Published online Jan 6, 2021. doi: 10.12998/wjcc.v9.i1.8
Peer-review started: September 8, 2020
First decision: October 18, 2020
Revised: October 30, 2020
Accepted: December 6, 2020
Article in press: December 6, 2020
Published online: January 6, 2021
Processing time: 114 Days and 21.1 Hours
The outbreak of coronavirus disease-2019 (COVID-19, previously known as 2019 nCoV) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Wuhan City, China, has spread rapidly around the world. Most patients from the first cluster had an epidemiological connection to the Wuhan's Huanan Seafood Wholesale Market. Available evidence has shown that SARS-CoV-2 can be easily transmitted from person to person through close contact and respiratory droplets, posing a substantial challenge to public health. At present, the research on SARS-CoV-2 is still in the primary stages. However, dexa-methasone and remdesivir are appeared to be promising medical therapies. Still, there is no definite specific treatment, and the mainstay of treatment is still focused on supportive therapies. Currently, over 150 vaccines are under investigation. It is necessary to understand the nature of the virus and its clinical characteristics in order to find effectively manage the disease. The knowledge about this virus is rapidly evolving, and clinicians must update themselves regularly. The present review comprehensively summarizes the epidemiology, pathogenesis, clinical characteristics, and management of COVID-19 based on the current evidence.
Core Tip: Coronavirus disease-2019 (COVID-19) is an emerging, rapidly evolving disease that spreads rapidly worldwide. Our understanding of COVID-19 is changing very rapidly, and the discovery of new findings occurs daily. So, clinicians must update themselves regularly. This review makes an effort to summarize the epidemiology, clinical manifestations, management of COVID-19 based on the current evidence.
- Citation: Krishnan A, Hamilton JP, Alqahtani SA, Woreta TA. COVID-19: An overview and a clinical update. World J Clin Cases 2021; 9(1): 8-23
- URL: https://www.wjgnet.com/2307-8960/full/v9/i1/8.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i1.8
The coronavirus disease-2019 (COVID-19) outbreak, which emerged in Wuhan, Hubei, China, in late 2019, has rapidly spread worldwide[1]. The pathogen responsible was identified as the 2019 novel coronavirus[2], which was subsequently renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the World Health Organization (WHO). Due to the rapid spread of this infection with global consequences, on March 11, 2020, WHO declared COVID-19 a pandemic and called for aggressive actions from all countries[3]. Epidemiological data suggest that initially reported cases in China had an exposure history to the Huanan Seafood Market[4]. With the escalated spread of the infection, it was discovered that SARS-CoV-2 could be transmitted from person to person through close contact and respiratory droplets, posing a substantial challenge to public health[5]. The clinical manifestations of patients with COVID-19, including fever, shortness of breath, cough, headache, myalgias, diarrhea, fatigue, sore throat, anosmia, ageusia, chest pain, hemoptysis, sputum production, rhinorrhea, nausea, vomiting, skin rash, impaired consciousness, and seizures[5,6]. Most cases have spontaneous recovery. Data from China had suggested that patients with underlying diseases had much higher fatality rates than those without any preexisting complications such as hypertension, diabetes, chronic respiratory disease, cardiovascular disease, and cancer[7]. The most common complications of COVID-19-related adverse respiratory distress syndrome (ARDS) include cardiac injury, acute kidney injury (AKI), liver dysfunction[8,9]. Since the viral pathogenesis and proliferation bases are unclear, there is still no vaccine or definitive treatment. Since the knowledge about this virus is rapidly evolving, clinicians must update themselves regularly. This present review aims to explore the epidemiology, clinical manifestations, and management of COVID-19 based on the current evidence.
Coronaviruses (CoVs) are enfolded, positive-sense, single-stranded RNA viruses with varying diameters (60-140 nm; 100 × smaller than an average human cell). The crown-like look of the spike-like superficial outgrowths under the electron microscope fetched the name coronavirus[10]. These viruses can infect birds, humans, and other mammals, developing respiratory, neurologic, hepatic, and enteric diseases[11]. Six CoVs are identified as pathogenic. The CoVs are divided into four genera: α-, β-, γ-, and δ-CoVs. The α- and β-CoVs can infect mammals, while γ- and δ-CoVs tend to affect birds. Four viruses, including HCoV-OC43, HCoV-NL63HCoV-HKU1, and HCoV-229E, have been transmitting in humans and commonly develop minor pulmonary infections[12]. The other two known β-CoVs, Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV), can cause severe, deadly pulmonary disease[13]. These fatal CoVs emerge periodically in different areas. The first SARS-CoV outbreak was in 2002[14], the second one, MERS-CoV, was in 2012[15], followed by the recent SARS-CoV-2 infection, which has threatened the global population. It appears that SARS-CoV-2 enters into host cells through angiotensin-converting enzyme 2 (ACE2), the same functional receptor as SARS-CoV[16].
On December 12, 2019[17], a case of viral pneumonia was observed, and other CoVs, influenza, and bacterial pathogens were ruled out by laboratory testing. The virus was ultimately recognized as a CoV, with over 95% and over 70% similarity with bat CoV and SARS-CoV[18], and Chinese authorities announced a new type of CoV (novel CoV) was isolated. Several initial patients had a general link to the Huanan Wholesale Seafood Market, and on January 1, this market was closed. Given the first cases originated in the market with a broad range of wild animals, the infection was possibly transmitted from animal to human. The number of new cases increased exponentially in Wuhan city and then internationally after the market was closed. These statistics were suggestive that human-to-human transmission occurred[19].
The CoV was isolated from the lower respiratory tract of patients with unidentified pneumonia in Wuhan and classified as a new type of CoV (SARS-CoV-2) belonging to the genus β[17]. The spreading of SARS-CoV-2 from a human to another is documented in health care and community settings, including among people sharing living quarters. Breathing-in of droplets having the virus or contacting contaminated surfaces and introducing to eyes, mouth, and nose can result in infection. The primary mode of transmission is from the respiratory tract indirectly via fomites or droplets, to a lesser extent, via aerosols. As MERS-CoV and SARS-CoV can infect the human gastrointestinal tract[18], it has been indicated that fecal-oral transmission may occur for SARS-CoV-2[19].
