Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5353
Peer-review started: March 27, 2020
First decision: September 24, 2020
Revised: September 27, 2020
Accepted: October 1, 2020
Article in press: October 1, 2020
Published online: November 6, 2020
Processing time: 223 Days and 23.3 Hours
Afferent loop syndrome (ALS) is a rare mechanical complication that occurs after reconstruction of the stomach or esophagus to the jejunum, such as Billroth II gastrojejunostomy, Roux-en-Y gastrojejunostomy, or Roux-en-Y esophagoje-junostomy. Traditionally, an operation is the first choice for benign causes. However, for patients in poor physical condition who experience ALS soon after R0 resection, the type of treatment remains controversial. Here, we present an efficient conservative method to treat ALS.
Case 1 was a 69-year-old male patient who underwent total gastrectomy with Roux-en-Y jejunojejunostomy. On postoperative day (POD) 10 he developed symptoms of ALS that persisted and increased over 1 wk. Case 2 was a 59-year-old male patient who underwent distal gastrectomy with Billroth II gastrojejunostomy. On postoperative day POD 9 he developed symptoms of ALS that persisted for 2 wk. Both patients underwent fluoroscopic-guided nasointestinal tube placement with maintenance of continuous negative pressure suction. Approximately 20 d after the procedure, both patients had recovered well and were discharged from hospital after removal of the tube. At 3-mo follow-up, there were no signs of ALS in these two patients.
This is the first report of treating postoperative ALS by fluoroscopic-guided nasointestinal tube placement. Our cases demonstrate that this procedure is an effective and safe method to treat ALS that relieves patients’ symptoms and avoids complications caused by other invasive procedures.
Core Tip: Afferent loop syndrome (ALS) is a rare complication following recon-struction of the stomach or esophagus to the jejunum. Traditionally, surgery is the cornerstone of treatment. However, for patients in poor physical condition who develop ALS soon after the operation, a secondary surgery may not be appropriate. Here, we present two patients who were successfully treated with fluoroscopic-guided nasointestinal tube placement without stent insertion or surgery. With continuous negative pressure suction for approximately 20 d, both patients recovered well and were discharged from hospital after removal of the tube. At 3-mo follow-up, the patients showed no symptoms of ALS.
- Citation: Hu HT, Ma FH, Wu ZM, Qi XH, Zhong YX, Xie YB, Tian YT. Treatment of afferent loop syndrome using fluoroscopic-guided nasointestinal tube placement: Two case reports. World J Clin Cases 2020; 8(21): 5353-5360
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5353.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5353
Afferent loop syndrome (ALS) is an uncommon complication following the reconstruction of the stomach or esophagus to the jejunum, such as Billroth II gastrojejunostomy, Roux-en-Y gastrojejunostomy, or Roux-en-Y esophagojejunostomy. The incidence of ALS is approximately 0.2%[1] after Billroth II and 1%[2] after Roux-en-Y reconstruction. ALS is a purely mechanical obstruction, usually caused by postoperative adhesion, kinking at the anastomosis, internal hernia, stomal stenosis, cancer recurrence, inflammation surrounding the anastomosis, or enteroliths, bezoars, and foreign bodies[3]. The causes can be classified into benign and malignant. Traditionally, for the patients diagnosed with ALS caused by a malignant obstruction, such as cancer recurrence, conservative methods are usually considered[4-6], while for benign causes, operation is recommended[3]. However, patients who develop ALS soon after R0 resection are usually in poor physical condition; therefore, the decision to perform a second operation remains controversial. Moreover, the second operation may cause more severe complications such as anastomotic fistula. Therefore, conservative treatment should be considered and provided first to relieve symptoms. To the best of our knowledge, there are limited case reports for such patients, and consensus regarding treatment is lacking.
Here, we present two cases of ALS, one patient who received total gastrectomy with Roux-en-Y anastomosis and the other who underwent distal gastrectomy with Billroth II anastomosis. Both patients developed ALS several days after surgery and were treated by fluoroscopic-guided nasointestinal tube placement. They obtained a favorable outcome. All study participants provided informed written consent prior to study enrollment.
