Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4793
Peer-review started: July 8, 2020
First decision: August 8, 2020
Revised: August 21, 2020
Accepted: September 25, 2020
Article in press: September 25, 2020
Published online: October 26, 2020
Processing time: 109 Days and 22.3 Hours
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) represent a relatively rare and heterogenous group of tumors. Currently available treatment options for patients with progressive GEP-NETs include lutetium (177Lu) oxodotreotide (177Lu-Dotatate) and everolimus [as well as sunitinib for patients with pancreatic NETs (P-NETs)].
To perform a health economic analysis to determine the cost-effectiveness of 177Lu-Dotatate compared with everolimus in patients with unresectable or metastatic midgut-NETs or P-NETs in both Sweden and Norway.
Simulations were performed using a three-state partitioned survival model and analyses were performed separately for patients with midgut-NETs and P-NETs. Clinical input data were sourced from an indirect comparison that utilized survival data from clinical trials of 177Lu-Dotatate and everolimus. The analyses were performed from the healthcare payer perspective over a time horizon of 20 years. For Sweden, future costs and clinical outcomes were discounted at 3% per annum. For Norway, a discount rate of 4% per annum was applied.
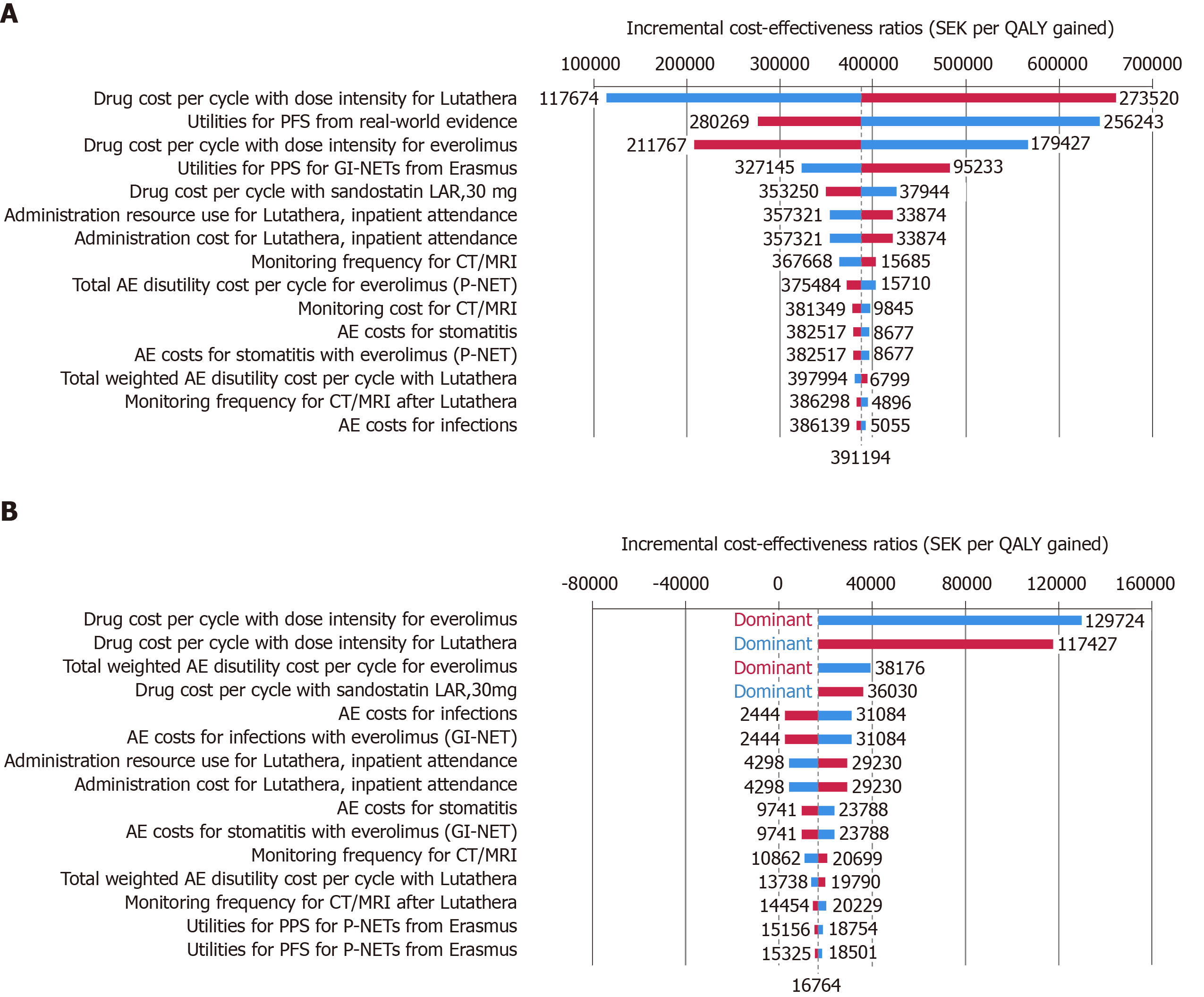
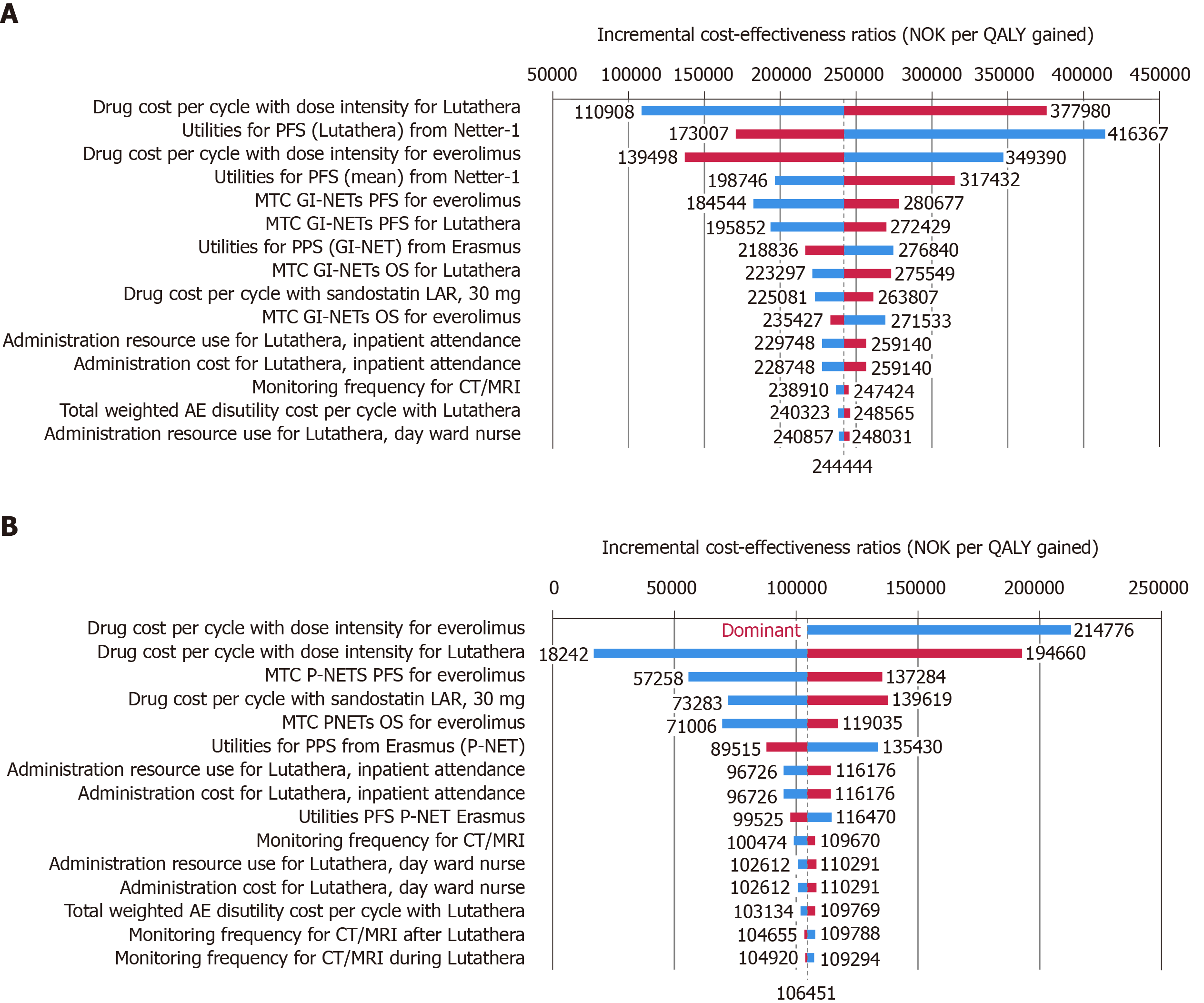
For Sweden, improved survival outcomes and higher lifetime costs with 177Lu-Dotatate resulted in an incremental cost-effectiveness ratio (ICER) of SEK 391194 per quality-adjusted life year (QALY) gained for midgut NETs and SEK 16764 per QALY gained for P-NETs for 177Lu-Dotatate compared with everolimus. For Norway, the corresponding ICERs were NOK 244444 per QALY gained and NOK 106451 per QALY gained, respectively. One-way sensitivity analyses revealed that the results were most sensitive to changes in drug acquisition costs and health state utility values.
In both Sweden and Norway, from a healthcare provider perspective, 177Lu-Dotatate is likely to be considered cost-effective relative to everolimus for the treatment of patients with unresectable or metastatic, progressive midgut-NETs or P-NETs.
Core Tip: Peptide receptor radionuclide therapy with 177lutetium oxodotreotide