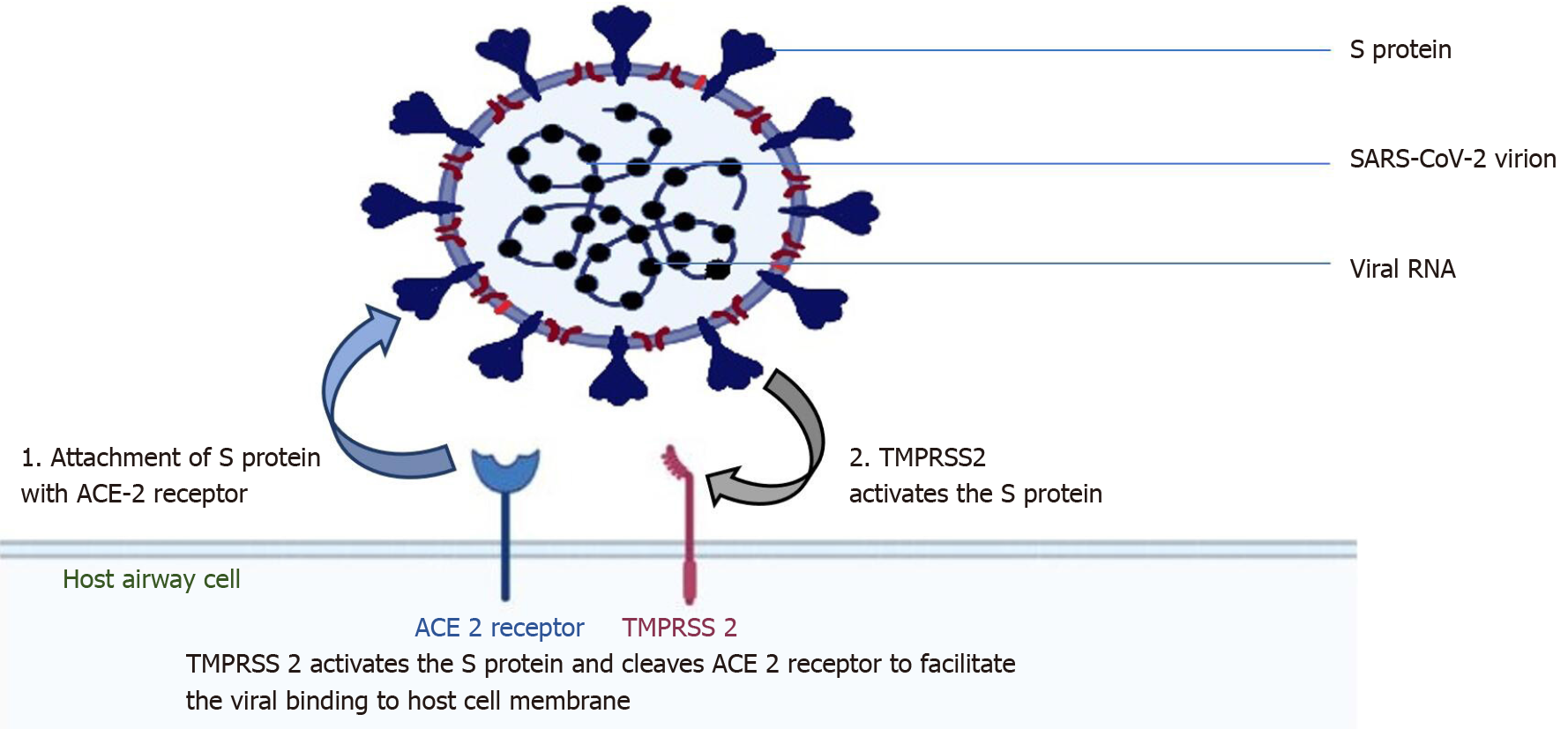
The surface spike protein ("S" protein) of the SARS-CoV-2 supports a strong interaction with human ACE2 as the receptor to infect human cells, which means that the virus poses a significant public health risk for human transmission by the S-protein-ACE2 binding pathway[20]. SARS-CoV-2 targets these ACE2 receptors in cells lining the upper airway: The nasal and bronchial epithelial cells and pneumocytes (Figure 1). ACE2 is also expressed in the upper esophagus, cholangiocytes, enterocytes of the small intestine, colon, renal proximal tubule cells, myocardial cells, and bladder[21]. The type II transmembrane serine protease (TMPRSS2), existing superficially on the host cell, supports viral uptake by slicing ACE2 and stimulating the S protein[22]. Activation of the S protein mediates the SARS-CoV-2 entry into host cells[23].
Therefore, TMPRSS2 and ACE2 are the principal components of viral entry, and activation of TMPRSS2 is required for S protein attachment. ACE2 and TMPRSS2 are also present in type II alveolar epithelial cells[24]. Exposed people are susceptible to SARS CoV-2, with an incubation period of generally 3-7 d (within 14 d); most patients (97.5%) present with symptoms within 11.5 d of getting infected[25]. The degree of viral load elevation correlates with the virus's transmissibility, but no significant difference in viral loads between symptomatic and asymptomatic patients has been reported, indicating the potential of virus transmission from asymptomatic carriers[26].
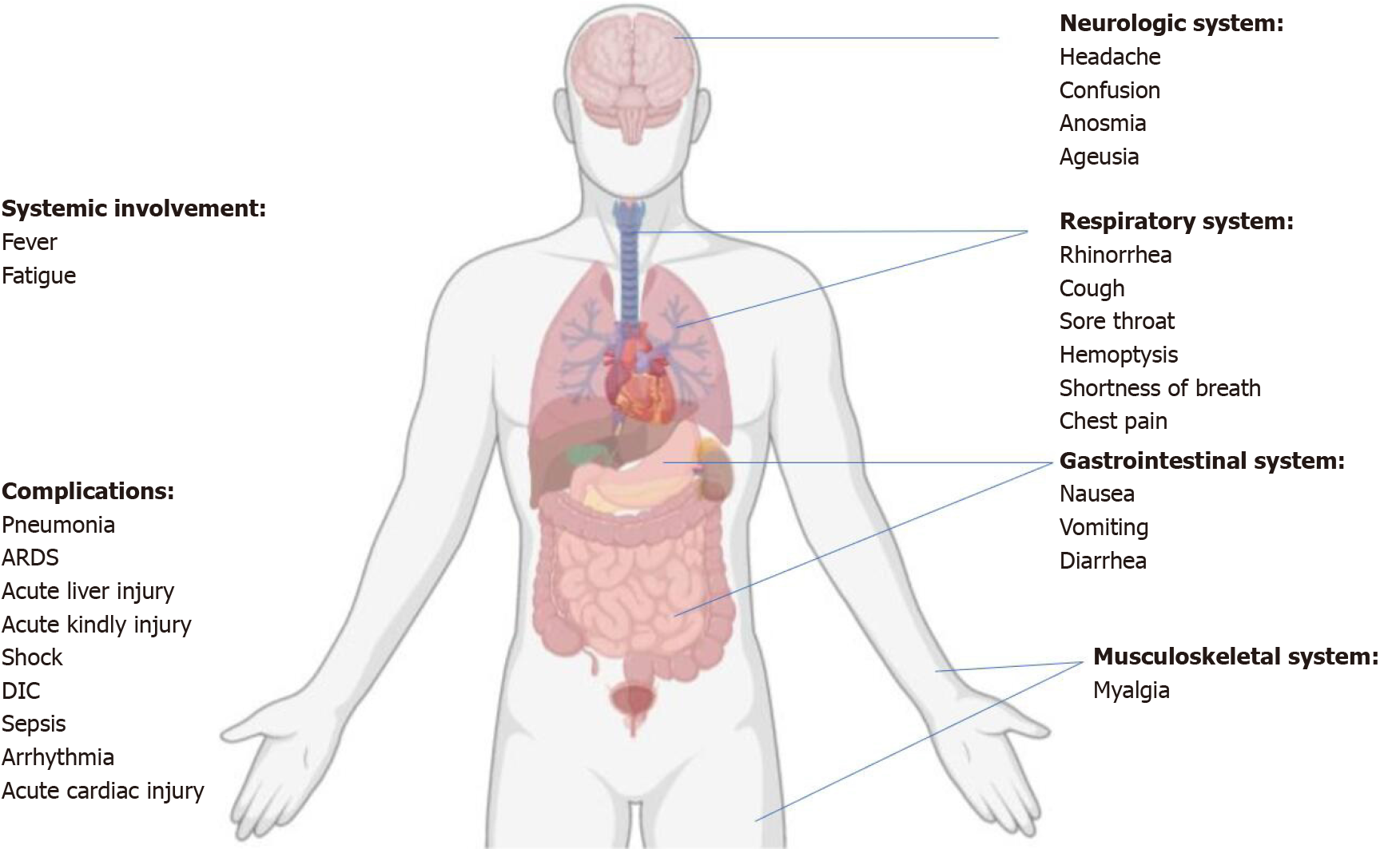
The symptoms of SARS-CoV-2 infection can be nonspecific. The most common clinical manifestations include pyrexia (88.7%), cough (67.8%), fatigue/tiredness (38.1%), sputum production (33.4%), dyspnea (18.6%), sore throat (13.9%), and headache (13.6%)[26,27]. Especially, some patients were afebrile or confirmed to have an asymptomatic infection[28]. Multiple systems are involved, including respiratory (rhinorrhea, cough, sore throat, chest pain, shortness of breath, and hemoptysis), gastrointestinal (diarrhea, nausea, and vomiting), and neurologic (confusion, headache, anosmia, and ageusia), musculoskeletal (myalgia)systems (Figure 2). Most adult patients with COVID-19 present with mild flu-like symptoms, while 14% progress to a severe condition involving oxygen support and hospitalization, and 5% may require admission to the intensive care unit (ICU)[6,29]. The definitions of asymptomatic, mild, moderate, severe, and critical are summarized in Table 1[30].
