Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2326
Peer-review started: October 22, 2020
First decision: December 13, 2020
Revised: December 26, 2020
Accepted: February 4, 2021
Article in press: February 4, 2021
Published online: April 6, 2021
Processing time: 154 Days and 5.8 Hours
Bedaquiline is among the prioritized drugs recommended by the World Health Organization for the treatment of extensively drug-resistant tuberculosis (XDR-TB). Many patients have not achieved better clinical improvement after bedaquiline is stopped at 24 wk. However, there is no recommendation or guideline on bedaquiline administration beyond 24 wk, which is an important consideration when balancing the benefit of prognosis for XDR-TB against the uncertain safety concerning the newer antibiotics.
This paper reported 2 patients with XDR-TB (a female of 58 years of age and a female of 18 years of age) who received bedaquiline for 36 wk, as local experience to be shared. The 2 cases had negative cultures after 24 wk of treatment, but lung imaging was still positive. After discussion among experts, the consensus was made to bedaquiline prolongation by another 12 wk. The 36-wk prolonged use of bedaquiline in both cases achieved a favorable response without increasing the risk of cardiac events or new safety signals.
Longer regimen, including 36-wk bedaquiline treatment, might be an option for patients with XDR-TB. More studies are needed to explore the effectiveness and safety of prolonged use of bedaquiline for 36 wk vs standard 24 wk in the treatment of multidrug-resistant/XDR-TB or to investigate further the biomarkers and criteria indicative for extension of bedaquline to facilitate clinical use of this novel drug.
Core Tip: In the 2 patients reported here, the longer regimen, including 36-wk bedaquiline treatment, might be an option for patients with extensively drug-resistant tuberculosis. More studies are needed to explore the effectiveness and safety of prolonged use of bedaquiline for 36 wk vs standard 24 wk in the treatment of multidrug-resistant/ extensively drug-resistant tuberculosis and to investigate further the biomarkers and criteria indicative for extension of bedaquline to facilitate clinical use of this novel drug.
- Citation: Gao JT, Xie L, Ma LP, Shu W, Zhang LJ, Ning YJ, Xie SH, Liu YH, Gao MQ. Prolonged use of bedaquiline in two patients with pulmonary extensively drug-resistant tuberculosis: Two case reports. World J Clin Cases 2021; 9(10): 2326-2333
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2326.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2326
There are insufficient antibiotics that compose an effective combined regimen for extensively drug-resistant tuberculosis (XDR-TB), which is caused by Mycobacterium tuberculosis resistant to fluoroquinolones and at least one of the injectable second-line agents (amikacin, kanamycin, or capreomycin) except for isoniazid and rifampin and treatment success is no more than 30%[1,2]. Novel drugs or regimens are urgently needed. Bedaquiline was approved in 2012 in the United States and has a novel mechanism to kill Mycobacterium tuberculosis[3]. It is among the prioritized drugs recommended by the World Health Organization (WHO) for the treatment of multidrug-resistant tuberculosis (MDR-TB) (group A agents)[4]. However, many patients do not achieve better clinical improvement when bedaquiline is stopped after a 24-wk standard treatment duration. It is known that there are a limited number of options available for the treatment of patients with highly-resistant TB. This paper reports 2 patients with XDR-TB and pre-XDR-TB who received bedaquiline for 36 wk. However, recent main experience and licensing of bedaquiline are limited to the 24-wk duration administration. Therefore, the effect of the prolonged use of bedaquiline needs to be explored. Considering the cardiac safety of bedaquiline treatment, the QT interval has to be monitored[5].
Case 1: In January 2018, a 58-year-old female was admitted to Beijing Chest Hospital due to intermittent cough and expectoration.
Case 2: The patient had enlarging pulmonary lesions in December 2016, and she was transferred to Beijing Chest Hospital in February 2017. During her hospitalization, nausea and vomiting occurred.
Case 1: She had a cough and expectoration for 7 mo and intermittent fever for more than half a year. Chest radiography was normal, and no treatment was performed. One month later, the patient developed a fever, with a maximum body temperature of 39 °C. Chest computed tomography (CT) revealed a patchy nodular shadow in the middle lobe of the right lung, with partial atelectasis. Lobar pneumonia was diagnosed at a local hospital, and the symptoms were relieved after cephalosporin treatment before she came to ours.
