Published online Feb 26, 2020. doi: 10.12998/wjcc.v8.i4.713
Peer-review started: November 29, 2019
First decision: December 30, 2019
Revised: January 3, 2020
Accepted: February 12, 2020
Article in press: February 12, 2020
Published online: February 26, 2020
Processing time: 89 Days and 12.5 Hours
The risk factors for patients with major postoperative complications immediately after liver resection have been identified; however, the intermediate and long-term prognoses for these patients have yet to be determined.
To evaluate the factors responsible for the long-term recurrence-free survival rate in patients with hepatocellular carcinoma (HCC) following anatomic hepatectomy.
We performed a retrospective analysis of 74 patients with HCC who underwent precise anatomic hepatectomy at our institution from January 2013 to December 2015. The observational endpoints for this study were the tumor recurrence or death of the HCC patients. The overall follow-up duration was three years. The recurrence-free survival curves were plotted by the Kaplan-Meier method and were analyzed by the log-rank test. The value of each variable for predicting prognosis was assessed via multivariate Cox proportional hazards regression analysis.
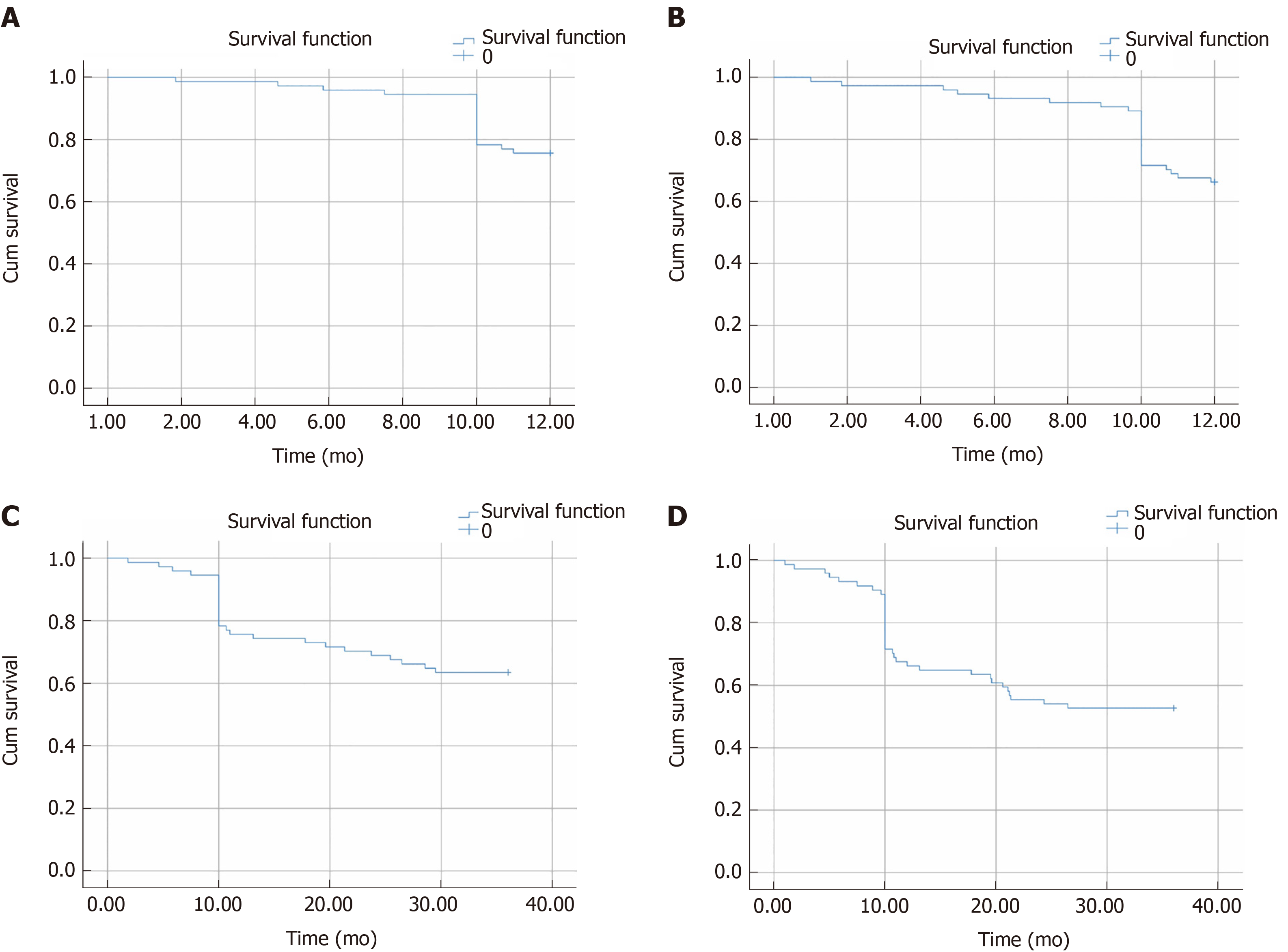
The 1-year and 3-year recurrence-free survival rates of HCC patients were 68.92% and 55.41%, respectively, following anatomic liver resection. The results showed that the 3-year recurrence-free survival rate in HCC patients was closely related to preoperative cirrhosis, jaundice level, tumor stage, maximal tumor diameter, complications of diabetes mellitus, frequency of intraoperative hypotensive episodes, estimated blood loss (EBL), blood transfusion, fluid infusion, and postoperative infection (P < 0.1). Based on multivariate analysis, preoperative cirrhosis, tumor stage, intraoperative hypotension, and EBL were identified to be predictors of 3-year recurrence-free survival in HCC patients undergoing anatomic hepatectomy (P < 0.05).
Tumor stage and preoperative cirrhosis adversely affect the recurrence-free survival rate in HCC patients following anatomic hepatectomy. The long-term recurrence-free survival rate of patients with HCC is closely related to intraoperative hypotension and EBL.
Core tip: This is a retrospective study of patients who underwent anatomic resection for hepatocellular carcinoma. Our study has some statistically significant findings regarding estimated blood loss and hypotension to survival. Simultaneously, tumor stage and preoperative cirrhosis adversely affected the recurrence-free survival rate in hepatocellular carcinoma patients following anatomic hepatectomy.
- Citation: Tian YL, Ji JJ, Chen LN, Cui XL, Liu ST, Mao L, Qiu YD, Li BB. Risk factors for long-term prognosis of hepatocellular carcinoma patients after anatomic hepatectomy. World J Clin Cases 2020; 8(4): 713-722
- URL: https://www.wjgnet.com/2307-8960/full/v8/i4/713.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i4.713
Primary hepatocellular carcinoma (HCC) associated with hepatitis B infection is the second most common cause of cancer-related death in China, with an estimated annual death of 383000, accounting for 45% of the total number of liver cancer deaths worldwide[1,2]. HCC is an insidious illness and has no specific or prominent symptoms, even in the later stage of this disease; HCC is commonly complicated by microscopic vascular invasion when a definitive diagnosis is first made. Liver surgery is still the mainstay for curing HCC, including liver resection or liver transplantation, but the prognosis of those patients is still poor[3,4]. Previous data have indicated that HCC has an estimated annual recurrence rate of 15%-20%, which is the leading factor responsible for the compromised therapeutic efficacy of hepatectomy[5]. Makuuchi et al[6] first introduced the procedure of precise anatomic hepatectomy in 1985; the procedure features complete excision of the tumor-bearing portal tributaries. With the practice of precise anatomic hepatectomy emerging in recent years, this procedure provides the maximal safety margin of tumor resection, playing a crucial role in reducing cancer metastasis and recurrence. Many studies have identified that the complete removal of tumor-bearing portal tributaries compared with non-anatomical resection improves survival in patients after hepatectomy[7,8]. The data from our institute indicated that the 3-year survival rate of solitary HCC patients undergoing anatomic hepatectomy was significantly improved compared with that of patients undergoing non-anatomical resection (64.9% vs 48.1%). The recurrence rate and median time to recurrence in the anatomic resection group were 41.9% and 48.0 mo, respectively, compared with 58.9% and 30.0 mo, respectively, after surgery in the non-anatomic resection group[9]. Regardless, the factors responsible affecting long-term prognosis in HCC patients treated by anatomic hepatectomy are still lacking. Therefore, the present retrospective study aimed to evaluate the risk factors for recurrence-free survival among HCC patients after anatomic liver resection.
This study was approved by the ethics committee of Drum Tower Hospital Affiliated with the Nanjing University Medical School.
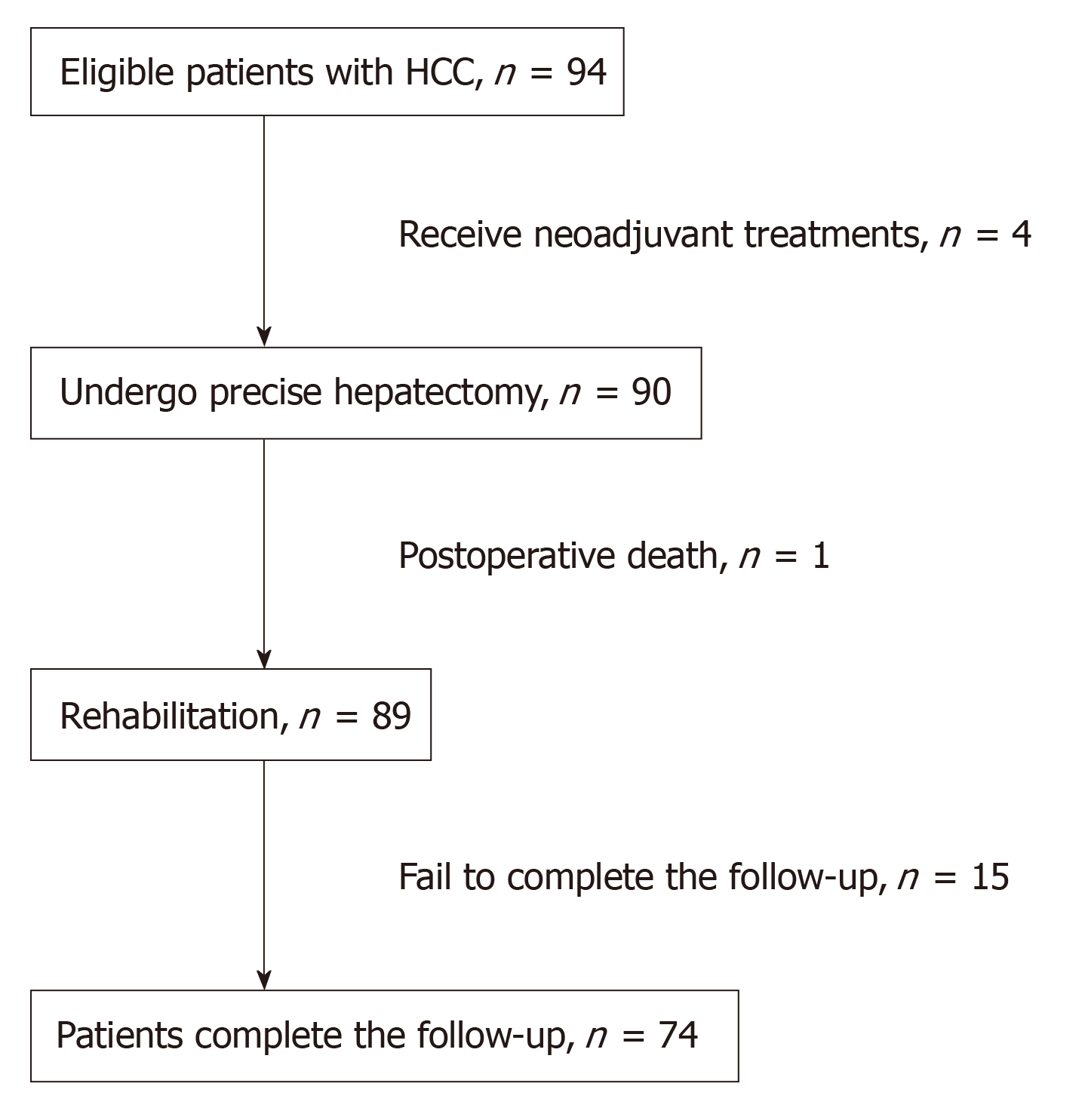
From January 2013 to December 2015, a total of 94 HCC patients who underwent precise anatomic hepatectomy at our institution were found, but ultimately only 74 included in this study. The Child-Pugh classification of these patients was not different prior to the surgery. Information, including patient age, sex, liver function grade, Barcelona clinic liver cancer (BCLC) stage, clinical stage, cancer grade on diagnostic biopsies, tumor size, surgical procedure, perioperative anesthesia management, and prognosis, was collected from the electronic charter system (Medisystem, Suzhou, China) in our hospital. The diagnosis of HCC was confirmed with image data and histopathological staining of the tumor specimen of the resected liver tissue. Patients who received any neoadjuvant treatments, such as transcatheter arterial chemoembolization or radiofrequency ablation, prior to surgery were excluded. Patients who had incomplete detailed clinical records or who did not have regular postoperative follow-up data were also excluded from this study. Anatomic hepatectomy was performed to achieve any type of complete excision of at least one segment based on Couinaud’s classification. After the precise dissection and control of the hepatic pedicle of the target part of the liver under the guidance of intraoperative ultrasound, the surgeons transected the liver parenchyma along the demarcation line. All surgical specimens were examined macroscopically and microscopically to determine surgical margins. All the operations were conducted by the same surgical team from the Department of Hepatobiliary Pancreatic Surgery at our institution.
Estimated blood loss (EBL) was estimated by the following formula: The weight difference of sterile gauze before and after surgery + the amount of fluid in the drainage bottle - the amount of saline flushed during the operation. Intraoperative hypotension (IOH) was defined as a decrease in the baseline mean arterial pressure over 20% for 5 min.
Follow-up examinations, which consisted of routine blood biochemistry, serum AFP measurements, and abdominal ultrasound or computed tomography (CT) if necessary, were performed once every 3 to 6 mo for the first 2 years and once every 6 to 12 mo thereafter. Once tumor recurrence was suspected, further examinations (CT or magnetic resonance imaging) were conducted to confirm or rule out. The diagnosis of HCC recurrence was based on the typical images of CT or magnetic resonance imaging scans, with or without elevated alpha-fetoprotein. The primary endpoint of this analysis was recurrence-free survival, defined as the interval from the date of surgery to that of diagnosis for the first recurrence, the date of death, or the deadline for follow-up.
The statistical methods of this study were reviewed by Professor Bi-Yun Xu at Department of Research, Drum Tower Hospital Affiliated with the Nanjing University Medical School, Nanjing, Jiangsu Province, China.
The statistical analyses were performed using Statistical Package for the Social Sciences version 22. Continuous variables are expressed as the mean ± SD or median with range. Categorical variables are expressed as proportions. Survival curves and the survival rate were calculated by the Kaplan-Meier method and analyzed using the log-rank test (P < 0.1). The prognostic value of each variable for affecting recurrence-free survival was assessed via multivariate Cox proportional hazards regression analysis. The threshold for significance was established at P < 0.05.
A total of 94 patients definitively diagnosed with HCC undergoing precise anatomic hepatectomy were identified during this study period. Four patients receiving TACE/interventions prior to surgery, one patient who died of circulatory failure after surgery, and fifteen patients not completing the follow-up were excluded from this study. As a result, 74 patients who underwent precise anatomic liver surgery were eligible for this study (Figure 1). The mean follow-up time was 27.82 mo (range, 1.84 to 36 mo). Of the 74 patients, the mean age was 58 years (range, 35 to 81 years). The proportion of hypertensive patients accounted for 30.7%. The mean 1-year and 3-year recurrence-free survival durations were 10.9 mo (range, 1 to 12 mo) and 24.7 mo (range, 1 to 36 mo), respectively. Their demographic and clinical data are summarized in Table 1.
| Category | Numbers of patients | 1 (yr) | 3 (yr) | ||
| χ2 | P | χ2 | P | ||
| Age, yr | 0.732 | 0.694 | 0.56 | 0.756 | |
| ≤ 60 | 42 | ||||
| > 60 | 32 | ||||
| Sex | 0.037 | 0.848 | 0.352 | 0.553 | |
| Male | 54 | ||||
| Female | 20 | ||||
| Diabetes | 0.073 | 0.788 | 3.044 | 0.081 | |
| Yes | 12 | ||||
| No | 62 | ||||
| Hypertension | 0.021 | 0.883 | 0.099 | 0.754 | |
| Yes | 23 | ||||
| No | 51 | ||||
| Hepatic cirrhosis | 3.653 | 0.056 | 8.864 | 0.003 | |
| Yes | 33 | ||||
| No | 41 | ||||
| Jaundice | 10.610 | 0.001 | 12.118 | 0.0001 | |
| Yes | 18 | ||||
| No | 56 | ||||
| Portal vein tumor thrombus | 1.602 | 0.206 | 0.749 | 0.387 | |
| Yes | 7 | ||||
| No | 67 | ||||
| Maximal tumor diameter, cm | 8.325 | 0.004 | 5.111 | 0.024 | |
| ≤ 5 | 41 | ||||
| > 5 | 33 | ||||
| Tumor number | 1.754 | 0.185 | 0.134 | 0.715 | |
| Single | 58 | ||||
| At least two | 16 | ||||
| Tumor stage | 22.402 | 0.0001 | 25.982 | 0.0001 | |
| 1 | 30 | ||||
| 2 | 15 | ||||
| 3 | 14 | ||||
| 4 | 15 | ||||
| BCLC stage | 0.265 | 0.607 | 0.125 | 0.724 | |
| 0 | 51 | ||||
| A | 23 | ||||
| Alpha-fetoprotein, ng/mL | 0.412 | 0.521 | 0.035 | 0.851 | |
| ≤ 400 | 62 | ||||
| > 400 | 12 | ||||
| Albumin, g | 0.053 | 0.818 | 1.004 | 0.316 | |
| ≤ 35 | 8 | ||||
| > 35 | 66 | ||||
| AST, U/L | 0.487 | 0.485 | 0.868 | 0.351 | |
| ≤ 80 | 67 | ||||
| > 80 | 7 | ||||
At the endpoint of the follow-up, the overall 1-year survival rate and the recurrence-free survival rate were 78.38% and 68.92%, respectively; the 3-year overall survival rate and the recurrence-free survival rate were 62.16% and 55.41%, respectively, after undergoing precise anatomic hepatectomy, as shown in Figure 2.