| Clinical types | Symptoms/clinical markers |
| Asymptomatic | Individuals who test positive for SARS-CoV-2 by COVID-19 nucleic acid test. Without any clinical symptoms and signs, and chest imaging is normal. |
| Mild | Presence of various signs and symptoms of COVID-19 (e.g., fever, fatigue, myalgia, cough, headache, sore throat, runny nose, sneezing), or digestive symptoms (nausea, vomiting, abdominal pain, diarrhea) without shortness of breath or abnormal chest imaging. |
| Moderate | Presence of pneumonia (frequent fever, cough) with no obvious hypoxemia (SpO2 ≥ 94% on room air at sea level); chest CT with lesions. |
| Severe | Patients with respiratory frequency > 30 breaths/minute; pneumonia with hypoxemia (SpO2 < 94%)on room air at sea level; a ratio of the arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mmHg or lung infiltrates > 50%. |
| Critical | Acute respiratory distress syndrome may have shock, encephalopathy, myocardial injury, heart failure, coagulation dysfunction, acute kidney injury, and/or multiple organ dysfunctions. |
The laboratory investigations are usually nonspecific. Most patients have a normal or decreased count of white blood cells; lymphocytes and platelet counts were lower, with extended activated thromboplastin time[25]. In patients with severe infection, the neutrophil count, blood urea, creatinine, and D-dimer values were significantly more, and the lymphocyte levels continued to decline, and the degree of lymphocytopenia correlates with disease severity. Co-infection by bacteria can be confirmed with increased procalcitonin levels. Initial plasma IL-1β, IL-1Rα, IL-7, IL-8, IL-9, IL-10, basic FGF, GCSF, GMCSF, IP10, IFN-γ, MCP1, MIP1A, MIP1B, PDGF, TNF-α, and vascular endothelial growth factor concentrations were higher in COVID-19 patients as compared to healthy controls[2]. Severe cases admitted to the ICU showed high levels of proinflammatory cytokines, including IL2, IL7, IL10, IP10, GCSF, TNFα MCP1, and MIP1α; all these could promote disease severity[2]. Confirmatory laboratory diagnosis usually relies on a real-time reverse transcriptase-polymerase chain reaction assay (RT-PCR) to identify viral RNA by targeting the E region of the pan beta-CoV or other more specific regions such as the N region (or RdRp)[2,17,30].
The full SARS-CoV-2 genome has been sequenced, and samples can be collected from the upper respiratory tract (nasopharyngeal and oropharyngeal) and lower respiratory tract (expectorated sputum, endotracheal aspirate, or bronchoalveolar lavage) of patients with suspected SARS-CoV-2 infection for diagnosis by real-time RT-PCR method[28,31]. Higher rates of positive findings occur in samples from the lower respiratory tract[32]. Chest x-ray typically displays bilateral infiltrates; however, the results may be normal in the initial stage. Multifocal ground glass changes on chest computed tomography (CT) scans, which are more specific and sensitive, are typical of viral pneumonia. As the infection progresses, bilateral, multilobular, and subsegmental areas of consolidation are seen on chest CT[2,7].
Complications of COVID-19 infections included ARDS, arrhythmia, shock, RNAaemia, AKI, acute cardiac injury, liver dysfunction, vascular thrombosis, and secondary infections (Figure 2)[2,32]. Most adult patients with COVID-19 have a good prognosis, but the patients aged ≥ 60 years and those with chronic underlying diseases such as respiratory disease, diabetes, obesity, and hypertensive heart disease, are at a greater risk for developing a severe or critical illness from COVID-19. The severity of the diseases is directly related to poor clinical outcomes, and the disease tends to progress more rapidly in older adults. In addition, the time interval between symptom onset and death is shorter among elderly patients (≥ 65 years)[30]. The immune status of newborns and the aged population may be poor and hence require special care.
At present, remdesivir has been recently recognized as a promising antiviral drug, and the Food and Drug Administration (FDA) approved emergency authorization for its use. However, currently, there are no FDA-approved antiviral treatments or vaccines against COVID-19. The first and foremost step is to isolate patients, as well as trace and quarantine contacts as early as possible because even asymptomatic infection may lead to disease transmission[26]. The main strategies are symptomatic treatment and supportive care, such as treating underlying diseases, maintaining vital signs, blood pressure, oxygen saturation, and treating complications like secondary infections or organ failure. Supportive therapy with respiratory and renal replacement support may be necessary, and the patients must maintain hydration and electrolyte balance. Maintaining nutrition and controlling fever and cough are critical. Patients have been found to have very high insulin requirements and require heavy sedation, need for anticoagulation, and extracorporeal membrane oxygenation[8]. Regular, irrational use of antibiotics and antivirals are not recommended, and they are needed only for suspected or proven cases. The most commonly used potential treatment agents and their mechanisms of action for COVID-19 infections are summarized in Table 2[33].
| Potential treatment agents | Mechanism of action |
| Corticosteroid | Anti-inflammatory effect |
| Remdesivirand ribavirin | Inhibition of the RNA-dependent RNA polymerase |
| Interferon therapy | Inhibition of viral entry, transcription, replication, translation, assembly |
| Proteaseinhibitors (lopinavir/ritonavir) | Inhibition of papain-like protease and 3C-like protease |
| Hydroxychloroquine and chloroquine | Inhibition of endosomal acidification and negatively influences virus–receptor binding, as well as interfere with the glycosylation of cellular receptors of SARS-CoV |
| Oseltamivir | Neuraminidase inhibitor |
| Tocilizumab | A recombinant monoclonal antibody that competitively inhibits the binding of IL-6 to its receptor. |
| Convalescent plasma | Neutralizing the SARS-CoV-2 antibodies |
At present, systemic corticosteroid administration is empirically used for severe complications, such as ARDS, acute cardiac injuries, acute kidney injuries, and patients with higher D-dimer levels, to suppress cytokine storm[2,7]. A decrease in mechanical ventilation duration and overall mortality could be brought about by early dexamethasone administration in patients with established moderate-to-severe ARDS[34]. So, Glucocorticoids can be considered for a short period of time according to the degree of dyspnea and the progression of chest imaging. In the recovery trial, the patients having confirmed COVID-19 infection were divided into two treatment arms: dexamethasone (n = 2104) and usual care (n = 4321). In patients who were symptomatic for more than seven days and required advanced respiratory support, dexamethasone reduced 28-d all-cause mortality compared to the usual care [22.9% vs 25.7%; 95%CI, 0.83 (0.75–0.93)][35]. Chloroquine has an immune-modulating activity and could effectively inhibit the pH-dependent replication pathways of many viruses and subdues the generation/discharge of TNF-α and IL-6[36]. It functions as an autophagy inhibitor and disrupts infection and replication of the virus[37]. Still, there is not enough data at this time to prove that hydroxychloroquine and chloroquine are effective treatments for COVID-19. The FDA recently specified that it was no longer reasonable to believe that chloroquine and hydroxychloroquine effectively treated COVID-19 and revoked their emergency use authorization for these medications[38].
