Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2296
Peer-review started: October 11, 2020
First decision: December 13, 2020
Revised: December 23, 2020
Accepted: January 12, 2021
Article in press: January 12, 2021
Published online: April 6, 2021
Processing time: 170 Days and 5.4 Hours
The management of vascular graft infections continues to be a significant challenge in a clinical situation. The aim of this report is to illustrate the novel vacuum sealing drainage (VSD) technique and rectus femoris muscle flap transposition for vascular graft infections, and to evaluate the prospective of future testing of this surgical procedure.
We report the case of a 32-year-old male patient, who presented a severe infected groin wound with biological vascular graft Acinetobacter baumannii infection resulting in extensive graft exposure. Using the VSD and muscle flap trans-position, the groin wound and vascular graft infection were finally treated successfully.
Our case report highlights that VSD technique and rectus femoris muscle flap transposition could be considered in patients presenting with a severe infected groin wound with biological vascular graft Acinetobacter baumannii infection resulting in extensive graft exposure, especially in consideration of treatable conditions.
Core Tip: Artificial vascular graft infected with Acinetobacter baumannii is extremely rare in the clinic and continues to be a significant challenge for clinicians. Vacuum sealing drainage technique and rectus femoris muscle flap transposition are of great value in patients presenting with a severe infected groin wound with biological vascular graft Acinetobacter baumannii infection resulting in graft exposure extensively.
- Citation: Zhang P, Tao FL, Li QH, Zhou DS, Liu FX. Salvage of vascular graft infections via vacuum sealing drainage and rectus femoris muscle flap transposition: A case report. World J Clin Cases 2021; 9(10): 2296-2301
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2296.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2296