Case 1: Patient 1 was a 69-year-old male who presented with jaundice, abdominal pain, fever, and vomiting bile-like liquid on postoperative day (POD) 10 after total gastrectomy with Roux-en-Y jejunojejunostomy. He experienced these symptoms over 1 wk.
Case 2: Patient 2 was a 59-year-old male who presented with upper abdominal pain and abdominal distention on POD 9 after distal gastrectomy with Billroth II gastrojejunostomy. He experienced these symptoms for 13 d.
Case 1: Patient 1 was admitted to the hospital with upper abdominal discomfort that occurred over the past 3 mo and was increased over the past 1 mo. He underwent gastroscopy that revealed an ulcerative mass in the esophagogastric junction. The pathological diagnosis was low-grade adenocarcinoma. No signs of any metastasis were found on chest and abdominal radiographs. On September 17, 2019, the patient underwent total gastrectomy with Roux-en-Y jejunojejunostomy.
Case 2: Patient 2 was admitted to the hospital with abdominal distention of more than 2 mo duration. He underwent gastroscopy that revealed an ulcer at the gastric angle. Pathology revealed signet-ring cells within the mucosal tissue. No signs of any metastasis were found. On August 25, 2019, the patient underwent distal gastrectomy with Billroth II gastrojejunostomy.