Case 2: An 18-year-old female presented with pulmonary shadow and positive sputum smear in September 2015. Tracheoscopy revealed congestion and edema of the bronchial mucosa in the dorsal segment of the lower left lobe, with stenosis of the orifice. Administration of isoniazid (300 mg/d, q.d., p.o.), rifampicin (450 mg/d, q.d., p.o.), ethambutol (750 mg/d, q.d., p.o.), and pyrazinamide (1500 mg/d, t.i.d., p.o.) was performed. A CT reexamination in a local general hospital in December 2015 showed that the patchy shadow of the lower left lung was larger compared with the last CT images, and lesions were shrunk after 2 wk of cephalosporin treatment. The anti-TB regimen was used for 9 mo. In May 2016, ethambutol and pyrazinamide were discontinued by herself, and isoniazid and rifampicin were continued. In July 2016, the CT reexamination showed enlarged lesions, but she did not go to the designated TB hospital for further treatment modification with only isoniazid and rifampicin ongoing.
Case 1: The patient had an unremarkable medical history.
Case 2: This patient had no significant medical history.
Case 1: The sputum smear and culture were positive. GeneXpert presented rifampicin resistance.
Case 2: Sputum Hain test showed isoniazid and rifampicin resistance.
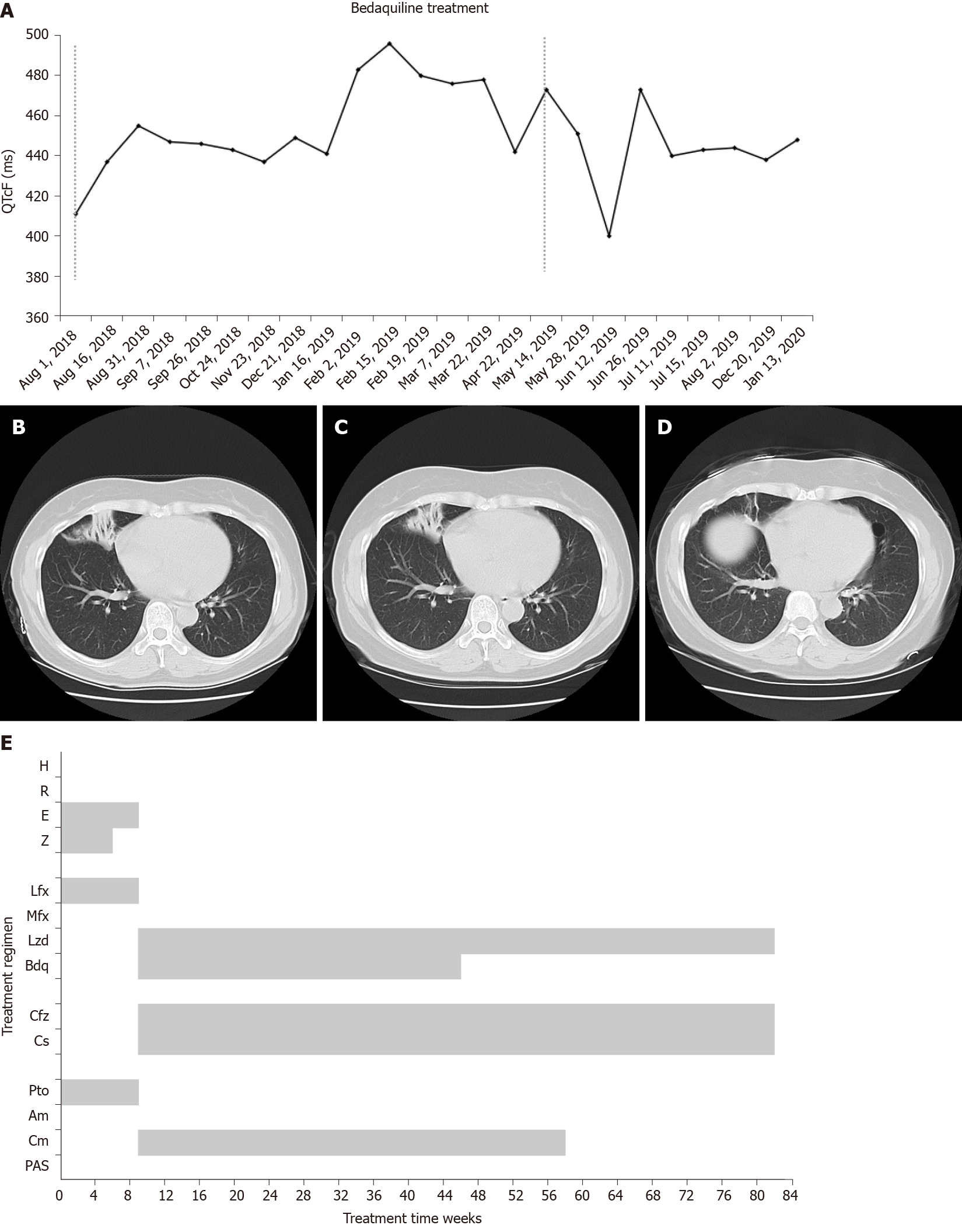
Case 1: In May 2018, chest CT showed the atelectasis shadow was larger compared with the last CT images (Figure 1B).