Twenty-three (31.1%) patients died within 1 year after the presence of tumor recurrence-related complications after precise hepatectomy. The mean 1-year recurrence-free survival duration was 10.9 mo (range, 1-12 mo). The analysis of diverse variables was conducted and is summarized in Table 2.
| Category | Number of patients | 1 (yr) | 3 (yr) | ||
| χ2 | P | χ2 | P | ||
| Operation duration, h | 0.026 | 0.873 | 0.045 | 0.832 | |
| ≤ 3 | 6 | ||||
| 3-5 | 17 | ||||
| > 5 | 51 | ||||
| Hepatic occlusion duration, min | 0.368 | 0.544 | 0.007 | 0.932 | |
| ≤ 30 | 41 | ||||
| > 30 | 33 | ||||
| Central venous pressure, mmHg | 1.335 | 0.248 | 0.089 | 0.765 | |
| < 5 | 37 | ||||
| ≥ 5 | 37 | ||||
| Hypotension | 5.479 | 0.019 | 4.963 | 0.026 | |
| Yes | 47 | ||||
| No | 27 | ||||
| Hypotension frequency | 3.855 | 0.050 | 3.490 | 0.062 | |
| ≤ 3 | 44 | ||||
| > 3 | 30 | ||||
| Hypotension duration, min | 3.342 | 0.068 | 2.134 | 0.144 | |
| ≤ 30 | 38 | ||||
| > 30 | 36 | ||||
| Blood loss, mL | 6.943 | 0.008 | 12.287 | 0.002 | |
| < 800 | 56 | ||||
| ≥ 800 | 18 | ||||
| Fluid infusion, mL | 11.453 | 0.022 | 9.936 | 0.042 | |
| ≤ 1500 | 3 | ||||
| 1500-3000 | 36 | ||||
| 3000-4500 | 20 | ||||
| 4500-6000 | 7 | ||||
| > 6000 | 8 | ||||
| Blood transfusion | 3.888 | 0.049 | 4.275 | 0.039 | |
| Yes | 24 | ||||
| No | 50 | ||||
| Vasoactive drugs | 0.089 | 0.766 | 0.049 | 0.824 | |
| Yes | 13 | ||||
| No | 61 | ||||
| Postoperative infection | 4.322 | 0.038 | 4.224 | 0.040 | |
| Yes | 29 | ||||
| No | 45 | ||||
| Adjuvant therapy | 3.204 | 0.202 | 1.654 | 0.437 | |
| None | 64 | ||||
| Chemotherapy | 7 | ||||
| Radiotherapy | 3 | ||||
Thirty-one (41.9%) patients died within 3 years after the presence recurrence-related major complications after hepatectomy. The mean survival duration of these patients after the operation was 24.7 mo (range, 1-36 mo). The analysis of diverse perioperative variables was conducted, and the results are summarized in Tables 1 and 2. The 3-year recurrence-free survival rate was significantly correlated with preoperative cirrhosis, jaundice (bilirubin level), diabetes, tumor stage, tumor size, IOH, frequency of hypotensive episodes, EBL, blood transfusion, intraoperative fluid volume, and postoperative infection (P < 0.1, Table 2). The significant risk factors were subjected to multivariate Cox proportional hazards regression analysis, which identified hepatic cirrhosis, tumor stage, and the incidence of IOH and EBL as independent risk factors for 3-year recurrence-free survival (P < 0.05, Table 3).