Case 1: Patient 1 had a cerebral infarction in 2008 and had recovered well. He denied any history of surgery, hypertension, diabetes, and coronary heart disease.
Case 2: Patient 2 had no remarkable previous medical history.
Both patients denied any family history of cancer.
During the physical examination, both patients showed marked tenderness, especially in the upper abdomen. No other special remarkable signs were found.
The laboratory results for patient 1 and patient 2 before fluoroscopic-guided nasointestinal tube placement are shown in Table 1.
| Test items | Patient 1 before placement | Patient 1 after placement | Patient 2 before placement | Patient 2 after placement |
| WBC | 11.32 × 109/L | 7.46 × 109/L | 7.33 × 109/L | 3.29 × 109/L |
| NEUT% | 83.2% | 60.2% | 74.1% | 52.6% |
| HGB | 93 g/L | 111 g/L | 127 g/L | 132 g/L |
| PLT | 287 × 109/L | 189 × 109/L | 224 × 109/L | 173 × 109/L |
| ALP | 200.3 U/L | 150.4 U/L | 34.2 U/L | 33.2 U/L |
| ALT | 71.2 U/L | 48.2 U/L | 30.1 U/L | 31 U/L |
| T-BIL | 82.78 μmol/L | 18.12 μmol/L | 10.25 μmol/L | 9.73 μmol/L |
| D-BIL | 78.08 μmol/L | 13.29 μmol/L | 4.16 μmol/L | 3.92 μmol/L |
| TP | 60.7 g/L | 68.2 g/L | 61.2 g/L | 66.0 g/L |
| ALB | 29.5 g/L | 41.4 g/L | 40.3 g/L | 45.2 g/L |