Convalescent plasma (CP) therapy is a classic adaptive immunotherapy and has been applied to prevent and treat many infectious diseases for more than one century. In SARS-CoV-2, the anticipated mechanism of action is to bind the transfused antibodies to the pathogen, resulting in antibody-dependent cellular cytotoxicity, phagocytosis, or direct viral neutralization. Neutralizing antibodies have a significant role in blocking viral infections, consequently supporting the virus's clearance while controlling disease or acute infection progression during the chronic phase. A multicenter, randomized trial reported no variance in achieving clinical betterment within 28 d of infection with COVID-19 (n = 103) in patients who randomly received CP (51.9%) compared to the usual treatment alone (43.1%)[39]. However, the potential clinical advantage and risk of convalescent blood products in COVID-19 remain uncertain. Alternative methods being studied include the use of monoclonal antibodies and CP-derived hyperimmune globulin targeting the SARS-CoV-2[40].
Remdesivir (GS-5734) is a broad-spectrum antiviral medication. It is a 1′-cyano-substituted adenosine nucleotide analog prodrug that can inhibit the Ebola virus[41] can act against many RNA viruses. Even it can inhibit MERS-CoV and SARS-CoV at a low dose. Remdesivir has been recently recognized as one of the most promising drugs against SARS-CoV-2 pneumonia. A placebo-controlled trial evaluated the efficacy of intravenously administered remdesivir over placebo in patients (n = 1063) with confirmed COVID-19 infection having lower pulmonary tract involvement. The patients in the remdesivir group recovered quickly (11 d) when compared to those who were administered with placebo (15 d)[42]. However, no significant difference in mortality between these two groups. Thus the beneficial effect of remdesivir on survival remains in question. Baricitinb, protease inhibitors (lopinavir/ritonavir), ribavirin, and interferon-α, have been indicated as potential therapies for patients with acute respiratory symptoms[43,44]. Earlier studies confirmed that the lopinavir and ritonavir were used to treat the human immunodeficiency virus and were also suggested as providing positive outcomes in MERS-CoV and SARS-CoV patients[45,46]. However, adverse reactions such as nausea, diarrhea, vomiting, elevated transaminase and lactate levels, icterus, and dyslipidemia can occur following combined therapy with lopinavir/ritonavir[32]. When combined therapy with lopinavir/ritonavir is used in combination, it is advised to monitor side effects. The interaction of these medicines with other concomitant drugs should be monitored carefully. Routine use of lopinavir, ritonavir, and oseltamivir is not recommended for COVID-19.
Therapeutic lifestyle interventions: Considering the numerous unknowns surrounding the COVID-19, the absence of treatment, and stopping fast-growing disease transmission, many countries have chosen strict lockdown measures. The development of new antiviral medication presents several challenges and involves effort and substantial time for drug design and validation. Hence, exploring the repurposing, lifestyle interventions can provide alternatives and support therapy against COVID-19 because an unhealthy lifestyle may substantially weaken the immune system and may more prone individuals to greater susceptibility to infectious diseases[47]. By lifestyle interventions, in this context, we are mainly representing to effects of nutrition, sleep, psychosocial stress, alcohol, and smoking on individual metabolic health factors now evidently revealed to be leading comorbidities associated with COVID-19 related deaths, including, but not limited to, metabolic, cardiovascular and renal disease[48,49]. A healthy lifestyle is also an essential measure that an individual can adapt to keep the immunity healthy and strong. For instance, available literature suggests that obesity could double the odds of hospital admission and worsen the outcome in COVID-19[50]. In addition, unhealthy lifestyle habits, like smoking, poor nutrition, and associated diseases, are strongly correlated to poor outcomes[49]. SARS-CoV-2 infection development mainly varies upon the interaction between the virus and the individual's immune system. On the other hand, viral factors like the type of virus, virus viability, mutation potential, and the individual immune system elements like gender, age, genetics, and nutritional status contribute to the severity of the diseases[51].
Nutrition: Healthy and balanced nutrition strengthens the immune system and supports the cells of the immune system by generating a significant reaction against pathogens and resolving the infections quickly[52]. Mounting evidence shows the impact of nutritional components on the immune system's functioning through various mechanisms, including modulation of intestinal microbiota[53]. As A Result, nutrition may directly impact the risk of SARS-CoV-2 infection and its prognosis[54,55]. Micronutrients, including several vitamins (A, B, C, D, and E) and minerals (minerals zinc, iron, magnesium, selenium, iodine, copper, and polyphenols), play essential roles in supporting the immune system functions. In addition, vitamins have antioxidant properties, affect the production and activity of antimicrobial proteins, and promote cytokine production[55]. The deficiency of micronutrients suppresses the immune system by dysregulating the host immune system and altering the T cell and antibody-mediated immune response[56]. The deficiency of vitamins negatively may decrease resistance to infection and affect the immune function[57]. In addition, several lines of evidence of reducing the risk of respiratory infections result from supplementation with vitamins, including C, D, and E. Furthermore, vitamin D deficiency has been proposed as a potential contributor to increased susceptibility to COVID-19 illness[57]. Also, the prevalence of vitamin D deficiency is 35% greater in individuals with obesity and 24% higher with overweight than individuals with normal weight[58]. However, there is not enough data to support the use of vitamin D supplements to prevent or treat COVID-19[59]. Still, It is worth to take vitamin D supplementation to prevent vitamin D deficiency and improve the management of bone and muscle strength during the COVID-19 pandemic, irrespective of any possible link with a respiratory infection[60]. However, it is worth acknowledging that dietary supplements may not prevent or treat the disease but may decrease symptoms and facilitate recovery. Still, we need further studies to evaluate the role of nutrients and its' beneficial synergistic effect against SARS-CoV-2.
Effect of microbiota on COVID-19 and the role of probiotics: Respiratory viral infections impact the composition and function of the gut microbiota(GM)[61]. GM's role in the severity of respiratory viral infections, such as those caused by the influenza virus, was recently recognized[62]. Changes in GM may also drive the symptoms of the gastrointestinal tract associated with influenza infection. Additionally, available studies have shown that the abundance of Firmicutes is decreased, whereas the abundance of Proteobacteria and Bacteroidetes increased during influenza infection[63-65]. This viral infection influence on the GM may be mediated by systemic immune signals, including types I and II interferon, physiologic changes, and increased susceptibility to colitis[62,63].