| Category | Multivariate | ||||||
| B | SE | χ2 | df | P value | Exp (B) | 95%CI | |
| Hepatic cirrhosis | -1.071 | 0.525 | 4.165 | 1 | 0.041 | 0.343 | 0.123-0.958 |
| Tumor stage | 0.639 | 0.178 | 12.842 | 1 | 0.001 | 1.894 | 1.336-2.687 |
| Hypotension | 0.905 | 0.477 | 3.607 | 1 | 0.050 | 2.472 | 0.971-6.292 |
| Blood loss | 0.904 | 0.448 | 4.069 | 1 | 0.044 | 2.469 | 1.026-5.944 |
Although much progress has been made in the past few decades in terms of surgical techniques, perioperative management, and the understanding of liver anatomy, hepatectomy remains a high-risk interventional procedure for HCC patients with a high 5-year mortality rate of approximately 33.3%-70%[10]. A recent study from Zhao et al[9] indicated that the application of precise anatomic hepatectomy rendered the beneficial effect in decreasing the recurrence of HCC. Patients who underwent non-anatomic liver resection were prone to early and marginal recurrence. The results from our retrospective study similarly showed that the long-term prognosis (1-year and 3-year recurrence-free survival) was better compared with that of patients undergoing the non-anatomic procedure from the previous data. Anatomic liver resection ensures that the tumor is resected en bloc in normal liver tissues out of the tumor mass with no tumor infiltration to improve the radical resection rate, which is thought to be one of the important determinants for reduced tumor metastasis and recurrence. Although prognostic factors or oncologic outcomes have been analyzed in patients after hepatectomy in multitudes of previous retrospective studies[11,12], the predictors of long-term outcomes in patients following precise anatomic hepatectomy are still lacking. Notably, in addition to several risk factors comprising tumor stage, preoperative cirrhosis, and intraoperative blood loss, which have also been identified to be relevant to the prognosis of HCC patients by other investigators[13,14], we found that the hypotension events during the surgery were highly associated with the poor outcome of HCC patients after precise anatomic hepatectomy in the present study.
IOH is actually not uncommon, and the incidence ranges from 5% to 99% depending on the threshold used to define hypotension. The deleterious effects of hypotension during surgery have been underestimated previously until a prospective observational study from Monk et al[15] indicated that the 1-year mortality of patients was most strongly correlated not only with preexisting comorbidity but also with the incidence of IOH (defined as systolic hypotension less than 80 mmHg), depending on the duration of hypotension. The association of IOH with 1-year mortality in noncardiac surgery has been identified in several studies[16]. However, the retrospective study conducted by Bijker et al[17] did not elicit the causative association between IOH and 1-year mortality despite using 48 distinct definitions for hypotension in noncardiac surgery. These conflicting results can be attributed to the nonunified threshold of IOH for a wide spectrum of surgeries and different populations because each individual is different in terms of the varying comorbid factors, baseline conditions, and thresholds of tolerance to hemodynamic insults. Therefore, we designated a decrease in the baseline mean arterial pressure over 20% for 5 min as IOH in the anatomic liver resection procedure in the present study. An intriguing finding was that the incidence of IOH could cause a detrimental effect on the 3-year survival of HCC patients, regardless notably of the time or duration of IOH during the operation. This suggests that IOH might render an unfavorable impact on the long-term outcome for carcinoma patients. Hypoperfusion status and hypoxic environment are favorable for tumor cell mitosis and angiogenesis, accounting for neoplasm recurrence. The adverse relationship between intraoperative hypotension and long-term survival in HCC patients needs to be validated in prospective randomized control trials.
Intraoperative blood loss is associated with immediate outcomes and mortality in patients undergoing noncardiac surgery. Several lines of evidence from multiple studies have suggested that massive bleeding (>1000 mL) can independently predict postoperative survival and recurrence after hepatectomy[18-20]. In accordance with these previous results, the multivariate analysis in this study identified estimated blood loss (>800 mL) as an independent risk factor responsible for decreased 3-year recurrence-free survival in HCC patients undergoing anatomic hepatectomy. It has generally been accepted that blood loss during hepatectomy is relevant to the extension of liver resection and the complexity of the procedure (vascular reconstruction), which, to some extent, represents the advanced stage of the tumor with a poor prognosis per se. Although blood loss did not correlate with tumor stage (P = 0.235, odds ratio [OR] = 1.378) or size (P = 0.729, OR = 1.250), EBL was confirmed to be one of the factors related to patient survival after hepatectomy. However, some reports have suggested that the impact of bleeding on prognosis is mainly caused by the event of blood transfusion, not the blood loss per se[21]. Therefore, whether perioperative blood transfusion will adversely affect the prognosis of cancer patients remains a controversial issue in humans[22,23]. Multivariate analysis in this study implicated that blood transfusion did not affect the recurrence-free survival of patients undergoing precise hepatectomy. We speculate that the composite effect of blood transfusion could be precisely delineated once considering the values of prompt rectification of anemia and maintenance of hemodynamic stability, not the blood transfusion-associated immunity repression effect alone[24].