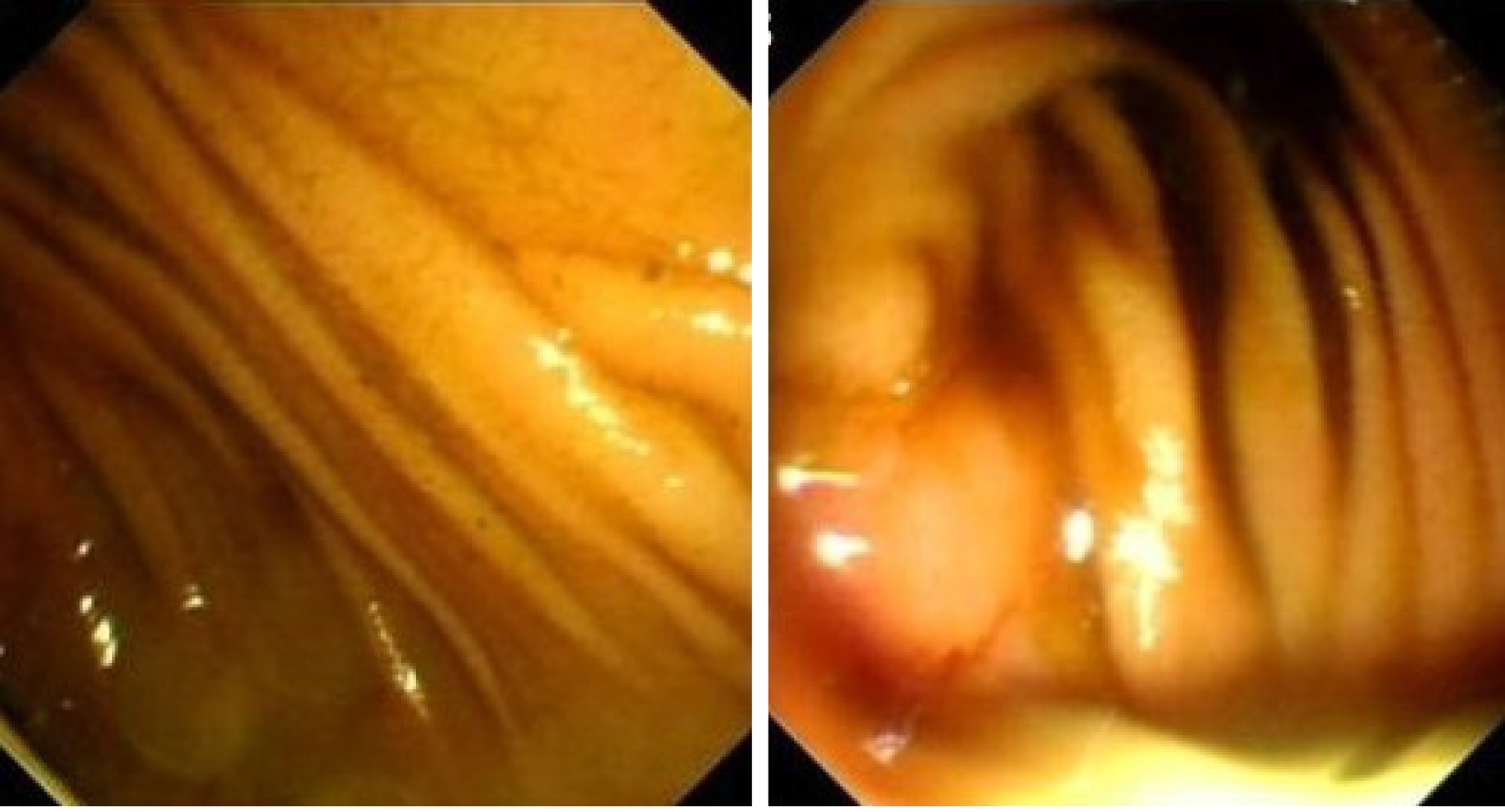
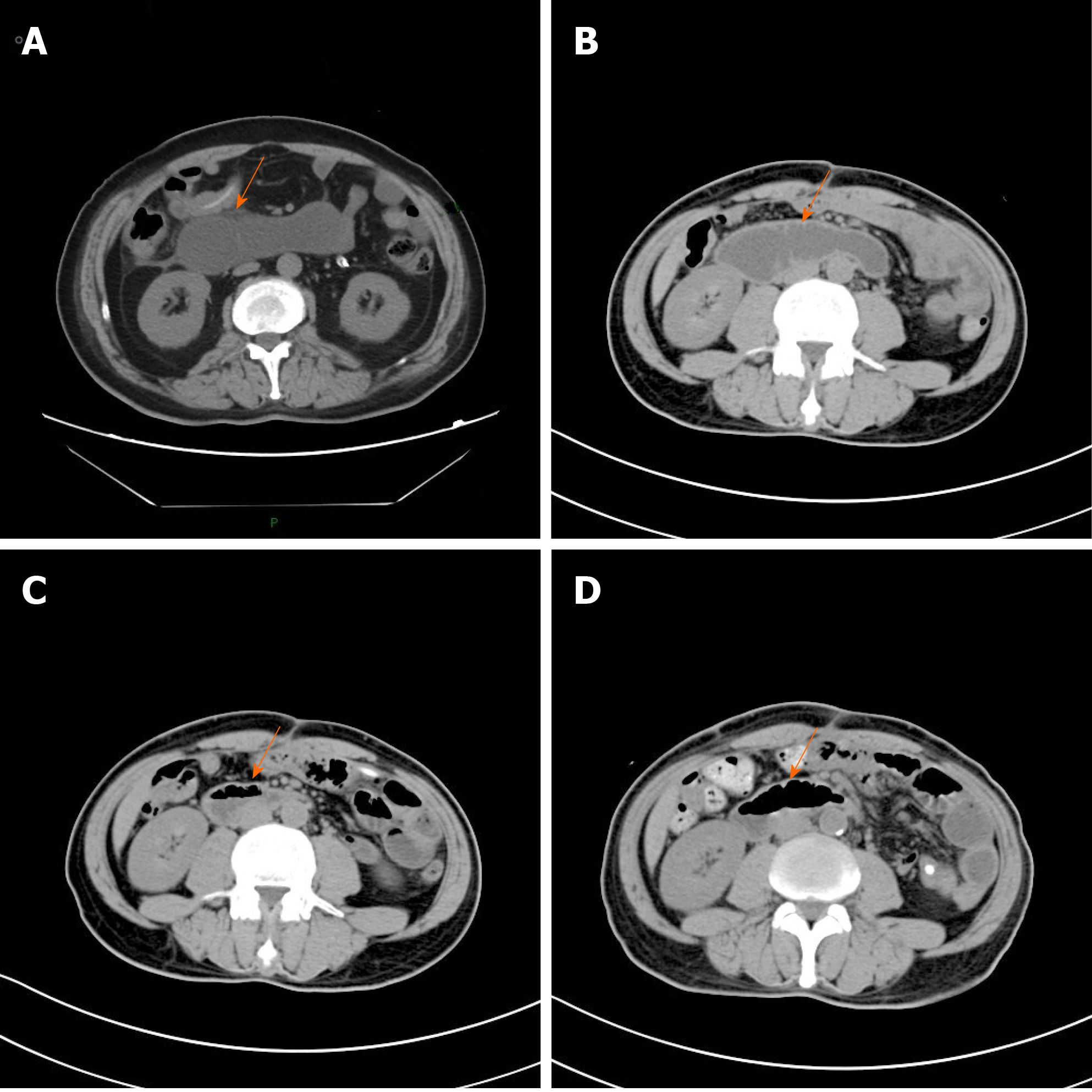
Case 1: In patient 1, gastroscopy revealed that the anastomosis of the esophagus and jejunum was unobstructed, but because of the severe kinking of the anastomosis, the scope failed to enter the afferent loop (Figure 1). The abdominal computed tomography (CT) showed apparent dilatation of the afferent loop (Figure 2A).
Case 2: In patient 2, abdominal CT showed apparent dilatation of the afferent loop (Figure 2B), which supported the diagnosis of ALS.
Considering the patients’ symptoms, laboratory examinations and imaging results, both patients were diagnosed with ALS.
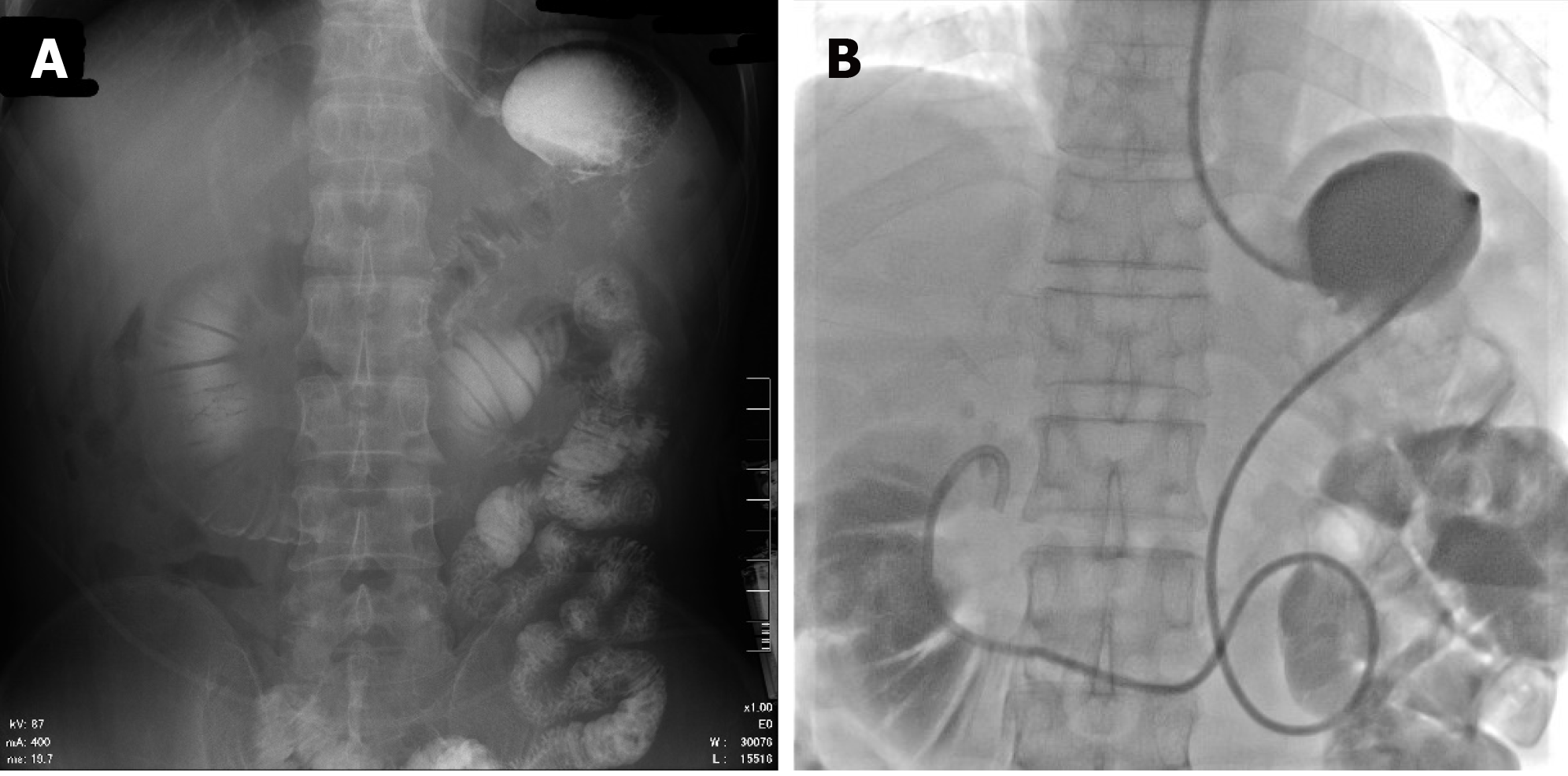
First, we administered Chinese medicine acupuncture treatment, which proved to be ineffective. Then, as previously reported[4], we first attempted endoscopic retrograde cholangiopancreatography (ERCP). However, the afferent loop was twisted such that the endoscope failed to pass. Therefore, we inserted a nasointestinal tube into the afferent loop through a guiding wire under fluoroscopic guidance. We expected that this procedure would relieve patients’ symptoms and improve their physical condition. The procedure included three steps. First, we injected contrast agent into the original stomach tube. The afferent and efferent loops were both developed, and the afferent loop was poorly peristaltic. Second, we adjusted the hard guide wire (RADIFOCUS®, 0.90 mm, 260 cm, Terumo Corporation, Tokyo, Japan) and guiding catheter (ETH201 V04, Cordis Corporation, Hialeah, FL, United States) into the afferent loop. The contrast agent could be seen in the afferent loop. Third, we removed the guiding catheter and the nasointestinal tube (145 cm, Nutricia, Wuxi, China) was exchanged and fixed (Figure 3). This procedure was performed on POD 20 for patient 1 and on POD 22 for patient 2. After the procedure, about 600 mL of bile-like liquid were obtained through the nasointestinal tube in 24 h with significant relief of pain and improvement in jaundice for patient 1. The amount of drainage fluid obtained in 24 h was about 1000 mL for patient 2. For patient 1, the decompression of the afferent loop continued for 16 d, at which time we intermittently clamped the nasointestinal tube. On POD 40, the nasointestinal tube was removed. For patient 2, the decompression of the afferent loop continued over 15 d, and then we intermittently clamped the nasointestinal tube. On POD 41, the nasointestinal tube was removed.