- Citation: Palmer J, Leeuwenkamp OR. Cost-effectiveness of lutetium (177Lu) oxodotreotide vs everolimus in gastroenteropancreatic neuroendocrine tumors in Norway and Sweden. World J Clin Cases 2020; 8(20): 4793-4806
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4793.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4793
Neuroendocrine tumors (NETs) are a heterogenous group of tumors that may arise from any cell with a neuroendocrine phenotype, although around 70% of NETs emanate from cells in the gastrointestinal tract or pancreas, collectively termed gastroenteropancreatic (GEP)-NETs[1]. Recent estimates relating to the prevalence and incidence of NETs or GEP-NETs specifically in Sweden are lacking, but in Norway in 2010 the overall incidence of all NET types was estimated at 21.3 per 100000 population and the median age at diagnosis was 65 years[2]. Moreover, analysis of temporal trends in incidence has also shown that in Norway over the period 1993–2010 the incidence of NETs increased by 5.1% per year in women and 2.1% per year in men[2], this increase has been attributed, in part, to increased physician and pathologist awareness of NETs, improved diagnostic techniques and improved classification[2,3]. With regard to the incidence of GEP-NETs specifically, this has been estimated at 5.25 per 100000 population[4]. For Sweden, this corresponds to an estimated 540 new cases per year (based on a population of 10.28 million[5]) and for Norway the corresponding figure is approximately 280 new cases per year (based on a population of 5.30 million[6]). Based on these estimates, GEP-NETs fulfil the European Medicines Agency (EMA) criteria for orphan disease status[7]. Additionally, the tumor biology and malignant potential of GEP-NETs is highly variable and prognosis is influenced by the location of the primary tumor, Ki-67 (a marker of cell proliferation rate), tumor size and tumor grade, metastases, and disease stage at diagnosis[8]. Indeed, in Norway over 40% of patients already had distant metastases at diagnosis[2] and 5-year survival for patients with pancreatic NETs (P-NETs) with distant disease at diagnosis was just 33%[2]. Similarly, 5-year survival rates for patients with small intestinal NETs with distant disease at diagnosis were 63% for tumors classified as low/intermediate aggressiveness[9].