Case 1: Tracheoscopy suggested stenosis of the middle lobe of the right lung, and necrosis and ulceration were found in the mucosa.
Pulmonary and bronchial TB was diagnosed.
She was diagnosed with pulmonary TB, intestinal TB, and intestinal obstruction.
Starting on June 1, 2018, ethambutol (750 mg/d, q.d., p.o.), pyrazinamide (1500 mg/d, t.i.d., p.o.), protionamide (600 mg/d, t.i.d., p.o.), and levofloxacin (500 mg, q.d., p.o.) were given. Her temperature gradually returned to normal, but she still had a cough and expectoration. During the period, the sputum examination for TB was not performed. Liver enzymes elevated after 40 d (alanine transaminase: 205 U/L, aspartate transaminase: 157 U/L), and pyrazinamide was discontinued with liver protection treatment strengthened.
Drug susceptibility testing on July 9 revealed that TB bacilli were resistant to isoniazid, rifampicin, streptomycin, levofloxacin, amikacin, moxifloxacin, and kanamycin; susceptibility to capreomycin and XDR-TB was confirmed. On August 1, a new regimen was initiated after expert group consultation with capreomycin (750 mg/d, q.d., ivgtt.), cycloserine (500 mg/d, b.i.d., p.o.), linezolid (600 mg/d, q.d., p.o.), clofazimine (100 mg/d, q.d., p.o.), and bedaquiline (400 mg/d, q.d., p.o. for the first 2 wk and 200 mg/d, 3 times a week, p.o. for 22 wk). Sputum culture converted to negative after 2 wk. After 24 wk, chest CT revealed improvements in lung lesions (Figure 1C).
The QT interval with Fridericia's correction (QTcF) increased during the intensive phase with the peak increased > 60 ms compared with a baseline of 411 ms but did not exceed 500 ms (Figure 1A). On January 16, 2019, after expert group consultation, bedaquiline (200 mg/d, 3 times a week, p.o.) was prolonged for another 12 wk and then followed with a 36-wk background regimen, which was completed on December 20 (Figure 1E).