Our results, which are in line with those of previous studies, also suggest that preoperative cirrhosis is a predictor of long-term recurrence-free survival in HCC patients. The 3-year recurrence-free survival rate and the average recurrence-free time in the cirrhotic group were lower than those in the noncirrhotic group. This might be due to the hepatic decompensation caused by ongoing cirrhosis itself in cirrhotic patients[25].
Our study has several limitations. First, this was a single-center retrospective study concerning the long-term outcome of patients undergoing anatomic hepatectomy by the same surgical team. The sample size was small, and selective bias may thus be inevitable. Second, inconsistent with the previous study, several tumor biological features, including tumor size with/without satellite foci, portal vein cancer thrombus, and preoperative AFP level, did not show a close correlation with 3-year recurrence-free survival in the present study. We cannot exclude the possibility that the aforementioned factors might have a relatively negligible impact on the prognosis of HCC patients undergoing anatomic hepatectomy. Third, the IOH criteria used in the present study were based on the definition of a decrease in the baseline mean arterial pressure over 20%, according to the majority of previous studies. However, it is uncertain whether a different definition would have served as a better predictor of disease recurrence for these HCC patients. Therefore, a multicenter and large-sample, randomized, controlled trial is warranted to validate the findings of our study.
In conclusion, tumor stage and preoperative cirrhosis adversely affect the postoperative recurrence-free survival rate in patients with hepatocellular carcinoma following anatomic hepatectomy. Furthermore, intraoperative hypotension and blood loss are the key determinants of long-term outcomes for HCC patients. Therefore, strict blood pressure control and reducing blood loss during surgery may improve the long-term prognosis of HCC patients following anatomic hepatectomy.
The risk factors for patients with major postoperative complications immediately after liver resection have been identified; however, the intermediate and long-term prognoses for these patients have yet to be determined.
The aim of the study was to evaluate the factors responsible for the long-term recurrence-free survival rate in patients with hepatocellular carcinoma (HCC) following anatomic hepatectomy.
We performed a retrospective analysis of 74 patients with HCC who underwent precise anatomic hepatectomy at our institution from January 2013 to December 2015. The observational endpoints for this study were the tumor recurrence or death of the HCC patients. The overall follow-up duration was 3 years. The recurrence-free survival curves were plotted by the Kaplan-Meier method and were analyzed by the log-rank test. The value of each variable for predicting prognosis was assessed via multivariate Cox proportional hazards regression analysis.
The 1-year and 3-year recurrence-free survival rates of HCC patients were 68.92% and 55.41%, respectively, following anatomic liver resection. The results showed that the 3-year recurrence-free survival rate in HCC patients was closely related to preoperative cirrhosis, jaundice level, tumor stage, maximal tumor diameter, complications of diabetes mellitus, frequency of intraoperative hypotensive episodes, estimated blood loss, blood transfusion, fluid infusion, and postoperative infection (P < 0.1). Based on multivariate analysis, preoperative cirrhosis, tumor stage, intraoperative hypotension, and estimated blood loss were identified to be predictors of 3-year recurrence-free survival in HCC patients undergoing anatomic hepatectomy (P < 0.05).
Tumor stage and preoperative cirrhosis adversely affect the postoperative recurrence-free survival rate in patients with hepatocellular carcinoma following anatomic hepatectomy. Furthermore, intraoperative hypotension and blood loss are the key determinants of long-term outcomes for HCC patients. Therefore, strict blood pressure control and reducing blood loss during surgery may improve the long-term prognosis of HCC patients following anatomic hepatectomy.
It is uncertain whether a different definition would have served as a better predictor of disease recurrence for these HCC patients. Therefore, a multicenter and large-sample, randomized, controlled trial is warranted to validate the findings of our study.
The authors would like to thank Professor Bi-Yun Xu for her kind assistance in data collection and statistical analysis.
| 1. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 373] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 2. | Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 981] [Article Influence: 81.8] [Reference Citation Analysis (4)] |
| 3. | Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, Inoue Y, Uchiyama K. Predictors of poor prognosis by recurrence patterns after curative hepatectomy for hepatocellular carcinoma in Child-Pugh classification A. Hepatogastroenterology. 2015;62:164-168. [PubMed] |
| 4. | Chen JY, Liu LP, Xu JF. Decrease of RBM4 indicates poor prognosis in patients with hepatocellular carcinoma after hepatectomy. Onco Targets Ther. 2017;10:339-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 6. | Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346-350. [PubMed] |
| 7. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 509] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 8. | Cho YB, Lee KU, Lee HW, Cho EH, Yang SH, Cho JY, Yi NJ, Suh KS. Anatomic versus non-anatomic resection for small single hepatocellular carcinomas. Hepatogastroenterology. 2007;54:1766-1769. [PubMed] |
| 9. | Zhao H, Chen C, Gu S, Yan X, Jia W, Mao L, Qiu Y. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: A propensity score matching analysis. J Gastroenterol Hepatol. 2017;32:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Ang SF, Ng ES, Li H, Ong YH, Choo SP, Ngeow J, Toh HC, Lim KH, Yap HY, Tan CK, Ooi LL, Cheow PC, Chung AY, Chow PK, Foo KF, Tan MH. Correction: The Singapore Liver Cancer Recurrence (SLICER) Score for Relapse Prediction in Patients with Surgically Resected Hepatocellular Carcinoma. PLoS One. 2015;10:e0128058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Lurje G, Bednarsch J, Czigany Z, Amygdalos I, Meister F, Schöning W, Ulmer TF, Foerster M, Dejong C, Neumann UP. Prognostic factors of disease-free and overall survival in patients with hepatocellular carcinoma undergoing partial hepatectomy in curative intent. Langenbecks Arch Surg. 2018;403:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Zhang TT, Zhao XQ, Liu Z, Mao ZY, Bai L. Factors affecting the recurrence and survival of hepatocellular carcinoma after hepatectomy: a retrospective study of 601 Chinese patients. Clin Transl Oncol. 2016;18:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Gupta R, Fuks D, Bourdeaux C, Radkani P, Nomi T, Lamer C, Gayet B. Impact of intraoperative blood loss on the short-term outcomes of laparoscopic liver resection. Surg Endosc. 2017;31:4451-4457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 14. | Sasaki K, Shindoh J, Margonis GA, Nishioka Y, Andreatos N, Sekine A, Hashimoto M, Pawlik TM. Effect of Background Liver Cirrhosis on Outcomes of Hepatectomy for Hepatocellular Carcinoma. JAMA Surg. 2017;152:e165059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 608] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 16. | Monk TG, Bronsert MR, Henderson WG, Mangione MP, Sum-Ping ST, Bentt DR, Nguyen JD, Richman JS, Meguid RA, Hammermeister KE. Association between Intraoperative Hypotension and Hypertension and 30-day Postoperative Mortality in Noncardiac Surgery. Anesthesiology. 2015;123:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 427] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 17. | Bijker JB, van Klei WA, Vergouwe Y, Eleveld DJ, van Wolfswinkel L, Moons KG, Kalkman CJ. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Taketomi A, Toshima T, Kitagawa D, Motomura T, Takeishi K, Mano Y, Kayashima H, Sugimachi K, Aishima S, Yamashita Y, Ikegami T, Gion T, Uchiyama H, Soejima Y, Maeda T, Shirabe K, Maehara Y. Predictors of extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2010;17:2740-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Sasaki K, Matsuda M, Ohkura Y, Kawamura Y, Inoue M, Hashimoto M, Ikeda K, Kumada H, Watanabe G. Factors associated with early cancer-related death after curative hepatectomy for solitary small hepatocellular carcinoma without macroscopic vascular invasion. J Hepatobiliary Pancreat Sci. 2014;21:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Schiergens TS, Stielow C, Schreiber S, Hornuss C, Jauch KW, Rentsch M, Thasler WE. Liver resection in the elderly: significance of comorbidities and blood loss. J Gastrointest Surg. 2014;18:1161-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Pang QY, An R, Liu HL. Perioperative transfusion and the prognosis of colorectal cancer surgery: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Wu HL, Tai YH, Lin SP, Chan MY, Chen HH, Chang KY. The Impact of Blood Transfusion on Recurrence and Mortality Following Colorectal Cancer Resection: A Propensity Score Analysis of 4,030 Patients. Sci Rep. 2018;8:13345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Carson JL, Sieber F, Cook DR, Hoover DR, Noveck H, Chaitman BR, Fleisher L, Beaupre L, Macaulay W, Rhoads GG, Paris B, Zagorin A, Sanders DW, Zakriya KJ, Magaziner J. Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet. 2015;385:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 24. | Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, Salloum R, Meredith UW, Osler TM. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 25. | Zhou YM, Sui CJ, Zhang XF, Li B, Yang JM. Influence of cirrhosis on long-term prognosis after surgery in patients with combined hepatocellular-cholangiocarcinoma. BMC Gastroenterol. 2017;17:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gumbs A, Sirin G S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Ma YJ