Both patients recovered well after the fluoroscopic-guided nasointestinal tube placement with relief of symptoms and improved laboratory characteristics and CT signs. The laboratory results after fluoroscopic-guided nasointestinal tube placement for both patients are shown in Table 1.
As for imaging examinations, on POD 28 and POD 34, patient 2 received abdominal CT (Figure 2C and D), which showed that the degree of the dilatation had significantly decreased.
Both patients were discharged to home after the nasointestinal tube was removed, with no abdominal pain, jaundice, or other abdominal discomfort. At 3-mo follow-up, both patients showed no symptoms of ALS and did not complain of any discomfort.
ALS is a rare and purely mechanical complication, for which there are few case reports, and multiple center clinical research studies are necessary. There is a lack of a consensus to guide treatment for patients who develop ALS soon after R0 resection. Here, we present two cases, one who developed ALS after distal gastrectomy with Billroth II reconstruction, and one who developed ALS after total gastrectomy with Roux-en-Y reconstruction, both of whom represent the main causes of ALS that are associated with surgery. Both patients achieved a good therapeutic effect with the fluoroscopic-guided nasointestinal tube placement. To the best of our knowledge, this is the first time anyone has reported an attempt to treat ALS with fluoroscopic-guided nasointestinal tube placement.
Surgery is one of the common causes of ALS[7] and is due to an excessively long extra afferent loop[3]. Two retrospective cohort studies showed that the incidence of ALS in gastric cancer patients undergoing Billroth II and Roux-en-Y reconstruction was 1.01% and 0.2% respectively, and all were caused by adhesion, internal herniation, and peritoneal recurrence[1,2]. As for treatment, traditionally, surgery is the cornerstone of treatment for ALS, which includes converting a Billroth II to a Roux-en-Y[8], creating a Braun anastomosis between the afferent and efferent loops in a Billroth II[9], or excising redundant loops and reconstruction[10]. However, surgery is not suitable for every patient with ALS[11,12]. A considerable proportion of patients could not undergo an operation because of poor physical condition, extensive peritoneal adhesions, or disseminated tumor[13]. On the other hand, for patients with acute ALS soon after operation, a secondary operation could increase the risk of anastomotic fistula, stenosis, stomatitis, and other postoperative complications. In our patients, considering their poor physical condition and the risks associated with a secondary operation, we decided to provide a conservative treatment to alleviate symptoms after discussion with several experts.
For those not suitable for surgery, percutaneous transhepatic biliary drainage is an effective method to provide palliative treatment[14-16]; however, we should notice the risk of leakage of bowel gases or contents into the peritoneum, which could induce severe infection or septicemia[17]. Cha et al[6] reported treatment of ALS using self-expanding metal stent (SEMS), which achieved a satisfied result. However, the temporary stoma rate of the placement of SEMS for acute malignant colonic obstruction is about 33%[18,19]. Recently, with the wide application of endoscopic ultrasonography (EUS), endoscopic ultrasound-guided gastrojejunostomy using a metal stent for the treatment of ALS has been reported[20-22]. A multicenter study[23] showed that the technical success of ultrasound-guided entero-enterostomy (EUS-EE) following the availability of the lumen-apposing metal stent was almost 100% in 18 patients, and the mean procedure time was 29.7 min. However, three patients (16.7%) experienced advent events, two mild, and one moderate, which reflected the risk of this technique. Besides, to ensure the safety of this technique, the targeted site within the duodenum or jejunum needs to be close to the stomach, duodenum, or jejunum, which limits the application of EUS-EE.