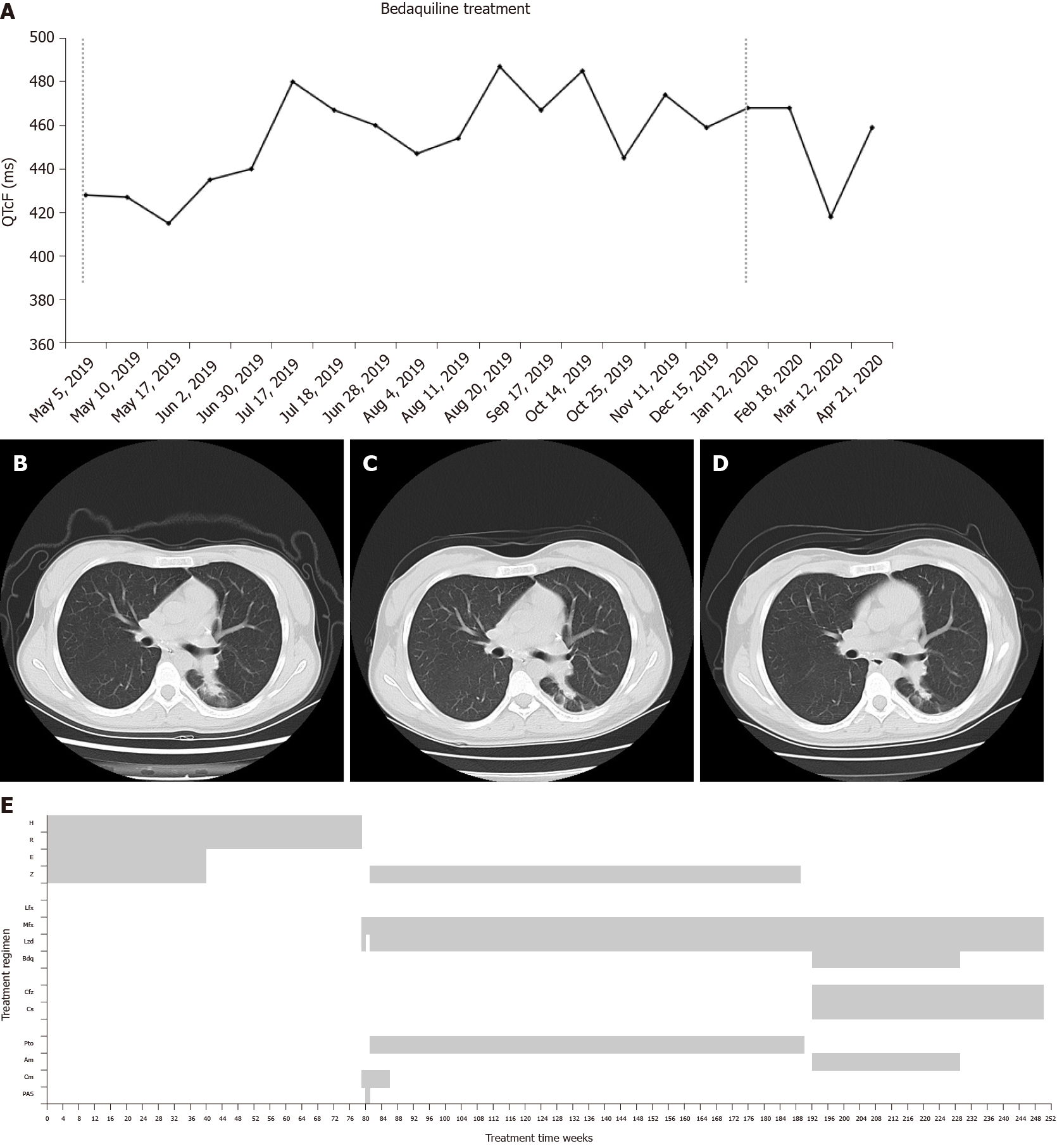
Capreomycin (750 mg/d, q.d., ivgtt.), moxifloxacin (400 mg/d, q.d., ivgtt.), and linezolid (600 mg/d, q.d., ivgtt.) via vein route were given on March 1 because of intestinal obstruction. Linezolid was discontinued for 1 wk instead of para-aminosalicylic acid (PAS, 8 g/d, b.i.d., p.o.) due to low platelet counting and was restarted on March 15 due to her nausea and vomiting with PAS. She was treated with capreomycin (750 mg/d, q.d., ivgtt.), moxifloxacin (400 mg/d, q.d., p.o.), linezolid (600mg/d, q.d., p.o.), pyrazinamide (1500 mg/d, t.i.d., p.o.), and protionamide (600 mg/d, t.i.d., p.o.) on March 20 with improvement of intestinal obstruction. Linezolid was reduced to 300 mg/d from April 8 for safety consideration. Drug susceptibility testing on April 18 (submitted on February 15) revealed that TB bacilli were susceptible to ethambutol, kanamycin, amikacin, moxifloxacin, clofazimine, PAS, protionamide, and levofloxacin; resistant to clarithromycin, isoniazid, rifampicin, rifapentine, rifabutin, capreomycin, and streptomycin. She was diagnosed as pre-XDR-TB, and capreomycin was discontinued and the remaining four drugs continued to be used. CT showed lesions in the lower left lung were slightly enlarged, and new lesions appeared in the lower right lung in August 2018. CT on April 10, 2019 showed improvement in lesions of the right lung but increased lesions in the lower left lung (Figure 2B). However, sputum smear and culture consistently presented negative, and the regimen was continued without change.
Because of the repeated new lesions or enlarged lesions seen at CT evaluation, a new treatment regimen was started on May 5, 2019. After expert group consultation, this regiment included amikacin (600 mg, q.d., ivgtt.), clofazimine (100 mg/d, q.d., p.o.), moxifloxacin (400 mg/d, q.d., p.o.), linezolid (300 mg/d, q.d., p.o.), cycloserine (750 mg/d, t.i.d, p.o.), and bedaquiline (400 mg/d, q.d. for the first 2 wk and 200 mg/d, 3 times a week, p.o. for 22 wk). By 24 wk, the lung lesions were gradually improved (Figure 2C). From a baseline of 428 ms before bedaquiline treatment, QTcF increased to 480 ms but did not increase > 60 ms compared with baseline or exceeding 500 ms (Figure 2A). On October 28, 2019, bedaquiline (200 mg/d, 3 times a week, p.o.) was prolonged for 12 wk after expert group consultation. The 36-wk bedaquiline-containing treatment was completed on January 12, 2020 (Figure 2E). A median QTcF of 468 ms was observed during the prolonged treatment period of bedaquiline. No cardiac arrhythmias or other adverse reactions occurred during treatment.
Consecutive sputum cultures were negative, and the lung lesions were greatly absorbed and remained stable (Figure 1D). The patient was evaluated as cured. No cardiac arrhythmias or other adverse reactions were reported during treatment. Drug adherence was managed by the responsible nurse with an App, and the patient presented good adherence.
The patient was receiving a background treatment regimen, and injectable was discontinued in the middle of March 2020, and her CT presented stable (Figure 2D). No recurrence of positive sputum culture was found at that time. Drug adherence was also managed by the responsible nurse, same as in case 1, and good adherence was recorded since she was strictly managed by our hospital.
Previous studies have shown that adding bedaquiline to an optimized background regimen can achieve a high treatment success rate in patients with XDR-TB[6,7]. WHO recommends that all three group A agents (levofloxacin or moxifloxacin, bedaquiline, and linezolid) and at least one group B agent (clofazimine and cycloserine or terozodone) should be included in the longer regimens for the treatment of MDR-TB at the start, and at least three agents are needed after bedaquiline is stopped[4]. For patients with a lack of effective drug combinations after the standard 24-wk of bedaquiline, prolongation of it is worthy of discussion. The 2 typical cases described here demonstrate the Chinese experts’ exploration and perspective for the prolonged use of bedaquiline when the bacteriological evidence remains negative, but imaging development presents the need for intensified treatment.
Although the sputum culture for the 2 patients remained negative during the 24-wk bedaquiline-containing treatment, CT revealed that the TB lesions were gradually absorbed but were not yet stable. After expert group consultation, it was considered that the prolonged use of bedaquiline might achieve additional benefits with tolerable toxicities for patients, and bedaquiline was prolonged for an additional 12 wk with strict monitoring of QTcF. The prognosis of the 2 patients was good, indicating that the prolonged use of bedaquiline might have a promising effect on select patients. This was supported by recent editorials and discussion, suggesting that bedaquiline could be used beyond the recommended 24 wk in patients with XDR-TB[8,9]. Lewis et al[9] even reported a patient with XDR-TB who received potent bedaquiline treatment for up to 18 mo. Although the optimal prolonged strategy of bedaquiline treatment needs further evidence, previous and the present reports showed that there was an opportunity for patients with XDR-TB to achieve better treatment outcomes. Last but not least, treatment adherence of the 2 patients was quite important for their favorable responses.
A major concern with prolonged bedaquiline treatment is cardiac toxicity. In the present reports, the peak QTcF of the middle-aged woman with unremarkable medical history increased > 60 ms compared with baseline but did not exceed 500 ms, which was not found in the young girl. In addition, bedaquiline was administered with other QT-prolonging agents (clofazimine and moxifloxacin), but no cardiac events or other adverse reactions were reported. Nevertheless, the potential side effects should be noted when treating an XDR-TB patient with a bedaquiline-containing regimen, especially when 2 or more QT-prolonging agents are included or in older patients with cardiac comorbidities.