Thus, changes in the GM seem to consequence not from the direct effects of the virus. However, from systemic inflammatory signals that travel from the lung and trigger local inflammatory responses in the gut. Current evidence suggests that SARS-CoV-2-infected individuals present deregulated GM[66,67], which might contribute to the poor outcomes in older patients and COVID-19 patients underlying preexisting conditions associated with inflammation, such as obesity, diabetes mellitus, cardiovascular and renal disorders[68,69]. Interestingly, these patients were also reported to have a lower abundance of Bacteroides compared to healthy individuals[70,71]. These results recommend that an individual's gut microbiome configuration may affect the subjects shown to be susceptibility and response to SARS-CoV-2 infection. Moreover, gut microbiota can influence antiviral immune response, thereby affecting the disease progression and may play a role in SARS-CoV-2 infection[66].
Moreover, SARS-CoV-2 infection is involved with both the innate and adaptive immune systems. There is also evidence that supplementation with probiotics has beneficial effects on the adaptive immune system by modulating both T and B cells' functions while preventing an autoimmune inflammatory response[61]. The administration of probiotics can also enhance the host's resistance against infection for older subjects and reduce the severity of viral infection in both the gastrointestinal tract and the respiratory tract[72,73]. However, the inflammatory shift's exact mechanism from the gut to the lung is not yet completely revealed. So It is essential to understand gut-lung axes and relevant to the association between proinflammatory functional dysbiosis and SARS-CoV-2, including disease progression, the importance of underlying chronic conditions, and the risk for developing complications[74]. Further clinical research trials are required to assess the effects of using probiotic administration as adjuvant therapy to manage COVID-19 patients.
Smoking: Currently, there no peer-reviewed studies that precisely assess the risk of hospitalization with COVID-19 among smokers. A meta-analysis with 2986 patients found a pooled prevalence of smoking of 7.6% (3.8%-12.4%)[75], whereas another analysis with 5960 hospitalized patients observed a pooled prevalence of 6.5% (1.4%-12.6%)[76]. However, available data suggest that smokers constituted 1.4%-18.5% of hospitalized adult patients with COVID-19[5,9,26,27,77]. Furthermore, a study from china observed that COVID-19 patients with a smoking history had a 14% higher risk of developing COVID-19 pneumonia, while the odds of the disease progressing to severe diseases and in the end to death were 14 times higher compared to patients without a smoking history[78].
On the other hand, Smoking increases the expression of ACE2 in smokers' lungs, which might facilitate host cell entry of SARS-CoV-2. The ACE2 protein levels are not only increased in the bronchial but also in the alveolar epithelium. However, this does not certainly translate into a higher risk for developing COVID-19 pneumonia[79]. Ultimately, smoking could increase the risk of contracting COVID-19 because the smokers' are more expected to touch their mouths with their fingers. Furthermore, critical conditions predispose to poor COVID-19 outcomes (respiratory, neoplastic, and cardiovascular diseases) are mostly related to smoking[80]. Also, several lines of available evidence have shown a positive correlation between smoking cessation causes an improvement in lung function and better clinical outcomes of possible COVID-19 comorbidities, which may, in turn, lead to better prognosis[81]. However, the effect of current smoking on COVID-19 is a complex and delicate matter. So, further studies are required to determine the reasons behind the reported low prevalence of current smokers among hospitalized patients with COVID-19.
Alcohol abuse: Acute and chronic alcohol use tend to compromises the immune system, increase the susceptibility to viral infections, and increase the risk of ARDS, more likely requires mechanical ventilation, have a longer intensive care unit (ICU) stay and have a higher risk of death from ARDS and a potential complication of COVID-19[82,83]. These consequences of alcohol misuse could undoubtedly complicate the prevention, treatment, and clinical recovery from COVID-19. Alcohol use induces significant defects in the defense mechanism against microorganisms by interfering with the immune system's cellular, humoral, and structural components[84]. Alcohol misuse may affect the ability to adhere to prevent the infection and regulate recommendations on physical distancing. In addition to negative effects on physical health associations, alcohol abuse may lead to or worsen preexisting conditions related to mental health problems, such as anxiety or depression, which may increase during COVID-19. Furthermore, alcohol misuse can substantially increase the risk of suicide in self-isolating COVID-19 patients and alter thoughts, judgment, behavior, and decision-making capacity, some of which could be critical factors for managing patients with COVID-19[81]. So, everyone should avoid excessive alcohol consumption, particularly in patients with COVID-19 and suspected individuals for COVID-19.
The ultimate approach for controlling this pandemic will depend on the development of an effective vaccine. In this present situation, vaccination will be the most efficient and cost-effective to prevent and control COVID-19. Clinical trials are currently in progress to facilitate the development of vaccines against COVID-19. Specifically, the S protein of SARS-CoV-2 remains a potential target for vaccine development. Several other types of vaccines, such as recombinant protein, nucleic acid (DNA and mRNA), live attenuated, and adenovirus-vectored vaccines, are in the pipeline[85]. Shown in Table 3 is a summary of the major COVID-19 vaccines under development[86-93]. Over 150 vaccines are currently under investigation, and several countries are trying to find the vaccine sooner[94-96]. It is anticipated that the first vaccines will be available in mid-2021. However, The development of the vaccine process will continue for the next few years until more clinical trials are completed, additional vaccine strategies are assessed, and host defense against SARS-CoV-2, which includes the immunity of the postinfection, is better understood. Probably not until then will global mass immunization become a reality. The groups of people receiving the first round of vaccines may have waning immunity and require boosting dose using improved second-generation generation vaccines to generate lasting COVID-19 immunity. In addition to unexposed individuals, some patients who have recovered from COVID-19 who develop poor or waning immunity may also require vaccination[97]. Another concern is that obesity becomes a worldwide phenomenon; the effects obesity has on pharmacokinetics processes (drug absorption, distribution, metabolism, and elimination) are not entirely understood[98]. On the other hand, obesity has been shown to impair immunological memory development and is also associated with T cell dysfunction. Moreover, the impaired response means a proportion of individuals with obesity remains at risk of influenza despite vaccination[99]. A similar risk with the COVID-19 vaccine would be concerning. Notably, the normal-weight individuals were the typical participant in the recently published early phase trials of vaccines against SARS-CoV-2[100-102]. These clinical trials have proven encouraging findings, using their results to adult populations with a high prevalence of obesity, 40% in the United States, 29% in England, and 13% globally carries a level of uncertainty, which may expectantly be addressed next phase of the trial. So, it is essential to understand the molecular pathologic epidemiology (MPE) of the COVID-19, which could help us understand etiologic heterogeneity, and the importance of tailored preventive strategies, depending on different risk factors and individuals' profiles. MPE can give clues to this vexing global public health problem because MPE investigation has high disease prevention relevance. After all, such research has shown that different risk factors have influenced the risks of different subtypes of one disease[103,104]. Also, It is widely accepted that a significant inter-individual variation exists in responding to an immune-based intervention. There are several factors-intrinsic host factors, extrinsic, environmental, behavioral, nutritional, and even vaccine-related factors-that may influence the human body to respond to a vaccine.
| Vaccines types | Candidate vaccine |
| Whole virus vaccines | Adenovirus-vectored vaccine |
| Live-attenuated vaccine | |
| Nucleic acid vaccines | mRNA vaccine |
| DNA vaccine | |
| Subunit vaccines | Oral recombinant protein vaccine |
| Coronavirus RBD protein-based vaccine | |
| Protein-based vaccine | |
| S-trimer recombinant protein |
Moreover, Sleep, diet, lifestyle, smoking habit, alcohol consumption, drug abuse, season, genetic differences, microbiome, immune system, and pathogenic mechanisms are among such factors[105]. The close relationship between the microbiota and the immune system is often regarded as vital in modulating the host immunity and influencing vaccine immunology targeted against a viral infection like COVID-19[106,107]. However, further research is necessary to understand the mechanisms that control our immune system's interplay and gut microbiota. Furthermore, research on molecular pathological epidemiology would help improve the prediction of response to vaccines or other forms of immune-based interventions.
In the meantime, it is important to emphasize preventative strategies to mitigate the further spread of the virus. Preventive strategies are primarily focused on the isolation of patients and careful infection control measures, including the use of personal protective equipment (PPE) in the settings of the patient care unit with suspected or confirmed infection. The Centers for Disease Control and Prevention (CDC) recommend routine use of face masks/cloth face coverings even for healthy individuals and maintaining physical distancing and personal hygiene. The course of this pandemic has rapidly changed, which also required the change from containment methods to mitigation. The current context recommendations remain regarding using a facial mask in public, but its optimization is essential for health care providers. Wearing a face mask is an additional precaution to stop infected droplets from getting into the environment, but the face masks are not a substitute for other measures meant to prevent the spread of COVID-19, such as frequent hand hygiene measures and social distancing, as these together allow avoiding droplets or aerosols of the viral particles[108]. When used together, these measures are adequate to protect the public.
Furthermore, with the increasing evidence of presymptomatic spread of COVID-19, masks may help protect people from transmission risk[109]. The WHO considers frequent hand washing with soap and water for at least 20 seconds as one of the most useful actions for COVID-19 containments. Patients with COVID-19 who have acute respiratory tract infection symptoms should maintain physical distancing, sneeze, cough with disposable tissues or clothes, and wash their hands at regular intervals.
The COVID-19 pandemic has confronted the financial, clinical, societal, and public health framework of many countries globally. As the outbreak multiplies, the global understanding of this infection has increased. However, several characteristics of the infection, transmission, and treatment remain unclear. More evidence is needed to develop public health and clinical interventions to prevent and treat infections successfully. Widespread testing to identify infections, contact tracing, and quarantine of infected patients is critical to control the spread of the virus. The development of an efficacious vaccine is essential for the prevention and limitation of COVID-19 transmission.
| 1. | World Health Organization. Coronavirus disease 2019 (COVID-19) situation report–41. [cited August 3 2020]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200301-sitrep-41-covid-19.pdf?sfvrsn=6768306d_2. |
| 2. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14346] [Article Influence: 2391.0] [Reference Citation Analysis (10)] |
| 3. | World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - March 11 2020. [cited August 3 2020]. Available from: https://www.who.int/dg/speeches/detail/whodirector-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. |
| 4. | World Health Organization. Novel Coronavirus—China. January 12 2020. [cited August 3 2020]. Available from: https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en. |
| 5. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9405] [Article Influence: 1567.5] [Reference Citation Analysis (0)] |
| 6. | Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2274] [Cited by in RCA: 1979] [Article Influence: 329.8] [Reference Citation Analysis (0)] |
| 7. | The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)-China. China CDC Weekly. 2020;2:113–122. |
| 8. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6709] [Article Influence: 1118.2] [Reference Citation Analysis (1)] |
| 9. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 13063] [Article Influence: 2177.2] [Reference Citation Analysis (4)] |
| 10. | Richman DD, Whitley RJ, Hayden FG. Clinical Virology, 4th ed. Washington: ASM Press; 2016. |
| 11. | Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 590] [Cited by in RCA: 534] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 12. | Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24:490-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1725] [Cited by in RCA: 1913] [Article Influence: 191.3] [Reference Citation Analysis (1)] |
| 13. | Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 632] [Cited by in RCA: 651] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 14. | Chan-Yeung M, Xu RH. SARS: epidemiology. Respirology. 2003;8 Suppl:S9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 421] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Wong G, Liu W, Liu Y, Zhou B, Bi Y, Gao GF. MERS, SARS, and Ebola: The Role of Super-Spreaders in Infectious Disease. Cell Host Microbe. 2015;18:398-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 16. | Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2710] [Cited by in RCA: 2662] [Article Influence: 126.8] [Reference Citation Analysis (0)] |
| 17. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17899] [Article Influence: 2983.2] [Reference Citation Analysis (2)] |
| 18. | Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 296] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 19. | Cowling BJ, Leung GM. Epidemiological research priorities for public health control of the ongoing global novel coronavirus (2019-nCoV) outbreak. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 20. | Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157:104833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 21. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4194] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 22. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14580] [Article Influence: 2430.0] [Reference Citation Analysis (3)] |
| 23. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19022] [Article Influence: 3170.3] [Reference Citation Analysis (9)] |
| 24. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1286] [Cited by in RCA: 1544] [Article Influence: 257.3] [Reference Citation Analysis (1)] |
| 25. | Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172:577-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4286] [Cited by in RCA: 3391] [Article Influence: 565.2] [Reference Citation Analysis (2)] |
| 26. | Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1252] [Cited by in RCA: 1325] [Article Influence: 220.8] [Reference Citation Analysis (0)] |
| 27. | Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6483] [Cited by in RCA: 5455] [Article Influence: 909.2] [Reference Citation Analysis (0)] |
| 28. | World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease is Suspected – Interim Guidance. 1 March 2020. [cited 3 August 2020]. Available from: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf?sfvrsn=bc7da517_2. |
| 29. | National Institutes of Health. COVID-19 Treatment Guidelines. Clinical Presentation of People with SARS-CoV-2 Infection. [cited 3 August 2020]. Available from: https://www.covid19treatmentguidelines.nih.gov/ overview/management-of-covid-19/. |
| 30. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8473] [Cited by in RCA: 7692] [Article Influence: 1282.0] [Reference Citation Analysis (0)] |
| 31. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14869] [Article Influence: 2478.2] [Reference Citation Analysis (1)] |
| 32. | Memish ZA, Al-Tawfiq JA, Makhdoom HQ, Assiri A, Alhakeem RF, Albarrak A, Alsubaie S, Al-Rabeeah AA, Hajomar WH, Hussain R, Kheyami AM, Almutairi A, Azhar EI, Drosten C, Watson SJ, Kellam P, Cotten M, Zumla A. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210:1590-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 33. | Bimonte S, Crispo A, Amore A, Celentano E, Cuomo A, Cascella M. Potential Antiviral Drugs for SARS-Cov-2 Treatment: Preclinical Findings and Ongoing Clinical Research. In Vivo. 2020;34:1597-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA, Martín-Rodríguez C, Díaz-Domínguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Añón JM, Fernández RL, González-Martín JM; dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 809] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 35. | RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7578] [Article Influence: 1515.6] [Reference Citation Analysis (7)] |
| 36. | Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 840] [Cited by in RCA: 843] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 37. | Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1228] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 38. | U. S. Food & Drug Administration. News Release: Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. 15 June 2020. [cited 11 Aug 2020]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and. |
| 39. | Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, Kong Y, Ren L, Wei Q, Mei H, Hu C, Tao C, Yang R, Wang J, Yu Y, Guo Y, Wu X, Xu Z, Zeng L, Xiong N, Chen L, Wang J, Man N, Liu Y, Xu H, Deng E, Zhang X, Li C, Wang C, Su S, Zhang L, Wang J, Wu Y, Liu Z. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:460-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 905] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 40. | Wang C, Li W, Drabek D, Okba NMA, van Haperen R, Osterhaus ADME, van Kuppeveld FJM, Haagmans BL, Grosveld F, Bosch BJ. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11:2251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 673] [Cited by in RCA: 757] [Article Influence: 126.2] [Reference Citation Analysis (0)] |
| 41. | Mulangu S, Dodd LE, Davey RT Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe-Tamfum JJ; PALM Writing Group; Sivahera B; Camara M; Kojan R; Walker R; Dighero-Kemp B; Cao H; Mukumbayi P; Mbala-Kingebeni P; Ahuka S; Albert S; Bonnett T; Crozier I; Duvenhage M; Proffitt C; Teitelbaum M; Moench T; Aboulhab J; Barrett K; Cahill K; Cone K; Eckes R; Hensley L; Herpin B; Higgs E; Ledgerwood J; Pierson J; Smolskis M; Sow Y; Tierney J; Sivapalasingam S; Holman W; Gettinger N; Vallée D; Nordwall J; PALM Consortium Study Team. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med. 2019;381:2293-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1131] [Article Influence: 161.6] [Reference Citation Analysis (0)] |
| 42. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5829] [Cited by in RCA: 5222] [Article Influence: 870.3] [Reference Citation Analysis (0)] |
| 43. | Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56:258-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 44. | Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1086] [Cited by in RCA: 1194] [Article Influence: 119.4] [Reference Citation Analysis (0)] |
| 45. | Arabi YM, Asiri AY, Assiri AM, Aziz Jokhdar HA, Alothman A, Balkhy HH, AlJohani S, Al Harbi S, Kojan S, Al Jeraisy M, Deeb AM, Memish ZA, Ghazal S, Al Faraj S, Al-Hameed F, AlSaedi A, Mandourah Y, Al Mekhlafi GA, Sherbeeni NM, Elzein FE, Almotairi A, Al Bshabshe A, Kharaba A, Jose J, Al Harthy A, Al Sulaiman M, Mady A, Fowler RA, Hayden FG, Al-Dawood A, Abdelzaher M, Bajhmom W, Hussein MA; and the Saudi Critical Care Trials group. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 46. | Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1117] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 47. | Harvard Medical School. How to boost your immunity. Harvard Health Publishing; 2014. Available from: https://www/health.harvard.edu/staying-healthy/how-to-boost-your-immunity. |
| 48. | Kalantar-Zadeh K, Moore LW. Impact of Nutrition and Diet on COVID-19 Infection and Implications for Kidney Health and Kidney Disease Management. J Ren Nutr. 2020;30:179-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Nasrawi CW, Pangborn RM. Temporal gustatory and salivary responses to capsaicin upon repeated stimulation. Physiol Behav. 1990;47:611-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, Mathieu D, Subtil F, Frobert E, Alligier M, Delaunay D, Vanhems P, Laville M, Jourdain M, Disse E; COVID Outcomes HCL Consortium and Lille COVID–Obesity Study Group. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 51. | Chatterjee SK, Saha S, Munoz MNM. Molecular Pathogenesis, Immunopathogenesis and Novel Therapeutic Strategy Against COVID-19. Front Mol Biosci. 2020;7:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Childs CE, Calder PC, Miles EA. Diet and Immune Function. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 275] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 53. | Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2116] [Cited by in RCA: 1872] [Article Influence: 124.8] [Reference Citation Analysis (0)] |
| 54. | Dhar D, Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285:198018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 404] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 55. | Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 512] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 56. | Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 725] [Article Influence: 120.8] [Reference Citation Analysis (1)] |
| 57. | Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1018] [Cited by in RCA: 1116] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 58. | Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015;16:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 620] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 59. | Covid 19 rapid evidence summary: vitamin D for covid-19 evidence summary. London: National Institute for Health and Care Excellence; 2020. [accessed 2020 Jul 1]. Available from: https://www.nice.org.uk/advice/es28/evidence. |
| 60. | SACN vitamin D and health report. London: Scientific Advisory Committee on Nutrition; 2016. [accessed 2020 May 3]. Available from: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report. |
| 61. | He Y, Wang J, Li F, Shi Y. Main Clinical Features of COVID-19 and Potential Prognostic and Therapeutic Value of the Microbiota in SARS-CoV-2 Infections. Front Microbiol. 2020;11:1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 62. | Bradley KC, Finsterbusch K, Schnepf D, Crotta S, Llorian M, Davidson S, Fuchs SY, Staeheli P, Wack A. Microbiota-Driven Tonic Interferon Signals in Lung Stromal Cells Protect from Influenza Virus Infection. Cell Rep 2019; 28: 245-256. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 63. | Groves HT, Cuthbertson L, James P, Moffatt MF, Cox MJ, Tregoning JS. Respiratory Disease following Viral Lung Infection Alters the Murine Gut Microbiota. Front Immunol. 2018;9:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 64. | Deriu E, Boxx GM, He X, Pan C, Benavidez SD, Cen L, Rozengurt N, Shi W, Cheng G. Influenza Virus Affects Intestinal Microbiota and Secondary Salmonella Infection in the Gut through Type I Interferons. PLoS Pathog. 2016;12:e1005572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 65. | Bartley JM, Zhou X, Kuchel GA, Weinstock GM, Haynes L. Impact of Age, Caloric Restriction, and Influenza Infection on Mouse Gut Microbiome: An Exploratory Study of the Role of Age-Related Microbiome Changes on Influenza Responses. Front Immunol. 2017;8:1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 66. | Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, Lu H, Zheng B, Zhang J, Yan R, Zhang H, Jiang H, Xu Q, Guo J, Gong Y, Tang L, Li L. Alterations of the Gut Microbiota in Patients with COVID-19 or H1N1 Influenza. Clin Infect Dis. 2020;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 589] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 67. | Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020; 159: 944-955. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 739] [Cited by in RCA: 1106] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 68. | Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, He Q, Wang Z, Liu Y, Liu L, Chen J, Xu L. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care. 2020;43:1392-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 69. | Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1758] [Cited by in RCA: 1959] [Article Influence: 326.5] [Reference Citation Analysis (0)] |
| 70. | Emoto T, Yamashita T, Sasaki N, Hirota Y, Hayashi T, So A, Kasahara K, Yodoi K, Matsumoto T, Mizoguchi T, Ogawa W, Hirata K. Analysis of Gut Microbiota in Coronary Artery Disease Patients: a Possible Link between Gut Microbiota and Coronary Artery Disease. J Atheroscler Thromb. 2016;23:908-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 71. | Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1129] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 72. | Sundararaman A, Ray M, Ravindra PV, Halami PM. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl Microbiol Biotechnol. 2020;104:8089-8104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 73. | Hufnagl K, Pali-Schöll I, Roth-Walter F, Jensen-Jarolim E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin Immunopathol. 2020;42:75-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 74. | van der Lelie D, Taghavi S. COVID-19 and the Gut Microbiome: More than a Gut Feeling. mSystems. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 75. | Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: a Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 2020;8:e35. [PubMed] |
| 76. | Farsalinos K, Barbouni A, Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med. 2020;15:845-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 77. | Han L, Ran J, Mak YW, Suen LK, Lee PH, Peiris JSM, Yang L. Smoking and Influenza-associated Morbidity and Mortality: A Systematic Review and Meta-analysis. Epidemiology. 2019;30:405-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Yu G. How smoking, vaping and drug use might increase risks from Covid-19. CNN 2020. Available from: https://edition.cnn.com/2020/03/20/health/coronavirus-vaping-drugs/index.html. |
| 79. | Polverino F. Cigarette Smoking and COVID-19: A Complex Interaction. Am J Respir Crit Care Med. 2020;202:471-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4318] [Article Influence: 719.7] [Reference Citation Analysis (1)] |
| 81. | Glantz SA. Reduce your risk of serious lung disease caused by corona virus by quitting smoking and vaping. University of California San Francisco—Centre for Tobacco Control Research and Education, 2020. Available from: https://tobacco.ucsf.edu/reduce-your-risk-serious-lungdisease-caused-corona-virus-quitting-smoking-and-vaping. |
| 82. | Simou E, Leonardi-Bee J, Britton J. The Effect of Alcohol Consumption on the Risk of ARDS: A Systematic Review and Meta-Analysis. Chest. 2018;154:58-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 83. | World Health Organization. Alcohol and COVID-19: what you need to know. Available from: https://www.euro.who.int/__data/assets/pdf_file/0010/437608/Alcohol-and-COVID-19-whatyou-need-to-know.pdf?ua=1. |
| 84. | Molina PE, Happel KI, Zhang P, Kolls JK, Nelson S. Focus on: Alcohol and the immune system. Alcohol Res Health. 2010;33:97-108. [PubMed] |
| 85. | Chen WH, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 Vaccine Pipeline: an Overview. Curr Trop Med Rep. 2020;Epub ahead of print 1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 322] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 86. | Johnson & Johnson. Novel Coronavirus. 2020. [cited 11 August 2020]. Available from: https://www.jnj.com/coronavirus. |
| 87. | BioSpace. Codagenix and Serum Institute of India Initiate Co-Development of a Scalable, Live-Attenuated Vaccine Against the 2019 Novel Coronavirus, COVID-19. 13 February 2020. [cited 11 August 2020]. Available from: https://www.biospace.com/article/releases/codagenix-and-serum-institute-of-india-initiate-co-development-of-a-scalable-live-attenuated-vaccine-against-the-2019-novel-coronavirus-covid-19/. |
| 88. | Park A. Inside the Company That's Hot-wiring Vaccine Research in the Race to Combat the Coronavirus. Time. 31 January 2020. Cited 11 August 2020. Available from https://time.com/5775784/coronavirus-vaccine-research/. |
| 89. | Smith J. CureVac bids to develop first mRNA coronavirus vaccine. 3 February 2020. Cited 11 August 2020. Available from https://www.labiotech.eu/medical/curevac-coronavirus-outbreak-cepi/. |
| 90. | Hennessy J. Australia’s been asked to make a coronavirus vaccine at ‘unprecedented speed’. Science Alert. 25 January 2020. Cited 11 August 2020. Available from https://www.sciencealert.com/australian-scientists-asked-to-make-coronavirus-vaccine-at-unprecedented-speed. |
| 91. | Pharmaceutical Technology. Coronavirus: Vir Biotechnology and Novavax announce vaccine plans. 13 February 2020. Cited 11 August 2020. Available from https://www.pharmaceutical-technology.com/news/coronavirus-vir-biotechnology-novavax-vaccine/. |
| 92. | Mukherjee S. The first coronavirus drug candidate is set for testing in China. Fortune. 4 February 2020. Cited 11 August 2020. Available from https://fortune.com/2020/02/03/coronavirus-vaccine-testing-in-china/. |
| 93. | Vaxart. PipelineReview.com Vaxart Announces Initiation of Coronavirus Vaccine Program. 2 February 2020. Cited 11 August 2020. Available from: https://pipelinereview.com/index.php/2020020273689/Vaccines/Vaxart-Announces-Initiation-of-Coronavirus-Vaccine-Program.html. |
| 94. | Coronavirus Vaccine Tracker: How they Work – Latest Developments. National Geographic. Cited August 3, 2020. Available from: https://www.nationalgeographic.com/science/health-and-human-body/human-diseases/coronavirus-vaccine-tracker-how-they-work-latest-developments-cvd. |
| 95. | China's coronavirus vaccines are leaping ahead – but face challenges as virus wanes. Nature News. July 31, 2020. Cited August 3, 2020. Available from https://www.nature.com/articles/d41586-020-02244-1. |
| 96. | Coronavirus: Russia plans a mass vaccination campaign in October. BBC News. August 1, 2020. Cited August 3, 2020. Available from https://www.bbc.com/news/world-europe-53621708. |
| 97. | Burton DR, Walker LM. Rational Vaccine Design in the Time of COVID-19. Cell Host Microbe. 2020;27:695-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 98. | Morrish GA, Pai MP, Green B. The effects of obesity on drug pharmacokinetics in humans. Expert Opin Drug Metab Toxicol. 2011;7:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 99. | Green WD, Beck MA. Obesity Impairs the Adaptive Immune Response to Influenza Virus. Ann Am Thorac Soc. 2017;14:S406-S409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 100. | Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1802] [Article Influence: 300.3] [Reference Citation Analysis (0)] |
| 101. | Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Wu SP, Wang BS, Wang Z, Wang L, Jia SY, Jiang HD, Wang L, Jiang T, Hu Y, Gou JB, Xu SB, Xu JJ, Wang XW, Wang W, Chen W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845-1854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1029] [Cited by in RCA: 1019] [Article Influence: 169.8] [Reference Citation Analysis (0)] |
| 102. | Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O'Dell S, Schmidt SD, Swanson PA 2nd, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH; mRNA-1273 Study Group. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med. 2020;383:1920-1931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2837] [Cited by in RCA: 2475] [Article Influence: 412.5] [Reference Citation Analysis (0)] |
| 103. | Bondarenko NA, Bondarenko NA. [Behavior disorder in rats induced by Madopar and its pharmacological correction]. Farmakol Toksikol. 1985;48:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 104. | Ogino S, Nishihara R, VanderWeele TJ, Wang M, Nishi A, Lochhead P, Qian ZR, Zhang X, Wu K, Nan H, Yoshida K, Milner DA Jr, Chan AT, Field AE, Camargo CA Jr, Williams MA, Giovannucci EL. Review Article: The Role of Molecular Pathological Epidemiology in the Study of Neoplastic and Non-neoplastic Diseases in the Era of Precision Medicine. Epidemiology. 2016;27:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 105. | Zimmermann P, Curtis N. Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 657] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 106. | Vlasova AN, Takanashi S, Miyazaki A, Rajashekara G, Saif LJ. How the gut microbiome regulates host immune responses to viral vaccines. Curr Opin Virol. 2019;37:16-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 107. | Ciabattini A, Olivieri R, Lazzeri E, Medaglini D. Role of the Microbiota in the Modulation of Vaccine Immune Responses. Front Microbiol. 2019;10:1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 108. | Centers for Disease Control and Prevention. Use of Masks to Help Slow the Spread of COVID-19. June 28, 2020. [cited August 3, 2020]. Available from https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/diy-cloth-face-coverings.html. |
| 109. | Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323:1406-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3006] [Cited by in RCA: 2696] [Article Influence: 449.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: United States
Peer-review report's scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ogino S S-Editor: Zhang H L-Editor: A P-Editor: Liu JH