With the progress of endoscopic treatment and interventional radiology, treating ALS with endoscopic interventions has been reported recently[24,25]. A single-center study evaluated the effectiveness and safety of endoscopic nasogastric tube insertion and found that this technique was a conservative treatment for patients with benign ALS[13]. However, we attempted this procedure first with our patients, but the afferent loop was so twisted that the endoscope failed to pass, which is a common occurrence. Kim et al[26] in their retrospective study, reported that 19 patients underwent fluoroscopic stent placement because tentative endoscopic stent placement had failed. In our patients, we inserted the nasointestinal tube with continuous negative pressure suction, without placement of a mental stent, which also achieved a satisfactory outcome. Zhang et al[27] analyzed 74 patients with malignant bowel obstruction treated with the fluoroscopy-guided long intestinal tube placement and showed that the symptoms of 58 patients improved significantly. At 1-mo follow-up, the symptoms of obstruction did not deteriorate. At our 3-mo follow-up, both patients showed no signs of ALS after the removal of the nasointestinal tube, which provided evidence for the treatment of acute postoperative ALS with fluoroscopic-guided nasointestinal tube placement.
Our patients developed ALS several days after surgery; therefore, it might not be beneficial to perform a secondary operation immediately. Here, we present a conservative method, fluoroscopic-guided nasointestinal tube placement, which could be considered a stable method to treat and observe and also a step to improve the patient’s physical condition before a secondary operation. This study is limited by the number of cases and short follow-up, and the long-term efficacy of this method needs to be verified.
This is the first study to report treatment of postoperative ALS by fluoroscopic-guided nasointestinal tube placement. For patients with ALS that occurs soon after surgery, multiple treatment options should be considered, including conservative treatment and secondary surgery. Our findings suggest that, by the 3-mo follow-up, fluoroscopic-guided nasointestinal tube placement was an effective and safe method to treat ALS that relieved patients’ symptoms and avoided complications caused by other invasive procedures.
| 1. | Kim DJ, Lee JH, Kim W. Afferent loop obstruction following laparoscopic distal gastrectomy with Billroth-II gastrojejunostomy. J Korean Surg Soc. 2013;84:281-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 2. | Aoki M, Saka M, Morita S, Fukagawa T, Katai H. Afferent loop obstruction after distal gastrectomy with Roux-en-Y reconstruction. World J Surg. 2010;34:2389-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Blouhos K, Boulas KA, Tsalis K, Hatzigeorgiadis A. Management of afferent loop obstruction: Reoperation or endoscopic and percutaneous interventions? World J Gastrointest Surg. 2015;7:190-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Burdick JS, Garza AA, Magee DJ, Dykes C, Jeyarajah R. Endoscopic management of afferent loop syndrome of malignant etiology. Gastrointest Endosc. 2002;55:602-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Kim YH, Han JK, Lee KH, Kim TK, Kim KW, Choi BI. Palliative percutaneous tube enterostomy in afferent-loop syndrome presenting as jaundice: clinical effectiveness. J Vasc Interv Radiol. 2002;13:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Cha RR, Cho SB, Kim WS, Kim JJ, Lee JM, Lee SS, Kim HJ, Cho JK. Self-expanding metal stent procedure for afferent loop syndrome with ascending cholangitis caused by remnant gastric cancer: A case report. Medicine (Baltimore). 2018;97:e13072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Bolton JS, Conway WC 2nd. Postgastrectomy syndromes. Surg Clin North Am. 2011;91:1105-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Lee SY, Lee JC, Yang DH. Early Postoperative Retrograde Jejunojejunal Intussusception after Total Gastrectomy with Roux-en-Y Esophagojejunostomy: A Case Report. J Gastric Cancer. 2013;13:263-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Nageswaran H, Belgaumkar A, Kumar R, Riga A, Menezes N, Worthington T, Karanjia ND. Acute afferent loop syndrome in the early postoperative period following pancreaticoduodenectomy. Ann R Coll Surg Engl. 2015;97:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Azevedo F, Canhoto C, Tralhão JG, Carvalho H. Management of afferent loop syndrome after Roux-en-Y subtotal gastrectomy and choledocolithiasis with recurrent cholangitis. BMJ Case Rep. 2020;13:e232498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Hakuta R, Kogure H, Nakai Y, Sato T, Takahara N, Mizuno S, Koike K. Treatment of afferent loop syndrome using digital cholangioscopy through the percutaneous transhepatic biliary drainage route. Endoscopy. 