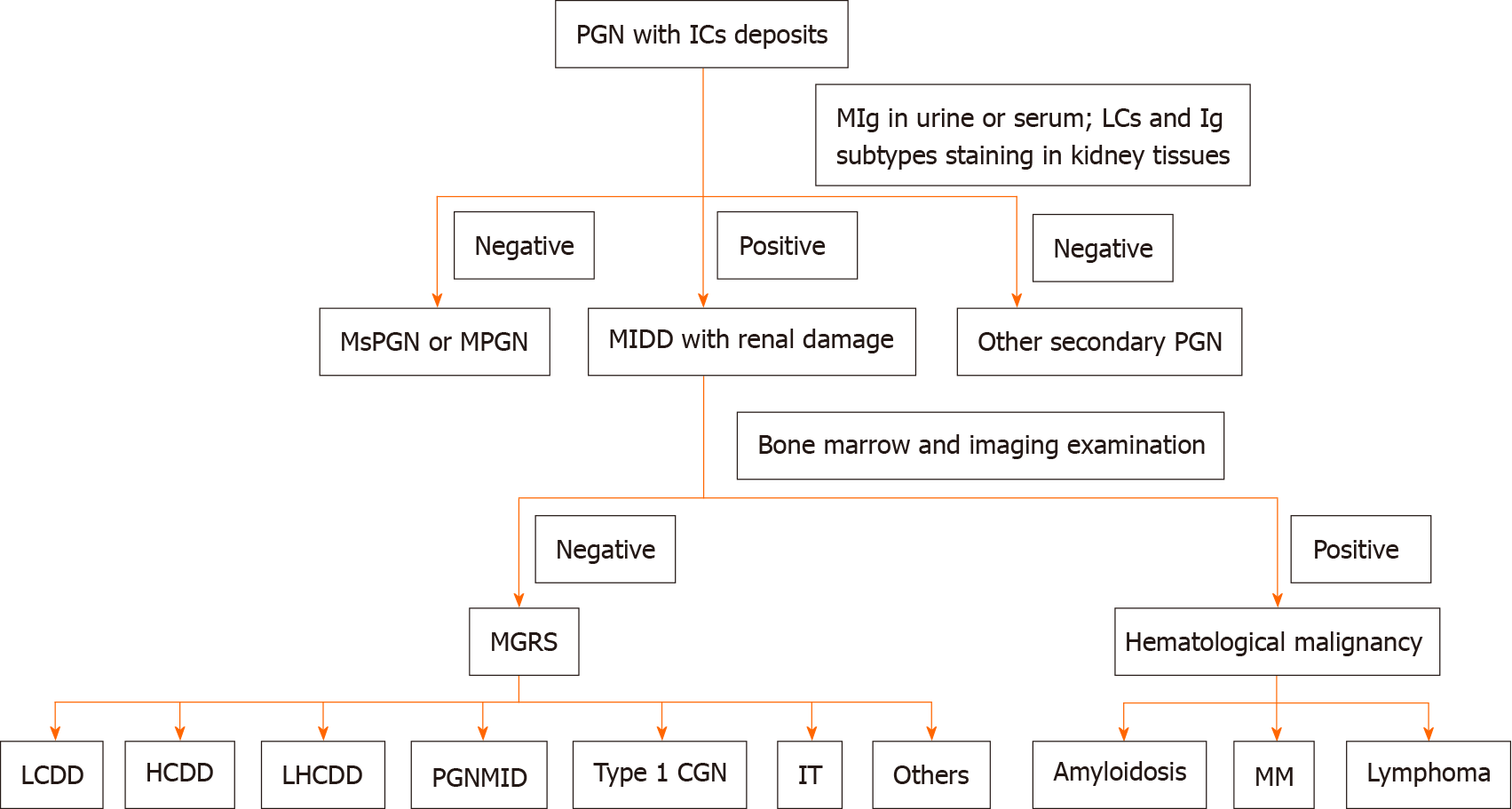
Although PGNMID is a rare disease that often occurs in middle-aged and elderly individuals, it cannot be easily excluded in young people, especially in those whose pathological type is MPGN or MsPGN, even when serum IFE is negative. Renal biopsy should be repeated if necessary. An accurate diagnosis at the earliest stage may avoid the overuse of glucocorticoids and immunosuppressants as well as any subsequent serious side effects (Figure 5).
| 1. | Nasr SH, Markowitz GS, Stokes MB, Seshan SV, Valderrama E, Appel GB, Aucouturier P, D'Agati VD. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int. 2004;65:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, Nadasdy T, D'Agati VD. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 323] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 3. | Masai R, Wakui H, Komatsuda A, Togashi M, Maki N, Ohtani H, Oyama Y, Sawada K. Characteristics of proliferative glomerulo-nephritis with monoclonal IgG deposits associated with membranoproliferative features. Clin Nephrol. 2009;72:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Leung N, Drosou ME, Nasr SH. Dysproteinemias and Glomerular Disease. Clin J Am Soc Nephrol. 2018;13:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Preud'homme JL, Aucouturier P, Touchard G, Striker L, Khamlichi AA, Rocca A, Denoroy L, Cogné M. Monoclonal immunoglobulin deposition disease (Randall type). Relationship with structural abnormalities of immunoglobulin chains. Kidney Int. 1994;46:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 80] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Andréau K, Lemaire C, Souvannavong V, Adam A. Induction of apoptosis by dexamethasone in the B cell lineage. Immunopharmacology. 1998;40:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Zhu LP, Cupps TR, Whalen G, Fauci AS. Selective effects of cyclophosphamide therapy on activation, proliferation, and differentiation of human B cells. J Clin Invest. 1987;79:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Bonaud A, Bender S, Touchard G, Lacombe C, Srour N, Delpy L, Oblet C, Druilhe A, Quellard N, Javaugue V, Cogné M, Bridoux F, Sirac C. A mouse model recapitulating human monoclonal heavy chain deposition disease evidences the relevance of proteasome inhibitor therapy. Blood. 2015;126:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Cheungpasitporn W, Leung N, Rajkumar SV, Cornell LD, Sethi S, Angioi A, Fervenza FC. Bortezomib-induced acute interstitial nephritis. Nephrol Dial Transplant. 2015;30:1225-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Bhutani G, Nasr SH, Said SM, Sethi S, Fervenza FC, Morice WG, Kurtin PJ, Buadi FK, Dingli D, Dispenzieri A, Gertz MA, Lacy MQ, Kapoor P, Kumar S, Kyle RA, Rajkumar SV, Leung N. Hematologic characteristics of proliferative glomerulonephritides with nonorganized monoclonal immunoglobulin deposits. Mayo Clin Proc. 2015;90:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Noto R, Kamiura N, Ono Y, Tabata S, Hara S, Yokoi H, Yoshimoto A, Yanagita M. Successful treatment with bortezomib and dexamethasone for proliferative glomerulonephritis with monoclonal IgG deposits in multiple myeloma: a case report. BMC Nephrol. 2017;18:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Fermand JP, Bridoux F, Kyle RA, Kastritis E, Weiss BM, Cook MA, Drayson MT, Dispenzieri A, Leung N; International Kidney and Monoclonal Gammopathy Research Group. How I treat monoclonal gammopathy of renal significance (MGRS). Blood. 2013;122:3583-3590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Arabey AA S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Liu JH