Vascular graft infection resulting from injury is a rare involving in the femoral region. It remains one of the most challenging complications in vascular trauma surgery. The gradual and irreversible deterioration of the vessel wall is the most serious issue on the conservative treatment of major vascular graft infections, significantly increasing bleeding risk[1-3].
Active wound treatment with vacuum sealing drainage (VSD) therapy is considered a very important strategy to accelerate wound healing and improve its prognosis[4,5]. Several studies demonstrated the excellent performance in the use of muscle flaps for treating chronically wound beds infected with various pathogenic organisms[6-8].
In the presented study, we report a patient who suffered from a severe vascular graft Acinetobacter baumannii infection and was cured successfully by using VSD to cover the infected wound and the rectus femoris muscle flap transposition is reported to share our experience with vascular graft salvage in the treatment of peripheral vascular graft infections involving the groin.
Vascular graft infections for 2 wk.
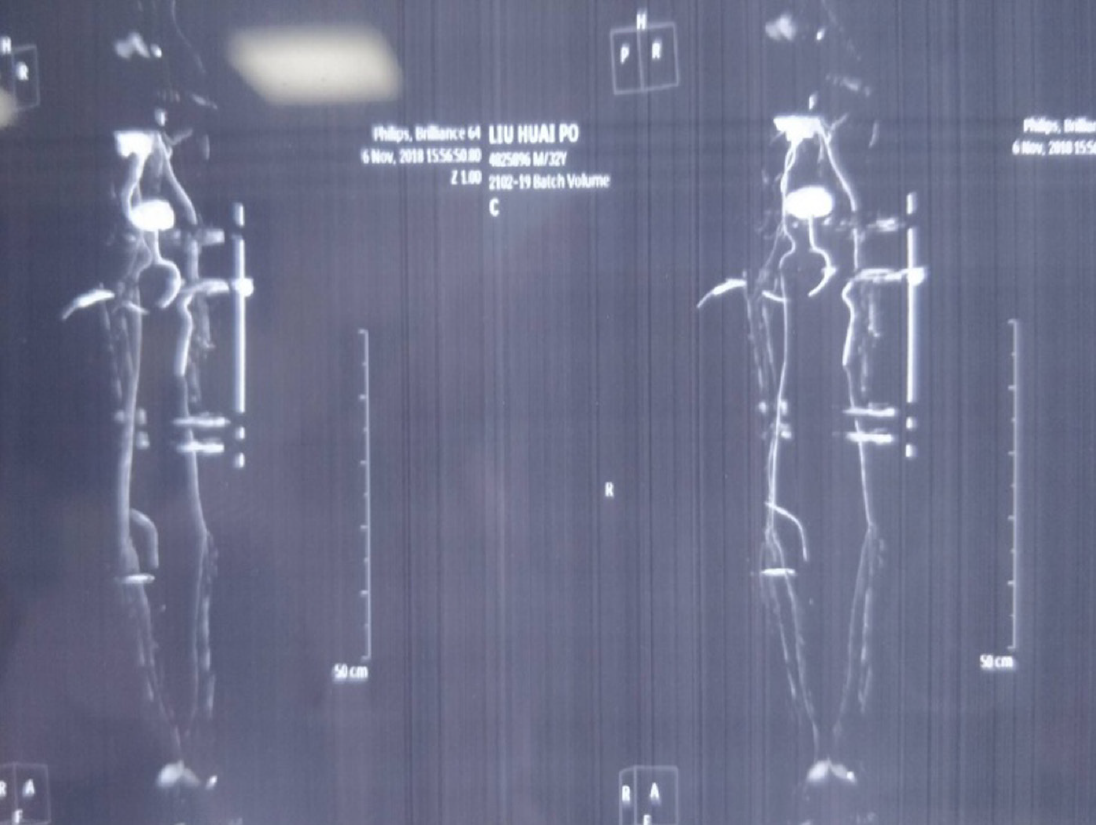
A 32-year-old male patient was transferred to our hospital because of hip and lower limb injuries caused by heavy objects. The initial diagnosis was left femoral artery defect injury and lower limb injuries. The left femoral artery defect injury was treated by anastomosis with artificial vascular graft (approximately 10 cm in length) and lower limbs injuries were managed using femoral external fixation. After 14 d, a recurrent deep right wound developed, with an exposure of the artificial vascular graft at the distal anastomosis level and underlying tissue necrosis. His family history and past history had nothing notable.
The patient had no major trauma or damage to the blood vessels before.
There is nothing special about the patient’s personal and family history.
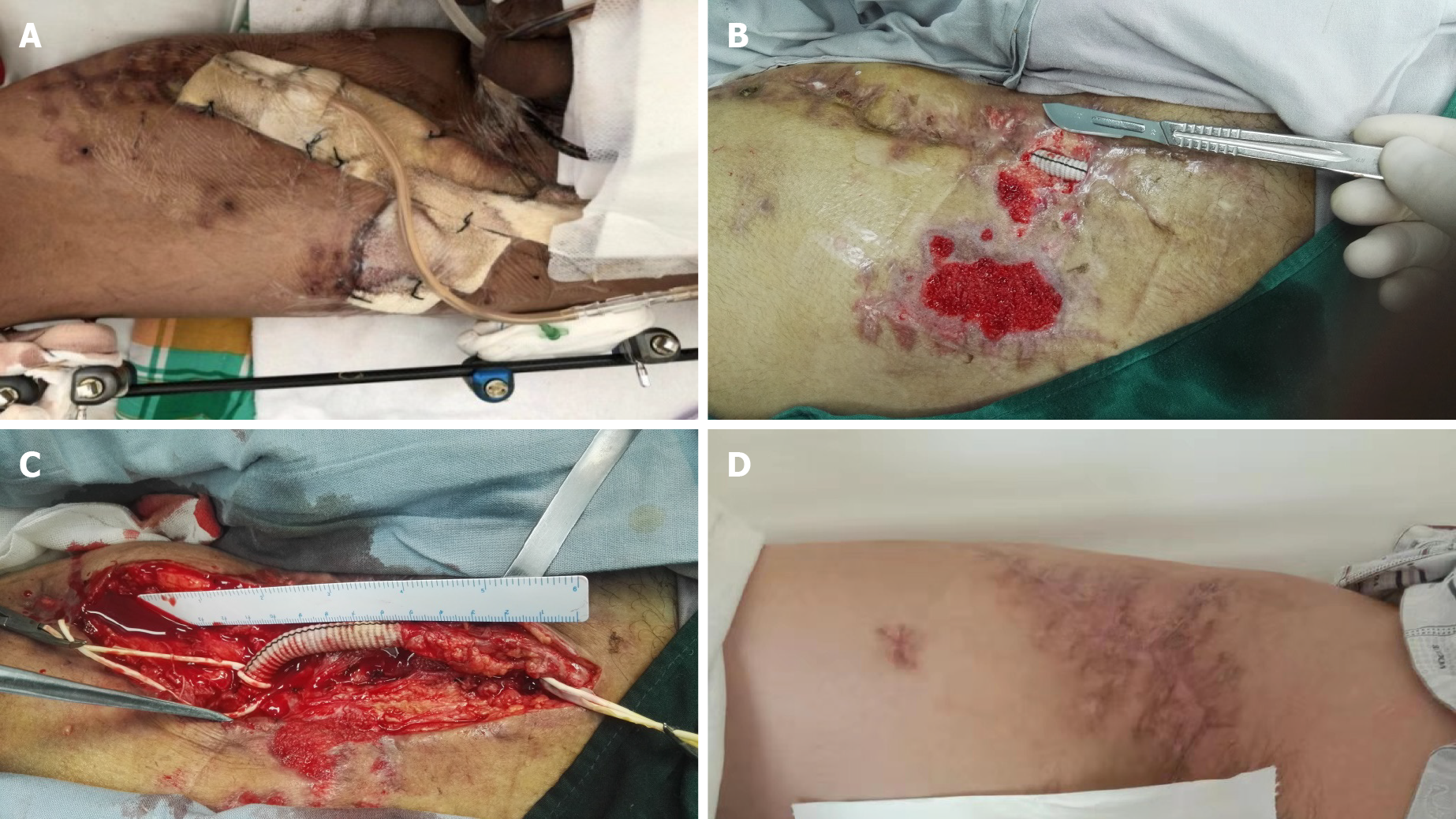
In the physical examination, the external fixator was firmly fixed. An obvious severe inguinal infection and extensive exposure of an artificial vascular graft (approximately 3.5 cm in length) that was not embedded in the surrounding tissues, with underlying tissue necrosis, were observed. The peripheral sensation of the left lower limb was normal, the peripheral blood supply of left lower limb was good, and left dorsalis pedis artery and posterior tibial artery pulsation was touched.
Repeated bacteriological cultures were positive for Acinetobacter baumannii.
The first computed tomography angiography was performed in another hospital, which revealed that the left femoral artery defect injury was repaired by anastomosis with an artificial vascular graft (Figure 1).
Based on imaging findings and the pathogenic microorganism identified, we finally diagnosed the patient with an obvious severe inguinal infection caused by Acinetobacter baumannii and extensive exposure of the artificial vascular graft (approximately 3.5 cm in length) that was not embedded in the surrounding tissues.
The artificial vascular graft was completely excised and the blood flow was unimpeded fortunately (Video). After initial debridement of the perivascular necrotic tissue, a continuous VSD device of 125 mmHg (17 kPa) was applied in the inguinal wound. A silicon-based dressing was used for coverage of the visible graft material and native artery in the wound; regular changes of the dressings were usually done in the ward (Figure 2).
Two weeks later, the wound was closed to protect both the anastomosis and the artificial vascular graft using rectus femoris muscle flap transposition. The large infected surgical wound was filled with a muscle flap adjacent to the biological graft and no bleeding was detected from both the anastomosis and the arterial wall. We did not add any antibiotic for Acinetobacter baumannii infection because of the antibiotic resistance. The muscle flap survived and the wound of the patient at risk healed successfully after 3 wk.
Vascular graft infection due to injury rarely involves the femoral region. It remains one of the most challenging complications in vascular trauma surgery. Local muscle flap transposition demonstrated the promising performance in the treatment of low-grade infections in the early stage but carried an unreasonable risk of failure in the high-grade infections sustained by high-virulence bacteria[1-3,5,9-11].
In our opinion, the careful analysis of patient’s medical history is particularly important. Infection occurring after the left femoral artery surgical revascularization, culture positivity for Acinetobacter baumannii and methicillin-resistant species, and exposure of the arterial-graft anastomosis were poor prognostic indicators for graft preservation. Furthermore, the wound infection in the groin had exposed the anastomosis and failed prior debridement and antibiotic treatment. Moreover, due to the poor general condition of the wound infected by Acinetobacter baumannii, surgeries including VSD and muscle flap transposition were not conducted simultaneously. Several studies have revealed a significantly increased risk for graft infection in the groin when multiple repeated surgeries were conducted[12-14].
Previous studies have reported many risk factors for vascular graft infections, such as groin incisions, wound infections, and comorbidities. A retrospective study[15] involving 39 of 438 patients with a vascular graft infection demonstrated that renal insufficiency, hemorrhage, incisional surgical site infections, and longer procedure time could increase the risk factor for vascular graft infections. A retrospective and descriptive study[16] including 223 patients receiving abdominal or lower extremity revascularization surgery identified risk factors for vascular graft infections and revealed that diabetes mellitus, hemoglobin A1c more than 7.0, blood glucose more than 180 mg/dL, and lack of mobility were preoperative risk factors; perioperative hypoxemia and hemostatic agents were intraoperative factors; admission to skilled nursing facility or acute rehabilitation facility and unscheduled clinic visits were postoperative factors. The warm, wet environment in the groin area combined with innumerable skin folds leads to a high burden of bacteria, which makes deep perivascular infected groin wounds particularly challenging to heal[17,18]. VSD strategy and graft preservation had been warned in case of Acinetobacter baumannii infection and sepsis; however, the wound of our patient at risk healed successfully. In our case, the initial operation was conducted to reduce infection of the groin wound and vascular graft. Our surgical team had long-standing experience in the treatment of various wounds by using the VSD method, which can be useful in the prevention of peripheral arterial injury in certain challenging cases and in the development of a hybrid approach.
To our knowledge, active wound treatment with VSD therapy is considered a vital important modality to accelerate wound healing and improve prognosis. Selective adjunctive strategies including muscle flap coverage probably contributed to the cover of dead space, and a decreased time of wound healing and risk of recurrent infections[4]. The muscle flap combined with VSD therapy can be useful for speeding up wound healing[5].
Several studies demonstrated the excellent performance in the use of muscle flaps for treating chronically wound beds infected with various pathogenic organisms[6-8]. These flaps have been shown to reduce healing time, lower wound bed bacterial count, improve antibiotic delivery, and obliterate dead space, thus decreasing the possibility of recurrent infection. In the present case, the muscle flap coverage was used for stable wound coverage regardless of the fate of the graft.
In addition to aggressive serial debridement and VSD, the rectus femoris muscle flap provided the best solution to promote healing of the infected field in this case. While the underlying mechanisms and biological effects of the muscle flap on the injured vessel wall remain to be proven, it is possible conduct trials with this technique to significantly improve limb salvage rates and avoid the related requirement to resort to extra-anatomic bypass, which carries high morbidity and mortality. In our opinion, with multidisciplinary treatment of malnutrition, infection, and local wound coverage along with the creative use of muscle flap closures, improved graft salvage rates and markedly increased limb salvage rates can be achieved.
This case highlights that VSD technique and rectus femoris muscle flap transposition could be considered in patients presenting with a severe infected groin wound with Acinetobacter baumannii biological vascular graft infection resulting in extensive graft exposure, especially in consideration of treatable conditions.
| 1. | Zetrenne E, McIntosh BC, McRae MH, Gusberg R, Evans GR, Narayan D. Prosthetic vascular graft infection: a multi-center review of surgical management. Yale J Biol Med. 2007;80:113-121. [PubMed] |
| 2. | Zetrenne E, Wirth GA, McIntosh BC, Evans GR, Narayan D. Managing extracavitary prosthetic vascular graft infections: a pathway to success. Ann Plast Surg. 2006;57:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Armstrong PA, Back MR, Bandyk DF, Johnson BL, Shames ML. Selective application of sartorius muscle flaps and aggressive staged surgical debridement can influence long-term outcomes of complex prosthetic graft infections. J Vasc Surg. 2007;46:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Illig KA, Alkon JE, Smith A, Rhodes JM, Keefer A, Doyle A, Serletti J, Shortell CK, Davies MG, Green RM. Rotational muscle flap closure for acute groin wound infections following vascular surgery. Ann Vasc Surg. 2004;18:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Seify H, Moyer HR, Jones GE, Busquets A, Brown K, Salam A, Losken A, Culbertson J, Hester TR. The role of muscle flaps in wound salvage after vascular graft infections: the Emory experience. Plast Reconstr Surg. 2006;117:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | De Santis F, Chaves Brait CM, Caravelli G, Pompei S, Di Cintio V. Salvage of infected vascular graft via 'perivascular venous banding' technique coupled with rectus abdominis myocutaneous muscle flap transposition. Vascular. 2013;21:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Herrera FA, Kohanzadeh S, Nasseri Y, Kansal N, Owens EL, Bodor R. Management of vascular graft infections with soft tissue flap coverage: improving limb salvage rates--a veterans affairs experience. Am Surg. 2009;75:877-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Andersson S, Monsen C, Asciutto G, Acosta S. EndoVAC hybrid therapy for salvage of patients with infected femoral artery reconstructions. Anaesthesiol Intensive Ther. 2019;51:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Evans GR, Francel TJ, Manson PN. Vascular prosthetic complications: success of salvage with muscle-flap reconstruction. Plast Reconstr Surg. 1993;91:1294-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Dacey LJ, Miett TO, Huntsman WT, Colen LB, Schned AR, McDaniel MD. Efficacy of muscle flaps in the treatment of prosthetic vascular graft infections. J Surg Res. 1988;44:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Maser B, Vedder N, Rodriguez D, Johansen K. Sartorius myoplasty for infected vascular grafts in the groin. Safe, durable, and effective. Arch Surg. 1997;132:522-5; discussion 525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Russell RC, Graham DR, Feller AM, Zook EG, Mathur A. Experimental evaluation of the antibiotic carrying capacity of a muscle flap into a fibrotic cavity. Plast Reconstr Surg. 1988;81:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Daigeler A, Dodic T, Awiszus F, Schneider W, Fansa H. Donor-site morbidity of the pedicled rectus femoris muscle flap. Plast Reconstr Surg. 2005;115:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Depuydt K, Boeckx W, D'Hoore A. The pedicled tensor fasciae latae flap as a salvage procedure for an infected abdominal mesh. Plast Reconstr Surg. 1998;102:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Anagnostopoulos A, Ledergerber B, Kuster SP, Scherrer AU, Näf B, Greiner MA, Rancic Z, Kobe A, Bettex D, Hasse B; VASGRA Cohort Study. Inadequate Perioperative Prophylaxis and Postsurgical Complications After Graft Implantation Are Important Risk Factors for Subsequent Vascular Graft Infections: Prospective Results From the Vascular Graft Infection Cohort Study. Clin Infect Dis. 2019;69:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Ratliff CR, Strider D, Flohr T, Moses D, Rovnyak V, Armatas J, Johnson J, Okerlund A, Baldwin M, Lawson M, Fuhrmeister S, Tracci MC, Upchurch GR, Cherry KJ. Vascular Graft Infection: Incidence and Potential Risk Factors. J Wound Ostomy Continence Nurs. 2017;44:524-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Masaki F, Shuhei Y, Riko K. Wound salvage with a fasciocutaneous flap after artificial vascular graft infection. Plast Reconstr Surg. 2008;121:1863-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Herrera FA, Easter D, Dobke M. Management of vascular graft infections with soft tissue coverage. J Surg Educ. 2008;65:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta SK S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Yuan YY