2020;52:E71-E72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Yane K, Hayashi T, Katanuma A. Successful emergency endoscopic drainage for afferent limb syndrome-induced severe acute cholangitis in a patient with altered Roux-en Y anatomy. Dig Endosc. 2018;30:802-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Cao Y, Kong X, Yang D, Li S. Endoscopic nasogastric tube insertion for treatment of benign afferent loop obstruction after radical gastrectomy for gastric cancer: A 16-year retrospective single-center study. Medicine (Baltimore). 2019;98:e16475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Kim KH, Lee HB, Kim SH, Kim MC, Jung GJ. Role of percutaneous transhepatic biliary drainage in patients with complications after gastrectomy. Int Surg. 2015;:. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Hosokawa I, Kato A, Shimizu H, Furukawa K, Miyazaki M. Percutaneous transhepatic metallic stent insertion for malignant afferent loop obstruction following pancreaticoduodenectomy: a case report. J Med Case Rep. 2012;6:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Laasch HU. Obstructive jaundice after bilioenteric anastomosis: transhepatic and direct percutaneous enteral stent insertion for afferent loop occlusion. Gut Liver. 2010;4 Suppl 1:S89-S95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Moriura S, Takayama Y, Nagata J, Akutagawa A, Hirano A, Ishiguro S, Matsumoto T, Sato T. Percutaneous bowel drainage for jaundice due to afferent loop obstruction following pancreatoduodenectomy: report of a case. Surg Today. 1999;29:1098-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Arezzo A, Passera R, Lo Secco G, Verra M, Bonino MA, Targarona E, Morino M. Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc. 2017;86:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | van den Berg MW, Sloothaak DA, Dijkgraaf MG, van der Zaag ES, Bemelman WA, Tanis PJ, Bosker RJ, Fockens P, ter Borg F, van Hooft JE. Bridge-to-surgery stent placement vs emergency surgery for acute malignant colonic obstruction. Br J Surg. 2014;101:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Ermerak G, Behary J, Edwards P, Abi-Hanna D, Bassan MS. EUS-guided enteroenterostomy for nonoperative management of afferent loop syndrome after Whipple resection. VideoGIE. 2019;4:461-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Yamamoto K, Tsuchiya T, Tanaka R, Mitsuyoshi H, Mukai S, Nagakawa Y, Itoi T. Afferent loop syndrome treated by endoscopic ultrasound-guided gastrojejunostomy, using a lumen-apposing metal stent with an electrocautery-enhanced delivery system. Endoscopy. 2017;49:E270-E272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Matsumoto K, Kato H, Tomoda T, Sakakihara I, Yamamoto N, Noma Y, Sonoyama T, Tsutsumi K, Okada H, Yamamoto K, Kawamoto H. A case of acute afferent loop syndrome treated by transgastric drainage with EUS. Gastrointest Endosc. 2013;77:132-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Brewer Gutierrez OI, Irani SS, Ngamruengphong S, Aridi HD, Kunda R, Siddiqui A, Dollhopf M, Nieto J, Chen YI, Sahar N, Bukhari MA, Sanaei O, Canto MI, Singh VK, Kozarek R, Khashab MA. Endoscopic ultrasound-guided entero-enterostomy for the treatment of afferent loop syndrome: a multicenter experience. Endoscopy. 2018;50:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Lim J, So H, Ko SW, Hwang JS, Song TJ. Endoscopic electrohydraulic lithotripsy of an enterolith causing afferent loop syndrome after Whipple's operation. Endoscopy. 2020;52:E176-E177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Sasaki T, Isayama H, Kogure H, Yamada A, Aoki T, Kokudo N, Koike K. Double-balloon enteroscope-assisted enteral stent placement for malignant afferent-loop obstruction after Roux-en-Y reconstruction. Endoscopy. 2014;46 Suppl 1 UCTN:E541-E542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Kim SH, Song HY, Park JH, Zhou WZ, Cho YC, Shin JH, Kim JH. Fluoroscopic-guided stent placement in failed tentative endoscopic approaches to malignant gastroduodenal obstructions. Acta Radiol. 2017;58:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Lai H, Wu K, Liu Y, Zeng Z, Zhang B. Fluoroscopy-guided long intestinal tube placement for the treatment of malignant bowel obstruction. Oncol Lett. 2019;17:5154-5158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Miethke A, Nakamoto N S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX