Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2344
Peer-review started: October 27, 2020
First decision: November 20, 2020
Revised: December 3, 2020
Accepted: February 11, 2021
Article in press: February 11, 2021
Published online: April 6, 2021
Processing time: 154 Days and 2.3 Hours
Granulomatosis with polyangiitis is a necrotizing inflammation of small and medium-sized vessels accompanied by formation of granuloma, involvement of primary granulomatous upper and lower respiratory tracts, glomerulonephritis, and vasculitis of small vessels.
Herein, we described a case of a 52-year-old man admitted with pulmonary nodules and high fever. Autoantibody workup revealed that the patient was positive for c-anti-neutrophil cytoplasmic antibodies and proteinase-3 anti-neutrophil cytoplasmic antibodies. Pulmonary biopsies revealed a local granulomatous structure. The patient received therapy with methylprednisolone and intravenous immunoglobulin, and his clinical symptoms improved.
Intravenous immunoglobulin may act on granulomatosis with polyangiitis similar to immunosuppressants.
Core Tip: By reviewing a patient diagnosed with granulomatosis with polyangiitis, we found that intravenous immunoglobin combined with methylprednisolone can reduce the symptoms of pulmonary hemorrhage. These findings differ from previous reports, and provide more possibilities for the treatment of similar conditions.
- Citation: Li XJ, Yang L, Yan XF, Zhan CT, Liu JH. Granulomatosis with polyangiitis presenting as high fever with diffuse alveolar hemorrhage and otitis media: A case report. World J Clin Cases 2021; 9(10): 2344-2351
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2344.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2344
Granulomatosis with polyangiitis (GPA) is a systemic disease characterized by necrotizing vasculitis and granulomatous inflammation. The inflammatory destructive lesions may develop in any organ. The evolution of GPA can be rapid and aggressive, and its mortality rate is 82% within 1 year when left untreated[1,2]. The classical clinical triad of GPA includes the upper respiratory tract, lower respiratory tract, and renal involvement[3]. Chronic sinusitis, epistaxis, and otitis media are the dominant clinical features of GPA in the upper respiratory tract, and 30%-50% of patients have otological involvement[4]. Approximately 8% of patients with GPA develop diffuse alveolar hemorrhage (DAH), a prominent and life-threatening pulmonary manifestation of this disease[5,6].
Herein, we report a rare case of GPA from China, which presented as high fever with DAH and otitis media.
A 52-year-old male patient of Zhuang ethnicity had a cough for 1 mo. The patient had previously taken antibiotic treatment for cough and otitis media, which presented as pain and impaired hearing in the outer court for 1.5 mo.
The patient’s symptoms started 1 mo prior with recurrent attacks of paroxysmal cough. At 10 d prior to admission, the patient had a fever and reported 3 kg weight loss in the past month.
The patient had no previous medical history, and no history of smoking or drinking.
The patient has no personal genetic history or family history.
Physical examination on admission revealed a temperature of 36.5 °C and heart rate of 106 bpm; and no lymphadenopathy was detected. The cardiovascular, and neurological examinations were unremarkable. The right eye had mild conjunctival hyperemia. Harsh breath sounds were heard in both lungs, and moist rales and voice tremor enhancement were heard in the left upper lobe.
Routine blood examination showed that white blood cell (WBC) count was 10.55 × 109/L, hemoglobin was 108.30 × 1012/L, neutrophil percentage was 0.828, procalcitonin was 0.428, erythrocyte sedimentation rate (ESR) was 91 mm, and C-reactive protein (CRP) level was > 200 mg/L. Liver function test showed that total bilirubin was 29.1 μmol/L, direct bilirubin was 23.3 μmol/L, and alanine aminotransferase and aspartate aminotransferase were within normal range. Coagulation function examination showed that prothrombin time was 16.7 s, activated partial thromboplastin time was 33.4 s, fibrinogen was 6.68 g/L, antithrombin was 74%, and D-dimer was 807 ng/mL. Electrolytes and renal function were within normal limits. Urine showed a pH of 5, specific gravity of 1.030, urine bilirubin + 1, urobilinogen + 2, urine protein ±, and no red blood cells or WBCs in the high-power field. Stool was positive for liver fluke, and blood culture was negative.
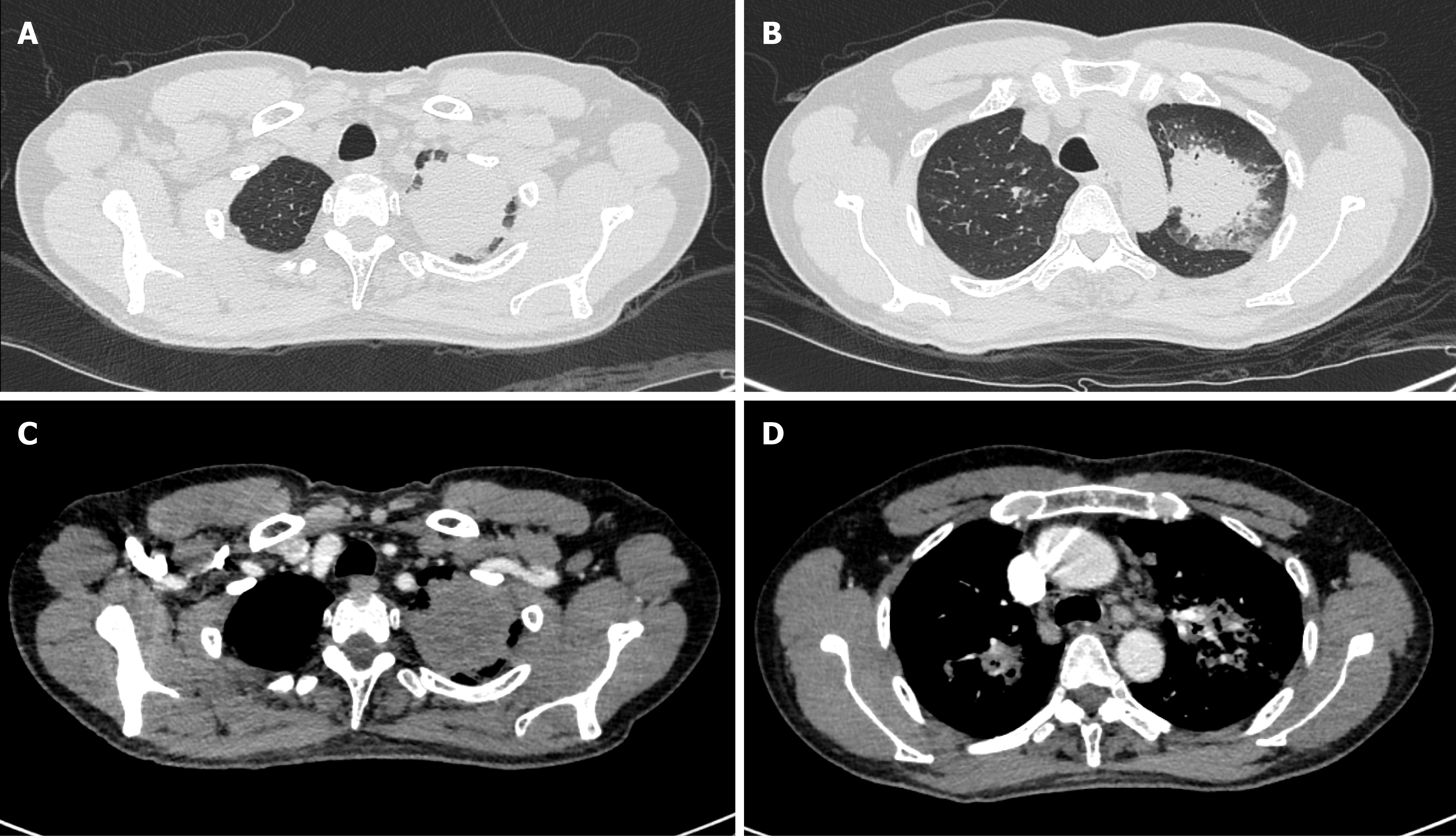
Bronchoscopy revealed remarkable tracheal and endobronchial mucosal erosion, congestion, and inflammation. Chest computed tomography (CT) revealed multiple pulmonary nodules, enlarged hilar and mediastinal lymph nodes, and pleural effusion in bilateral lungs. The lesions were not enhanced in the CT images (Figure 1). No enhancement was found in the susceptible sites of tuberculosis, so the possibility of lung cancer was temporarily ruled out.
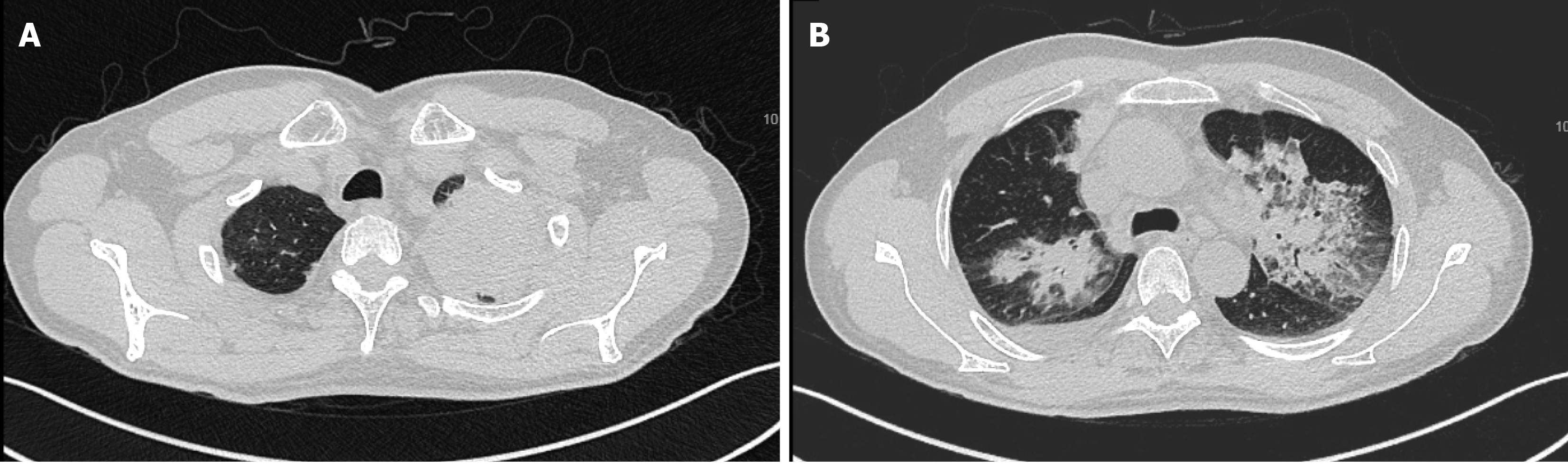
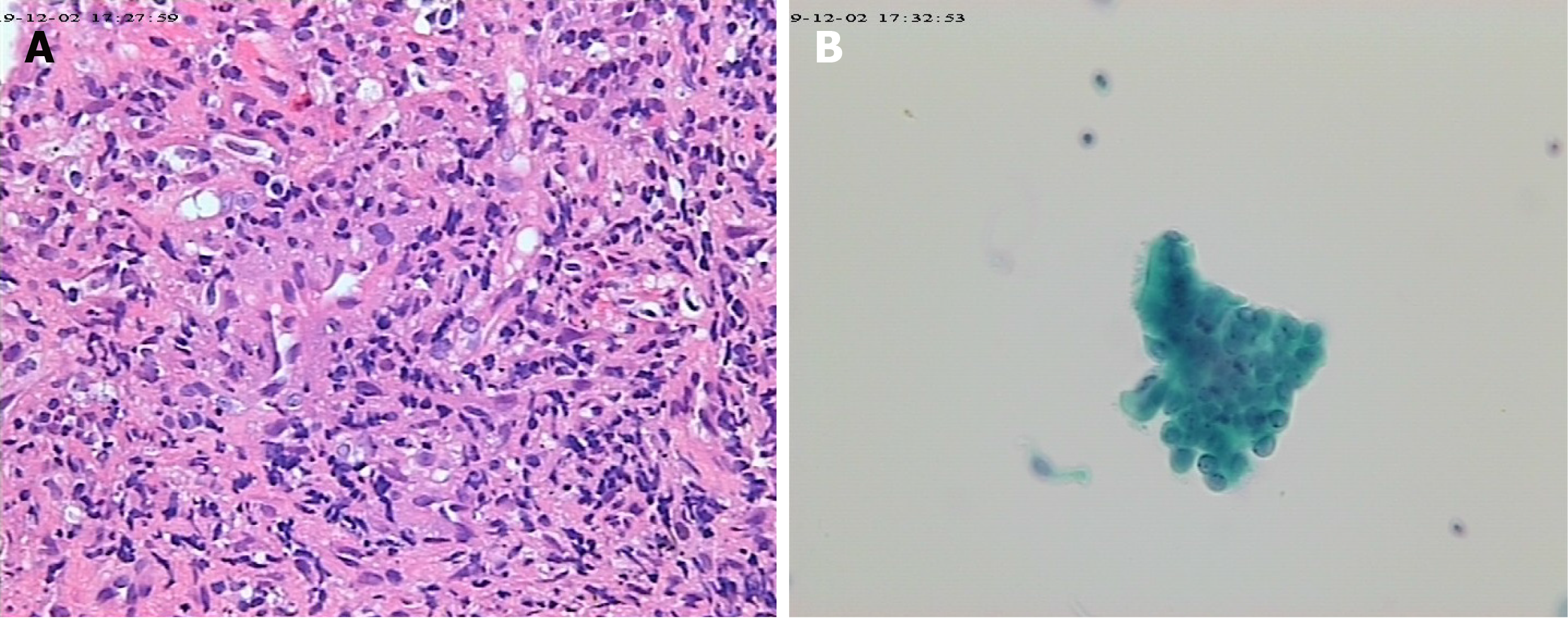
No acid-fast bacteria were observed on smear, and all blood cultures were negative. Chest CT scan reexamination (Figure 2) showed enlarged pulmonary nodules (the anti-tuberculosis drugs were discontinued). Pure tone audiometry revealed moderately high-frequency mixed hearing loss in the right ear, and normal hearing in the left ear. Ear examination showed intact tympanic membrane with congestion and invagination in the right ear, and normal tympanic membrane in the left ear. Biopsies revealed local granulomatous structure; peripheral fibrous hyperplasia; lymphocyte, plasma cell, and neutrophil infiltration around the structure; and no heterotypic cells (Figure 3). Bronchoscopy reexamination showed diffuse mucosal hemorrhage ending in the glottis. Therefore, the operation was discontinued. During this period, hemoglobin decreased progressively from 108.30 g/L to 53.10 g/L, antithrombin decreased from 74% to 20%, and fever persisted at > 39.0 °C (multiple blood transfusions and fresh frozen plasma transfusions were given). Rheumatoid factor was negative. Autoantibody examination showed positive antinuclear antibody (ANA), c-anti-neutrophil cytoplasmic antibodies (c-ANCA), and proteinase-3 anti-neutrophil cytoplasmic antibodies (PR3-ANCA).
The final diagnosis was GPA.
After the patient was admitted to the hospital, trial anti-tuberculosis treatment (0.3 g isoniazid + 0.45 g rifampicin + 0.75 g ethambutol) was initiated (from November 4, 2019 to November 10, 2019), supplemented with anti-infection (2.0 g cefoperazone sodium and tazobactam sodium and 0.4 g levofloxacin), antipyretic, and symptomatic supportive treatment. However, high fever and shortness of breath persisted. After the results of autoantibody examination, chest CT scan and biopsies, the patient received methylprednisolone combined with intravenous immunoglobulin (IVIG) therapy. Emergency bronchoscope-assisted tracheal intubation was performed on the second day of remission induction because the patient had a disturbance of consciousness and low pulse oxygen at 79%. Tracheal blood secretion was observed under bronchoscopy. During the full-dose full-period administration of methylprednisolone (80 mg, once a day [qd], from November 12, 2019 to November 18, 2019) and IVIG (20 g, qd, from November 12, 2019 to November 18, 2019), the patient’s breathlessness was significantly improved. Thereafter, the treatment plan was changed to cyclophosphamide (200 mg, every other day [qod], from November 18, 2019 to December 3, 2019) and methylprednisolone (80 mg, qd, from November 18, 2019 to December 3, 2019).
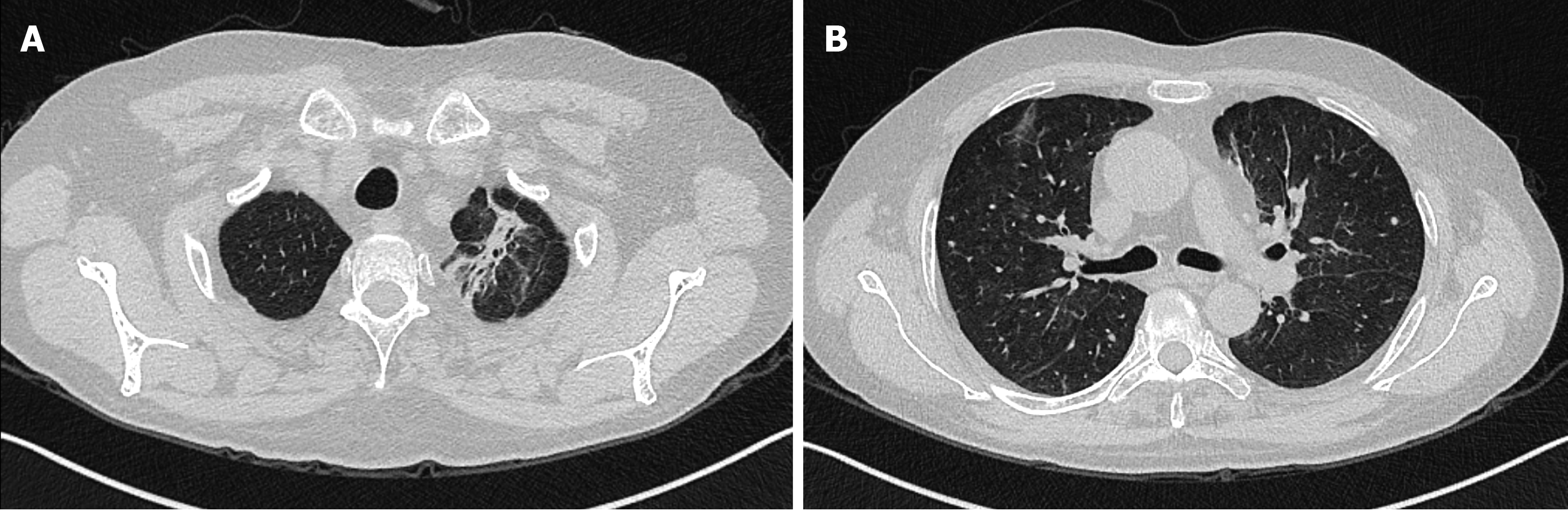
The patient was transferred to the rheumatology and immunology department, and subsequently discharged. During the last follow-up on May 6, 2020, except for bilateral vocal cord paralysis and tinnitus in the right ear with impaired hearing, there was no cough, fever or other discomfort. Reexamination showed no obvious increase in the inflammatory indexes (including WBCs, neutrophils, high-sensitivity CRP, and ESR). No obvious abnormality was found in the liver function, renal function and coagulation function. ANA and anti-neutrophil cytoplasmic antibodies (including pANCA, cANCA, MPO-ANCA, PR3-ANCA) were negative. Chest CT showed that the high-density shadows in both lungs were absorbed more obviously than before (Figure 4).
GPA, a new alternative name for Wegener’s granulomatosis, is a necrotizing inflammation of small and medium-sized vessels accompanied by formation of granuloma, involvement of primary granulomatous upper and lower respiratory tracts, glomerulonephritis, and vasculitis of small vessels. GPA is a heterogeneous disease in terms of severity and clinical manifestations[7,8]. Its annual incidence is 4-21 cases per million, with a higher incidence in Europe, particularly in Southern Sweden, Norway, New Zealand and the United States, and a lower incidence in China[5]. The mean age of patients at diagnosis is 40-50 years[9]. The most frequently affected organ is the lung, which is involved in more than 90% of patients during the course of the disease. The associated lung diseases range from asymptomatic pulmonary nodules to pulmonary infiltrates and fulminant alveolar hemorrhage. Lung nodules and masses are the most common radiographic and CT abnormalities[10]. DAH is a rare fatal complication of GPA that increases the mortality rate of affected patients by six times the normal value[11]. The skin, central nervous system, heart, salivary gland, eye and orbit, breast, gastrointestinal tract, spleen, pituitary gland, thyroid gland, and urogenital tract can also be involved in this disease[9]. GPA is diagnosed when two of the following four criteria are met: Nasal or oral inflammation with ulcers or purulent bloody discharge; chest radiograph showing nodules, cavities, or infiltrates; urinary sediment with red cell casts or microscopic hematuria; and granulomatous inflammation on lung biopsy. GPA has a strong and specific association with c-anti-neutrophil cytoplasmic antibodies, which in combination with anti-proteinase-3 testing, has great significance for early GPA diagnosis because their sensitivity and specificity are higher than 90% and 98%, respectively[5,12]. Early diagnosis and treatment are the key to GPA management. The therapeutic options for GPA are as follows: Cyclophosphamide + corticosteroids, methotrexate + corticosteroids, azathioprine, leflunomide, cotrimoxazole, mycophenolate, cyclosporine, or rituximab[9]. Glucocorticoids with cyclophosphamide are considered the first-line regimen[3,13]. However, treatment-related morbidity and toxicity from prolonged cyclophosphamide therapy remains a major concern. Therefore, safe and effective alternatives are urgently needed. IVIG can reduce the disease activity in diverse autoimmune diseases by interrupting T-cell- or B-cell-mediated immune responses[14]. The results of a French Vasculitis Study Group prospective trial showed that after treatment with IVIG as additional therapy, 16 of the 22 patients experienced complete remission after 6 mo (72.7; 15 with Wegener’s granulomatosis and 1 with microscopic polyangiitis), and 17 patients after 24 mo (77.3%)[15]. This is in agreement with Etienne Crickx’s retrospective study, which supported the use of IVIG in anti-neutrophil cytoplasmic antibody-associated vasculitides patients with refractory or relapsed disease[16]. In Fortin et al[17]’s study, 34 participants with GPA were randomly assigned to two groups: One group was treated with IVIG, systemic corticosteroids, and immunosuppressive drugs; and the other group was treated with systemic corticosteroids and immunosuppressive drugs. The IVIG group showed significant increase in total adverse events, but the two groups had no remarkable differences in mortality, serious adverse events, time to relapse, open-label rescue therapy, and infection rates (Table 1)[17]. Two patients diagnosed with eosinophilic granulomatosis with polyangiitis and drastically progressive neuropathy were successfully treated with IVIG, not steroids, and showed no apparent side effects[18]. Similarly, for chronic residual peripheral neuropathy in eosinophilic GPA (Churg-Strauss syndrome), the number of muscles with low manual muscle testing scores and the neuropathic pain scores also improved significantly 2 wk after IVIG administration[19], which indicated the practicality and effectiveness of IVIG treatment for eosinophilic patients with peripheral neuropathy. In addition, IVIG therapy has been safely used during pregnancy[20,21]. The frequency of cluster of differentiation-positive cells among the CD4+ T cells (which may promote remission in eosinophilic GPA) in the study group (IVIG therapy combined with conventional therapy) was lower than that of the control group (conventional therapy) before therapy, and was significantly increased after IVIG treatment (Table 2)[22].
| Treated with IVIG | Treated with systemic corticosteroids and immunosuppressive drug | Total adverse events | Mortality, serious adverse events, time to relapse, open-label rescue therapy, and infection rates | |
| Control group | (-) | (+) | - | No remarkable differences |
| Study group | (+) | (+) | Higher | No remarkable differences |
| IVIG therapy | Conventional therapy | Frequency of CD25+ (before therapy) | Frequency of CD25+ (after IVIG treatment) | |
| Control group | (-) | (+) | Higher | - |
| Study group | (+) | (+) | - | Higher |
Our case strengthens the validity of IVIG use. The combination of methylprednisolone and IVIG can considerably improve the symptoms, with less cytotoxic side effects. However, the length of remission induction, the potential side effects compared with cyclophosphamide use, and the best time for administration need further study.
GPA is an autoimmune disease that is rare and difficult to diagnose, and its treatment plan needs to be constantly optimized. Early diagnosis and treatment are the key to managing and treating this lethal disease.
The authors acknowledge the invaluable participation of the patient.
| 1. | Rossini BA, Bogaz EA, Yonamine FK, Testa JR, Penido Nde O. Refractory otitis media as the first manifestation of Wegener's granulomatosis. Braz J Otorhinolaryngol. 2010;76:541. [PubMed] |
| 2. | Chouhan A, Kulhari M, Amisha B, Kasliwal N. An Unusual Presentation of Wegener's Granulomatosis with Otologic Symptoms and Unilateral Parotid Gland Enlargement. Indian J Otolaryngol Head Neck Surg. 2019;71:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Apostolova M, Shoib M, Nasser S. Multiple pulmonary nodules: a complex case of Wegener's granulomatosis. Clin Pract. 2013;3:e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Lynch JP 3rd, Derhovanessian A, Tazelaar H, Belperio JA. Granulomatosis with Polyangiitis (Wegener's Granulomatosis): Evolving Concepts in Treatment. Semin Respir Crit Care Med. 2018;39:434-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Neves I, Marinho A, Melo N, Jesus JM, Moura CS, Bernardes M, Vaz C. Wegener's granulomatosis and alveolar hemorrhage - case report. Acta Reumatol Port. 2013;38:295-298. [PubMed] |
| 6. | Ishiguro T, Takayanagi N, Yamaguchi S, Shimizu Y, Yanagisawa T, Sugita Y, Kawabata Y. Pulmonary capillaritis in Wegener's granulomatosis detected via transbronchial lung biopsy. Intern Med. 2012;51:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Harabuchi Y, Kishibe K, Komabayashi Y. Clinical manifestations of granulomatosis with polyangiitis (Wegener's granulomatosis) in the upper respiratory tract seen by otolaryngologists in Japan. Clin Exp Nephrol. 2013;17:663-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Gadó K, Z Szabó L, Csákó L, Domján G. [Wegener's granulomatosis]. Orv Hetil. 2013;154:1083-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Almouhawis HA, Leao JC, Fedele S, Porter SR. Wegener's granulomatosis: a review of clinical features and an update in diagnosis and treatment. J Oral Pathol Med. 2013;42:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | de Oliveira RV, Zanetti G, Marchiori E. Multiple pulmonary nodules. Wegener's granulomatosis simulating pulmonary metastases. QJM. 2013;106:1143-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Arora R. Massive alveolar haemorrhage: a rare life threatening complication of Wegener's granulomatosis-report of a rare case. Quant Imaging Med Surg. 2014;4:509-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Liang J, Fan W, Yuan RD. Peripheral ulcerative keratitis and otitis media as initial symptoms of Wegener's granulomatosis: a case report. Int J Ophthalmol. 2019;12:873-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Karras A, Guiard E, Lévi C, Thervet E. [Granulomatosis with polyangiitis (Wegener's granulomatosis)]. Presse Med. 2012;41:1014-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Blum M, Andrassy K, Adler D, Hartmann M, Völcker HE. Early experience with intravenous immunoglobulin treatment in Wegener's granulomatosis with ocular involvement. Graefes Arch Clin Exp Ophthalmol. 1997;235:599-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Martinez V, Cohen P, Pagnoux C, Vinzio S, Mahr A, Mouthon L, Sailler L, Delaunay C, Sadoun A, Guillevin L; French Vasculitis Study Group. Intravenous immunoglobulins for relapses of systemic vasculitides associated with antineutrophil cytoplasmic autoantibodies: results of a multicenter, prospective, open-label study of twenty-two patients. Arthritis Rheum. 2008;58:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Crickx E, Machelart I, Lazaro E, Kahn JE, Cohen-Aubart F, Martin T, Mania A, Hatron PY, Hayem G, Blanchard-Delaunay C, de Moreuil C, Le Guenno G, Vandergheynst F, Maurier F, Crestani B, Dhote R, Silva NM, Ollivier Y, Mehdaoui A, Godeau B, Mariette X, Cadranel J, Cohen P, Puéchal X, Le Jeunne C, Mouthon L, Guillevin L, Terrier B; French Vasculitis Study Group. Intravenous Immunoglobulin as an Immunomodulating Agent in Antineutrophil Cytoplasmic Antibody-Associated Vasculitides: A French Nationwide Study of Ninety-Two Patients. Arthritis Rheumatol. 2016;68:702-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Fortin PM, Tejani AM, Bassett K, Musini VM. Intravenous immunoglobulin as adjuvant therapy for Wegener's granulomatosis. Cochrane Database Syst Rev. 2013;2013:CD007057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Matsumoto T, Otsuka K, Kawamoto M, Nagata K, Tachikawa R, Imai Y, Oka N, Tomii K. Efficacy of early intravenous immunoglobulin for eosinophilic granulomatosis with polyangiitis with drastically progressive neuropathy: a synopsis of two cases. Intern Med. 2013;52:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Koike H, Akiyama K, Saito T, Sobue G; Research Group for IVIg for EGPA/CSS in Japan. Intravenous immunoglobulin for chronic residual peripheral neuropathy in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): a multicenter, double-blind trial. J Neurol. 2015;262:752-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Daher A, Sauvetre G, Girszyn N, Verspyck E, Levesque H, Le Besnerais M. Granulomatosis with polyangiitis and pregnancy: A case report and review of the literature. Obstet Med. 2020;13:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Kim SY, Linton JM, Kolasinski SL. Successful treatment of new onset Wegener's granulomatosis with IVIG (intravenous immunoglobulin) during pregnancy: a case report. Mod Rheumatol. 2008;18:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Tsurikisawa N, Saito H, Oshikata C, Tsuburai T, Akiyama K. High-dose intravenous immunoglobulin treatment increases regulatory T cells in patients with eosinophilic granulomatosis with polyangiitis. J Rheumatol. 2012;39:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Otorhinolaryngology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Han YM S-Editor: Liu M L-Editor: Filipodia P-Editor: Xing YX