There are some additional considerations for this report. Firstly, the TB care and treatment services for the patients were provided by several facilities during the duration of the long treatment, which may inevitably cause the incorrect or incomplete recall of previous treatment history before the patient was given the bedaquiline-containing regimen in our hospital. Secondly, injectable agents are relatively broadly used in China for MDR-TB patients, and longer duration can be used if patients are able to tolerate it. Thirdly, the dosage of some drugs may differ from patients, such as cycloserin, and the amount of it will be given according to the drug plasma concentration of it.
Many patients cannot achieve clinical improvement when bedaquiline is stopped after the 24-wk standard treatment duration, which is the approved treatment duration. Since bedaquiline is among the anti-TB drugs prioritized by the WHO[3], the prolonged use of bedaquiline needs to be explored in terms of efficacy but also in terms of cardiac safety[5]. Future studies could be focused on exploring the effectiveness, including sputum culture conversion and final treatment outcomes, and safety of prolonged use of bedaquiline for 36 wk vs standard 24 wk in the treatment of MDR/XDR-TB or further exploring biomarkers and criteria indicative for extension of bedaquline to facilitate clinical use of this novel drug. Moreover, studies on shortened regimens composed of 36-wk use of bedaquiline are ongoing. We expect the rapid increase in evidence supporting the prolonged use of bedaquiline will provide additional treatment options for MDR/XDR-TB patients.
In conclusion, our experiences using prolonged use of bedaquiline demonstrated a favorable response, without increasing the risk of cardiac events or new safety signals for patients with XDR-TB. Both of the patients were satisfied with their favorable treatment responses, but the findings need to be confirmed by a large-scale study.
| 1. | Matteelli A, Roggi A, Carvalho AC. Extensively drug-resistant tuberculosis: epidemiology and management. Clin Epidemiol. 2014;6:111-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Global tuberculosis report: 2019 Global TB Report. Available from: http://www.who.int/tb/publications/global_report/en/. |
| 3. | Andries K, Verhasselt P, Guillemont J, Göhlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1654] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. WHO Consolidated Guidelines on Tuberculosis, Module 4: Treatment—Drug-resistant Tuberculosis Treatment. Available from: https://www.who.int/publications/i/item/9789240007048. |
| 5. | Pontali E, Sotgiu G, Tiberi S, D'Ambrosio L, Centis R, Migliori GB. Cardiac safety of bedaquiline: a systematic and critical analysis of the evidence. Eur Respir J. 2017;50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Borisov SE, Dheda K, Enwerem M, Romero Leyet R, D'Ambrosio L, Centis R, Sotgiu G, Tiberi S, Alffenaar JW, Maryandyshev A, Belilovski E, Ganatra S, Skrahina A, Akkerman O, Aleksa A, Amale R, Artsukevich J, Bruchfeld J, Caminero JA, Carpena Martinez I, Codecasa L, Dalcolmo M, Denholm J, Douglas P, Duarte R, Esmail A, Fadul M, Filippov A, Davies Forsman L, Gaga M, Garcia-Fuertes JA, García-García JM, Gualano G, Jonsson J, Kunst H, Lau JS, Lazaro Mastrapa B, Teran Troya JL, Manga S, Manika K, González Montaner P, Mullerpattan J, Oelofse S, Ortelli M, Palmero DJ, Palmieri F, Papalia A, Papavasileiou A, Payen MC, Pontali E, Robalo Cordeiro C, Saderi L, Sadutshang TD, Sanukevich T, Solodovnikova V, Spanevello A, Topgyal S, Toscanini F, Tramontana AR, Udwadia ZF, Viggiani P, White V, Zumla A, Migliori GB. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Ndjeka N, Schnippel K, Master I, Meintjes G, Maartens G, Romero R, Padanilam X, Enwerem M, Chotoo S, Singh N, Hughes J, Variava E, Ferreira H, Te Riele J, Ismail N, Mohr E, Bantubani N, Conradie F. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur Respir J. 2018;52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Furin J, Lessem E, Cox V. Recommending prolonged bedaquiline use for the treatment of highly resistant strains of tuberculosis. Eur Respir J. 2017;50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Lewis JM, Hine P, Walker J, Khoo SH, Taegtmeyer M, Squire SB, Sloan DJ. First experience of effectiveness and safety of bedaquiline for 18 months within an optimised regimen for XDR-TB. Eur Respir J. 2016;47:1581-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Novita BD S-Editor: Gao CC L-Editor: Filipodia P-Editor: Yuan YY