Currently available treatment options for patients with unresectable or metastatic GEP-NETs include everolimus, sunitinib (only indicated for patients with well-differentiated P-NETs with disease progression) and 177Lutetium oxodotreotide (177Lu-Dotatate; Lutathera). 177Lu-Dotatate was approved for use in Europe in 2017 and is a peptide receptor radionuclide therapy (PRRT) specifically targeting tumors overexpressing somatostatin receptors, particularly of type 2 (SSRT2) on the surface of tumor cells of most NETs[10]. 177Lu-Dotatate is radiolabeled with the β-emitter 177Lu, which has a maximum penetration range of 2.2 mm and binds with high affinity to SSRT2, thereby delivering targeted cytotoxic radiation to tumor cells overexpressing SSRT2 with minimal damage to neighboring healthy tissue[11]. In the phase III NETTER-1 trial, conducted in patients with advanced, progressive, somatostatin-receptor-positive midgut NETs 177Lu-Dotatate plus best supportive care (BSC) with octreotide long-acting release (LAR) 30 mg was associated with a statistically significant benefit in both progression-free survival (PFS) and overall survival (OS) compared with octreotide LAR 60 mg[12]. In the primary analysis median [95% confidence interval (CI)] PFS was not yet reached in the 177Lu-Dotatate group compared with 8.4 (5.8–9.1) months in the octreotide LAR 60 mg group and the hazard ratio (95%CI) for disease progression or death for 177Lu-dotatate-treated patients was 0.21 (0.13–0.33) (P < 0.001)[12]. In parallel with improved survival outcomes, treatment with 177Lu-Dotatate also significantly delayed deterioration in quality of life (QoL) relative to octreotide LAR 60 mg[13]. Further, the authors of a 2015 review on QoL in patients with GEP-NETs noted that whilst QoL has been shown to improve in patients treated with PRRT (irrespective of the radionuclide used), it remains relatively unchanged from baseline in patients undergoing treatment with everolimus[14].
In addition to clinical efficacy, novel treatments must also demonstrate cost-effectiveness relative to currently available treatments. The acquisition costs of novel treatments, particularly those used in oncology or for orphan indications are often higher than for the current standard of care. As such, healthcare policy makers and payers are increasingly stipulating that for granting reimbursement novel treatments must demonstrate not only better safety and efficacy, but also long-term cost-effectiveness relative to the current standard of care. On a European level, spending on cancer drugs has increased substantially in recent years. In 2005, spending on cancer drugs in the EU totaled EUR 7.6 billion and accounted for 12% of total healthcare spending on cancer, by 2014 these figures had increased to EUR 19.1 billion and 23%, respectively[15]. Consequently, to maintain access and equity throughout healthcare systems the acquisition costs associated with novel cancer treatments have been subject to increasing scrutiny from payers and policy makers[16]. In light of the importance of demonstrating the cost-effectiveness and substantiating the budget impact of new interventions the aim of the current analysis was to investigate the long-term cost-effectiveness of 177Lu-Dotatate compared with everolimus in populations of patients with unresectable or metastatic, progressive P-NETs and midgut NETs in both Sweden and Norway (analyses were performed based on health economic models utilized in successful reimbursement submissions for 177Lu-Dotatate in both Sweden and Norway). In addition, budget impact evaluations for Norway, Sweden and Denmark are presented to reflect the potential influence of the novel treatment options on the health care budget.
Long-term cost-effectiveness analysis was performed using a three-state partitioned survival model developed in Microsoft Excel, wherein the three states were PFS, post-progression survival and death, and analyses were performed separately for patients with midgut NETs and patients with P-NETs. This approach allows for independent modeling of PFS and OS based on the use of parametric survival models to extrapolate clinical data beyond the time frame of clinical studies[17]. A series of parametric survival functions (including exponential, Weibull, Gompertz, log-logistic and log-normal models) were fitted to the survival data obtained from the NETTER-1[12] and ERASMUS studies (AAA, data on file). The most appropriate function was determined based on a combination of visual inspection, Akaike’s Information Criterion and the Bayesian Information Criterion as well as both clinical and biological plausibility. On this basis the Weibull model, was considered to be the most appropriate and best fit (for both PFS and OS) and was utilized in the base case analysis (log-normal and log-logistic functions were excluded on the basis that both have long survival tails considered to be biologically implausible). Further, the Weibull model is flexible as has the advantage that the associated hazard rate is not constant over time in contrast to the exponential survival model.
In the analysis a cycle length of 4 wk was used and half-cycle correction was applied (note that 177Lu-Dotatate is administered once every 8 wk for a total of four courses and is therefore integrated into the model every second cycle).
For 177Lu-Dotatate-treated patients, survival data were sourced from the NETTER-1 trial for patients with midgut-NETs and from the ERASMUS study for patients with P-NETs. The analysis for Norway was performed prior to that for Sweden, consequently for the Swedish analysis updated post-hoc survival data were used. For the Norwegian analysis, survival data from the primary analysis were used as post-hoc survival data were not yet available at the time the Norwegian analysis was performed. Baseline cohort characteristics for the simulated patient cohort were based on the NETTER-1 trial and for patients treated with everolimus survival data were sourced from the RADIANT-4[18] trial in patients with gastrointestinal-NETs (GI-NETs) and the RADIANT-3[19] trial in patients with P-NETs. For the survival modeling approach, a hazard ratio was applied to the baseline risk curve to compare each active treatment to a control so that 177Lu-Dotatate and everolimus could be compared indirectly via a common comparator assuming constant proportionality.
For both treatments, missed doses or dose modifications due to toxicity were accounted for using relative dose intensity (RDI) and missed doses were also taken into account in terms of the acquisition costs for each treatment. For everolimus, an RDI of 79.4% was assumed based on data from the RADIANT-4 trial[18]. For 177Lu-Dotatate, RDI refers to the amount of the drug that is actually administered relative to the planned number of four doses, the RDI may be below 100% if patients either miss a dose or have the dose modified due to toxicity or an adverse event. A complete course of 177Lu-Dotatate consists of 4 doses, but based on the findings of the ERASMUS study an RDI of 84.4% was assumed, which corresponds to a mean of 3.4 doses per patient.
Only grade 3-4 adverse events (AEs) were conservatively included in the analysis. For 177Lu-Dotatate-treated patients the frequency of AEs was sourced from the NETTER-1 trial. For everolimus-treated patients the frequency of AEs was sourced from the RADIANT-4 study[18] for midgut-NETs and the RADIANT-3 study[19] for patients with P-NETs.
Drug acquisition costs and resource utilization costs associated with drug administration specific to each setting (e.g., for 177Lu-Dotatate specialist physician, biochemist and nurse as well as general inpatient and outpatient costs) were accounted in the analysis. For Sweden, drug acquisition costs were sourced from FASS (Pharmaceutical specialties in Sweden)[20] and for Norway drug acquisition costs were sourced from the Norwegian Medicines Agency[21] (Table 1). The costs of routine monitoring in each setting (e.g., CT scans, blood tests etc.) were also captured in the analysis (resource use was country-specific owing to differences between health care systems in terms of monitoring frequency). Applying a conservative assumption, costs associated with palliative care were not captured in the analysis.
| Treatment | Dose and frequency | Cost Sweden, SEK | Cost Norway, NOK |
| 177Lu-Dotatate 7.4 GBq (Lutathera®) | 4 administrations of 7.4 GBq, administered once every 8 wk | 200000 per dose | 228508 per dose |
| Octreotide LAR (Sandostatin®) | 60 mg or 30 mg administered once every 28 d | 11957.94 (30 mg) 23915.88 (60 mg) | 13673 (30 mg) 27346 (60 mg) |
| Everolimus (Afinitor®) | 10 mg once daily | 38089.19 (1 × 30 tablet pack, 10 mg) | 36525 (1 × 30 tablet pack, 10 mg) |
| Octreotide SC 50 μg/mL | If required | 283.41 per dose | 227.40 per dose |
| Granisetron1 | 3.1 mg/24 h depot patch administered prior to 177Lu-Dotatate | 913.25 (1 × patch) | 875.30 |
| Ondansetron1 | 8 mg administered prior to 177Lu-Dotatate | 1474.02 (1 × 30 tablet pack, 4 mg) | 985.70 (1 × 30 tablet pack, 8 mg) |
| Tropisetron1 | 5 mg administered prior to 177Lu-Dotatate | 1474.022 | 749.70 (1 × 5 tablet pack, 5 mg) |
| Vamin3 | 18%–18% infusion administered prior to/during 177Lu-Dotatate | 458.08 (2 × 500 mL infusions) | 202.8 (1 × 500 mL infusion) |
| Clinisol3 | 15% infusion administered prior to/during 177Lu-Dotatate | 458.084 | Not available in Norway |
The cost of AEs was calculated as a single cost based on a weighted probability of AEs in each treatment arm. For Sweden, costs for individual AEs were not identified, therefore Norwegian AE costs were used as a proxy and converted to SEK, which included a conversion factor that corrected for the relative cost level in specialist care and the average exchange rate in the previous 12 mo. For Norway, AE costs were calculated based on DRG codes. All costs are presented in 2017 values.
In the base case analysis, for Sweden, the health state utility value for the PFS state for patients with midgut-NETs was sourced from a real-world study in patients with GI-NETs conducted in the United Kingdom (AAA, data on file; Table 2). For Norway, the health state utility value for the PFS state in midgut-NET patients was sourced from NETTER-1 study. For both Sweden and Norway, utility values for all other health states were sourced from the real-world ERASMUS study conducted in the Netherlands. In both the NETTER-1 and ERASMUS studies, QoL was assessed using the European Organization for Research and Treatment of Cancer QLQ-C30 questionnaire[22] and subsequently mapped to the EQ-5D using the algorithm developed by Longworth et al[23], 2014).
| Health state | Utility (SE) | Source |
| Sweden | ||
| Midgut-NET progression-free survival | 0.79 (0.01) | Real world evidence1 |
| Midgut-NET post-progression survival | 0.74 (0.01) | ERASMUS study2 |
| P-NET progression-free survival | 0.80 (0.01) | ERASMUS study2 |
| P-NET post-progression survival | 0.79 (0.02) | ERASMUS study2 |
| Norway | ||
| Midgut-NET progression-free survival (Lutathera) | 0.75 (0.01) | NETTER-1[13] |
| Midgut-NET post-progression survival | 0.74 (0.01) | ERASMUS study2 |
| P-NET progression-free survival | 0.80 (0.01) | ERASMUS study2 |
| P-NET post-progression survival | 0.79 (0.02) | ERASMUS study2 |
Disutilities associated with treatment-related AEs were sourced from published literature[24-27] (Table 3). QoL decrements associated with palliative/end of life care were not included in the analysis.
| Event | Disutility | Ref. |
| Nausea | 0.05 | Nafees et al[24] |
| Vomiting | 0.05 | Nafees et al[24] |
| Diarrhea | 0.05 | Nafees et al[24] |
| Abdominal pain | 0.071 | Doyle et al[25] |
| Thrombocytopenia | 0.111 | Tolley et al[26] |
| Lymphopenia | 0.111 | Assumed2 |
| Leukopenia | 0.111 | Assumed2 |
| Stomatitis | 0.111 | Assumed2 |
| Fatigue | 0.201 | Swinburn et al[27] |
| Infections | 0.111 | Assumed2 |
| Asthenia | 0.201 | Assumed3 |
| Anemia | 0.121 | Swinburn et al[27] |
| Pyrexia | 0.111 | Assumed2 |
| Hyperglycemia | 0.111 | Assumed2 |
| Neutropenia | 0.091 | Nafees et al[24] |
| Hypertension | 0.111 | Assumed2 |
| Musculoskeletal pain | 0.111 | Assumed2 |
| Flushing | 0.111 | Assumed2 |
| Decreased appetite | 0.201 | Assumed3 |
For both Sweden and Norway, the analysis was performed from the perspective of the healthcare payer and the time horizon was 20 years reflecting a life-time analysis given the average age of NET patients at time of diagnosis. In line with national recommendations, for Sweden both future costs and clinical outcomes were discounted at a rate of 3% per annum, and for Norway a discount rate of 4% per annum was applied for both costs and outcomes.
A series of one-way sensitivity analyses were performed to determine key drivers of outcomes. For Sweden, the impact of changing key input values by ± 20% was explored. For Norway key input parameters were varied by ± 30%. Sensitivity analyses were performed around drug acquisition costs, health state utility values, efficacy, resource use and the overall disutility associated with AEs.
Budget impact analyses were performed to estimate the budget impact of introducing 177Lu-Dotatate into clinical practice in Sweden and Norway. For both countries, the treatment eligible GEP-NET population was based on national population figures, incidence estimates published by Öberg et al[4] (2012) and it was assumed that 40% of patients had metastasis at baseline[28]. Market share projections for each country were based on estimates from the manufacturer of 177Lu-Dotatate (AAA, data on file). In both Sweden and Norway, it was assumed that 177Lu-Dotatate would replace everolimus as a treatment option. Budget impact analyses were performed over a time horizon of 5 years, in line with good practice[29] and national guidance[30] and costs were not discounted.
In patients with midgut NETs, treatment with 177Lu-Dotatate was associated with a projected gain in quality-adjusted life expectancy of 0.48 quality-adjusted life years (QALYs) relative to everolimus (3.45 QALYs for 177Lu-Dotatate vs 2.97 QALYs for everolimus), which was driven by longer PFS and OS with 177Lu-Dotatate (Table 4). Total lifetime costs were SEK 189818 higher with 177Lu-Dotatate compared with everolimus (SEK 1,170,731 vs SEK 980913), which resulted in an incremental cost effectiveness ratio (ICER) of SEK 391194 (EUR 36154, September 2019 exchange rates) per QALY gained.
| 177Lu-Dotatate | Everolimus | Delta | |
| Sweden | |||
| Midgut-NETs | |||
| Total costs, SEK | 1170 731 | 980913 | 189818 |
| Quality-adjusted life expectancy, QALYs | 3.45 | 2.97 | 0.48 |
| ICER, SEK per QALY gained | 391194 | ||
| P-NETs | |||
| Total costs, SEK | 1398533 | 1376035 | 22498 |
| Quality-adjusted life expectancy, QALYs | 4.90 | 3.55 | 1.35 |
| ICER, SEK per QALY gained | 16764 | ||
| Norway | |||
| Midgut-NETs | |||
| Total costs, NOK | 985062 | 653066 | 331995 |
| Quality-adjusted life expectancy, QALYs | 3.15 | 1.79 | 1.36 |
| ICER, NOK per QALY gained | 244444 | ||
| P-NETs | |||
| Total costs, NOK | 1280254 | 1056767 | 223487 |
| Quality-adjusted life expectancy, QALYs | 5.31 | 3.21 | 2.10 |
| ICER, NOK per QALY gained | 106451 | ||
For patients with P-NETs, the difference in projected quality-adjusted life expectancy between the two treatments was more pronounced. Treatment with 177Lu-Dotatate resulted in a mean quality-adjusted life expectancy of 4.90 QALYs compared with 3.55 QALYs for patients treated with everolimus (difference 1.35 QALYs) (Table 4). Total mean lifetime costs were also SEK 22498 higher with 177Lu-Dotatate (SEK 1398533 vs SEK 1376034), resulting in an ICER of SEK 16764 (EUR 1549) per QALY gained.
One-way sensitivity analyses showed that for patients with midgut NETs, the findings of the analysis were most sensitive to changes in drug acquisition costs for both 177Lu-Dotatate and everolimus as well as assumptions around health state utility values for the PFS and PPS states (Figure 1). Similarly, for P-NET patients the ICER was most sensitive to changes in drug acquisition costs and changes in the total weighted disutility value for AEs experienced by everolimus-treated patients.
For patients with midgut-NETs, treatment with 177Lu-Dotatate led to a gain in quality-adjusted life expectancy of 1.36 QALYs relative to the use of everolimus (3.15 QALYs for 177Lu-Dotatate vs 1.79 QALYs for everolimus). Total mean lifetime costs were NOK 331995 higher with 177Lu-Dotatate compared with everolimus (NOK 985062 vs NOK 653066), which was primarily driven by the higher acquisition costs associated with 177Lu-Dotatate (Table 4). The incremental gain in quality-adjusted life expectancy combined with higher lifetime costs resulted in an ICER of NOK 244444 (EUR 24416; September 2019 exchange rates) per QALY gained for 177Lu-Dotatate vs everolimus.
For patients with P-NETs, the incremental gain in quality-adjusted life expectancy associated with the use of 177Lu-Dotatate compared with everolimus was 2.10 QALYs (5.31 QALYs for 177Lu-Dotatate vs 3.21 QALYs for everolimus) (Table 4), which was driven by the notably longer PFS and OS projected for patients treated with 177Lu-Dotatate. Lifetime costs were NOK 223487 higher with 177Lu-Dotatate than with everolimus (NOK 1280254 vs NOK 223487). This led to an ICER of NOK 106451 (EUR 10633) per QALY gained.
One-way sensitivity analyses revealed that for patients with midgut-NETs cost-effectiveness was most sensitive to changes in drug acquisition costs for both 177Lu-Dotatate and everolimus and also to the utility value assigned to the PFS health state (Figure 2). Similarly, for P-NET patients results were most sensitive to changes in drug acquisition costs and changes in assumptions around efficacy.
In Sweden it was assumed that the market share of 177Lu-Dotatate for the treatment of patients with GEP-NETs would increase from 30% in Year 1 to 90% in Years 4 and 5 (Table 5). In Year 1, the total budget impact associated with the introduction of 177Lu-Dotatate was SEK 17.3 million (EUR 1.6 million), which increased to SEK 52.2 million (EUR 4.8 million) in Years 4 and 5 (Table 5). Over a 5-year period the total budget impact associated with the introduction of 177Lu-Dotatate was SEK 202 million (EUR 19 million). In Norway, the market share of 177Lu-Dotatate was assumed to remain at 50% over the 5-year time horizon of the analysis and the annual budget impact associated with the introduction of 177Lu-Dotatate was NOK 23.5 million (EUR 2.4 million). Over a 5-year time horizon the total budget impact was estimated at NOK 117 million (EUR 11.8 million).
| Year | Cumulative budget impact over 5 yr | |||||
| 1 | 2 | 3 | 4 | 5 | ||
| Sweden | ||||||
| Total treatment-eligible patients, n1 | 158 | 158 | 158 | 158 | 158 | ― |
| Projected market share for 177Lu-Dotatate, %2 | 30 | 60 | 80 | 90 | 90 | ― |
| Total cost if 177Lu-Dotatate adopted, SEK | 85610153 | 102881530 | 114640765 | 120520382 | 120520382 | 544173212 |
| Total cost if 177Lu-Dotatate not adopted, SEK | 68338777 | 68338777 | 68338777 | 68338777 | 68338777 | 341693885 |
| Budget impact, SEK | 17271376 | 34542753 | 46301988 | 52181605 | 52181605 | 202479327 |
| Norway | ||||||
| Total treatment-eligible patients, n1 | 100 | 100 | 100 | 100 | 100 | ― |
| Projected market share for 177Lu-Dotatate, %2 | 50 | 50 | 50 | 50 | 50 | ― |
| Total cost if 177Lu-Dotatate adopted, NOK | 67920975 | 67920975 | 67920975 | 67920975 | 67920975 | 339604875 |
| Total cost if 177Lu-Dotatate not adopted, NOK | 44438750 | 44438750 | 44438750 | 44438750 | 44438750 | 222193750 |
| Budget impact, NOK | 23482225 | 23482225 | 23482225 | 23482225 | 23482225 | 117411125 |
An additional budget impact analysis was also performed for Denmark. In Denmark, it was assumed that 177Lu-Dotatate would wholly replace lanreotide as a treatment option for GEP-NETs (and that 60 GEP-NET patients per year would be treated with 177Lu-Dotatate) and the annual budget impact was estimated at DKK 27.2 million (EUR 3.6 million), which led to a total budget impact over 5 years of DKK 136 million (EUR 18.2 million).
Overall, the findings of long-term cost-effectiveness analyses suggest that in both Sweden and Norway, 177Lu-Dotatate is associated with substantial gains in quality-adjusted life expectancy and higher lifetime costs in patients with unresectable/metastatic midgut-NETs or P-NETs. In particular, in both countries 177Lu-Dotatate was most cost-effective in patients with P-NETs and was associated with an ICER below EUR 11000 per QALY gained relative to everolimus in both settings. There is no official willingness-to-pay (WTP) threshold in Sweden, in Norway an unofficial threshold of NOK 600000 (EUR 59939) per QALY gained is often cited[31] and in high income European countries a WTP threshold of EUR 50000 per QALY gained is also frequently cited. Consequently, applying these commonly applied thresholds, 177Lu-Dotatate is likely to be considered cost-effective for the treatment of patients with unresectable or metastatic midgut NETs or P-NETs in both Sweden and Norway.
Although 177Lu-Dotatate was shown to be cost-effective in both midgut-NETs and P-NETs the findings in patients with P-NETs may be particularly salient to healthcare payers as historically 5-year survival rates in patients with P-NET have been low relative to patients with GI-NETs[32]. In Sweden, total direct medical costs are also substantially higher for patients with P-NETs compared with patients with small intestine NETs. In 2013, annual per patient direct medical costs were EUR 24800 for patients with small intestine-NETs compared with EUR 37300 for patients with P-NETs[33]. Further, overall 54% of direct costs for patients with GEP-NETs were attributable to cancer drugs and on a national level, EUR 5.1 million was spent on pharmacologic agents for the treatment of patients with GEP-NETs[33]. In terms of budget impact, in Sweden and Denmark overall healthcare spending on cancer in 2014 was EUR 2.7 billion and EUR 1.2 billion, respectively (similar data for Norway were not available)[15]. As such, the budget impact associated with the introduction of 177Lu-Dotatate is likely to have only a minimal impact on overall spending on pharmacologic agents used in cancer treatment.
The findings of the cost-effectiveness analyses presented here align with previously published findings from the National Institute for Health and Care Excellence (NICE)[34] and the Scottish Medicines Consortium[35] in the United Kingdom as well as findings published by the Tandvårds-och läkemedelsförmånsverket (TLV; Dental and Pharmaceutical Benefits Agency) in Sweden. In particular, the NICE assessment reported that for patients with GI-NETs, when compared with everolimus or best supportive care 177Lu-Dotatate was considered to be cost-effective and associated with an ICER below the threshold of GBP 30000 per QALY gained. ICERs were also below GBP 30000 per QALY gained vs everolimus, sunitinib or best supportive care in patients with P-NETs, with the NICE appraisal also noting that for P-NETs 177Lu-Dotatate met the criteria for end of life care of extending life expectancy by at least 3 mo[34]. Additionally, the TLV conducted cost-minimization rather than cost-effectiveness analysis and reported that whilst 177Lu-Dotatate was associated with higher costs than everolimus in patients with GI-NETs it was associated with lower costs than both everolimus and sunitinib in patients with P-NETs[36].
A key limitation of the current analysis is the use of clinical input data obtained via an indirect comparison. Although everolimus was considered to be the most relevant comparator for 177Lu-Dotatate, to date, there are no head-to-head trials that directly compare the efficacy and safety of 177Lu-Dotatate with everolimus in patients with midgut NETs or P-NETs. Consequently, this necessitated the use of data sourced from indirect comparisons, which is inherently associated with a degree of uncertainty. The findings of the indirect comparison used here align with those of a recent systematic review and meta-analysis of 177Lu-Dotatate vs everolimus in advanced P-NETs, wherein 177Lu-Dotatate was associated with a significantly higher objective response rate, disease control rate and PFS relative to everolimus[37]. Additionally, although the analysis included the incidence of grade 3–4 AEs it did not account for long-term persistent hematologic dysfunction that has recently been reported in a small proportion of GEP-NET patients undergoing treatment with 177Lu-Dotatate. A recent real-world analysis reported that 4% of 177Lu-Dotatate-treated GEP-NET patients experienced persistent hematologic dysfunction, occurring at a median of 41 mo post-treatment[38], the costs and clinical implications of which should be considered in future analyses.
A further limitation in the analysis in patients with P-NETs is that only everolimus was included as a relevant comparator, based on clinical practice utilization patterns and input from local clinicians. In Europe, the tyrosine kinase inhibitor sunitinib is indicated for use in the treatment of patients with unresectable or metastatic, well differentiated P-NETs experiencing disease progression[39]. However, as with everolimus, head-to-head clinical data comparing 177Lu-Dotatate with sunitinib are currently lacking. Evidence from two different indirect comparisons, reported that sunitinib and everolimus were associated with similar PFS and OS in P-NET[40,41]. Drug acquisition costs for everolimus and sunitinib were also similar in both Sweden and Norway. It is therefore feasible that given the similar efficacy profile and costs the cost-effectiveness profile of 177Lu-Dotatate vs sunitinib would be similar to that of 177Lu-Dotatate vs everolimus.
Additionally, although Nordic guidelines[3] relating the treatment of patients with GEP-NETs were published prior to the approval of 177Lu-Dotatate, European guidelines advocate the use of PRRT as a treatment option for patients with metastatic midgut-NETs or P-NETs[28] and the findings of the NETTER-1 and ERAMUS trials lend further credence to the clinical evidence base for these recommendations.
For consistency, the analyses in both Sweden in Norway were performed from the healthcare payer perspective. In Sweden reimbursement decisions are based on analyses performed from a societal perspective, which takes into account indirect costs associated with lost productivity. However, the mean age of patients in the simulated cohort was 63.7 years. In Sweden, the minimum retirement age is 61 years and the mean age at retirement was 64.5 years in 2016[42]. As such is it unlikely that the inclusion of indirect costs associated with lost productivity in this population would have a notable impact on the conclusions of the analysis. Further, both PFS and OS were projected to be longer for 177Lu-Dotatate patients, suggesting that the inclusion of indirect costs would likely further reduce the ICER for 177Lu-Dotatate vs everolimus and as such the analysis was conservative.
Overall, the findings of life-long cost-effectiveness analyses for both Sweden and Norway indicate that owing to improved survival outcomes 177Lu-Dotate is likely to be cost-effective and associated with minimal budget impact relative to everolimus for the treatment of patients with unresectable or metastatic progressive midgut-NETs or P-NETs.
177Lu oxodotreotide (177Lu-Dotatate) is a peptide receptor radionuclide therapy that was approved for use in Europe in 2017. Findings from the phase III NETTER-1 trial, conducted in patients with advanced, progressive, somatostatin-receptor-positive midgut neuroendocrine tumors (NETs) showed that the use of 177Lu-Dotatate in combination with best supportive care with octreotide long-acting release (LAR) 30 mg resulted in a significant benefit in terms of both progression-free survival (PFS) and overall survival (OS) compared with octreotide LAR 60 mg.
Health systems frequently have to operate within finite budgetary constraints; therefore, it is important to establish not only the efficacy but also the long-term cost-effectiveness of new treatments as they are introduced into the healthcare system.
The objective of the analysis was to establish the long-term cost-effectiveness of 177Lu-Dotatate compared with everolimus in patients with unresectable or metastatic midgut-NETs or pancreatic-NETs (P-NETs) in both Sweden and Norway.
Cost-effectiveness analysis was performed using a three-state partitioned survival model, which allows for the independent modeling of PFS and OS and the extrapolation of clinical data beyond the time frame of a clinical trial. In the absence of head-to-head trials clinical input data used in the model for the P-NET analysis were sourced from an indirect treatment comparison using input data from trials of 177Lu-Dotatate and everolimus.
In both Sweden and Norway, treatment with 177Lu-Dotatate was associated with an incremental gain in quality-adjusted life expectancy relative to everolimus for both midgut-NETs and P-NETs, which was driven by projected improvements in PFS and OS associated with the use of 177Lu-Dotatate. For Sweden, improved survival outcomes and higher lifetime costs with 177Lu-Dotatate resulted in an incremental cost-effectiveness ratio (ICER) of SEK 391194 per quality-adjusted life year (QALY) gained for midgut NETs and SEK 16764 per QALY gained for P-NETs for 177Lu-Dotatate compared with everolimus. For Norway the corresponding ICERs were NOK 244444 per QALY gained for midgut NETs and NOK 106,451 per QALY gained for P-NETs.
In both Sweden and Norway, 177Lu-Dotatate is likely to be considered cost-effective relative to everolimus for the treatment of patients with unresectable or metastatic, progressive midgut-NETs or P-NETs when considered from the perspective of the healthcare provider.
Based on clinical data derived through indirect comparison 177Lu-Dotatate is likely to be a cost-effective treatment option relative to everolimus for patients with midgut NETs or P-NETs based in Sweden or Norway.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Switzerland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bergsma H, Takura T S-Editor: Ma YJ L-Editor: A P-Editor: Li JH
| 1. | Klöppel G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc Med. 2017;33:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 2. | Cetinkaya RB. Epidemiology of Neuroendocrine Neoplasms in Norway. Oslo University Hospital, Institute of Clinical Medicine, University of Oslo. 2017. [Last accessed June 18, 2019] Available from: https://www.duo.uio.no/bitstream/handle/10852/61617/Cetinkaya-PhD-2018.pdf?sequence=1&isAllowed=y. |
| 3. | Janson ET, Sorbye H, Welin S, Federspiel B, Grønbæk H, Hellman P, Ladekarl M, Langer SW, Mortensen J, Schalin-Jäntti C, Sundin A, Sundlöv A, Thiis-Evensen E, Knigge U. Nordic guidelines 2014 for diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol. 2014;53:1284-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Öberg K, Knigge U, Kwekkeboom D, Perren A; ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii124-vii130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 5. | Statistics Sweden. Population statistics. [Last accessed August 28, 2019] Available from: https://www.scb.se/en/finding-statistics/statistics-by-subject-area/population/population-composition/population-statistics/. |
| 6. | Statistics Norway. Key figures for the population. [Last accessed August 28, 2019] Available from: https://www.ssb.no/en/befolkning/nokkeltall/population. |
| 7. | European Medicines Agency. Orphan designation: Overview. [Last accessed September 02, 2019] Available from: https://www.ema.europa.eu/en/human-regulatory/overview/orphan-designation-overview. |
| 8. | Milione M. Prognostic factors for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): what's better? Endocrine. 2018;59:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Boyar Cetinkaya R, Aagnes B, Myklebust TÅ, Thiis-Evensen E. Survival in neuroendocrine neoplasms; A report from a large Norwegian population-based study. Int J Cancer. 2018;142:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Childs A, Vesely C, Ensell L, Lowe H, Luong TV, Caplin ME, Toumpanakis C, Thirlwell C, Hartley JA, Meyer T. Expression of somatostatin receptors 2 and 5 in circulating tumour cells from patients with neuroendocrine tumours. Br J Cancer. 2016;115:1540-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Lutathera, INN-lutetium (177Lu) oxodotreotide. European Summary of Product Characteristics. [Last accessed June 18, 2019] Available from: https://www.ema.europa.eu/en/documents/product-information/Lutathera-epar-product-information_en.pdf. |
| 12. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2421] [Article Influence: 269.0] [Reference Citation Analysis (0)] |
| 13. | Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, Baum RP, Kunz P, Hobday T, Hendifar A, Oberg K, Sierra ML, Thevenet T, Margalet I, Ruszniewski P, Krenning E; NETTER-1 Study Group. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial. J Clin Oncol. 2018;36:2578-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 290] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 14. | Jiménez-Fonseca P, Carmona-Bayonas A, Martín-Pérez E, Crespo G, Serrano R, Llanos M, Villabona C, García-Carbonero R, Aller J, Capdevila J, Grande E; Spanish Neuroendocrine Tumor Group (GETNE). Health-related quality of life in well-differentiated metastatic gastroenteropancreatic neuroendocrine tumors. Cancer Metastasis Rev. 2015;34:381-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Jönsson B, Hofmarcher T, Lindgren P, Wilking N. The cost and burden of cancer in the European Union 1995-2014. Eur J Cancer. 2016;66:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Aggarwal A, Ginsburg O, Fojo T. Cancer economics, policy and politics: What informs the debate? J Cancer Policy. 2014;2: 1–11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | National Institute for Health and Care Excellence Decision Support Unit. Partitioned survival analysis technical support document. [Last accessed September 02, 2019] Available from: http://nicedsu.org.uk/technical-support-documents/partitioned-survival-analysis-tsd/. |
| 18. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours; Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 938] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 19. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors; Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2160] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 20. | FASS (Pharmaceutical specialities in Sweden) website. [Last accessed August 28, 2019] Available from: https://www.fass.se/LIF/startpage?userType=2. |
| 21. | Norwegian Medicines Agency legemiddelsøk. [Last accessed September 02, 2019] Available from: https://www.legemiddelsok.no/. |
| 22. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11880] [Article Influence: 360.0] [Reference Citation Analysis (0)] |
| 23. | Longworth L, Yang Y, Young T, Mulhern B, Hernández Alava M, Mukuria C, Rowen D, Tosh J, Tsuchiya A, Evans P, Devianee Keetharuth A, Brazier J. Use of generic and condition-specific measures of health-related quality of life in NICE decision-making: a systematic review, statistical modelling and survey. Health Technol Assess. 2014;18:1-224. [PubMed] [DOI] [Full Text] |
| 24. | Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 25. | Doyle S, Lloyd A, Walker M. Health state utility scores in advanced non-small cell lung cancer. Lung Cancer. 2008;62:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Tolley K, Goad C, Yi Y, Maroudas P, Haiderali A, Thompson G. Utility elicitation study in the UK general public for late-stage chronic lymphocytic leukaemia. Eur J Health Econ. 2013;14:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Swinburn P, Wang J, Chandiwana D, Mansoor W, Lloyd A. Elicitation of health state utilities in neuroendocrine tumours. J Med Econ. 2012;15:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Pavel M, O'Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape UF, Öberg K; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 766] [Article Influence: 76.6] [Reference Citation Analysis (1)] |
| 29. | Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, Orlewska E, Penna P, Rodriguez Barrios JM, Shau WY. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 796] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 30. | International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomic guidelines around the world. [Last accessed September 25, 2019] Available from: https://tools.ispor.org/PEguidelines/. |
| 31. | Korman M, Wisløff T. Modelling the cost-effectiveness of PCSK9 inhibitors vs. ezetimibe through LDL-C reductions in a Norwegian setting. Eur Heart J Cardiovasc Pharmacother. 2018;4:15-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 33. | Lesén E, Granfeldt D, Houchard A, Berthon A, Dinet J, Gabriel S, Björstad Å, Björholt I, Elf AK, Johanson V. Cost-of-illness of metastatic gastroenteropancreatic neuroendocrine tumours in Sweden-A population-based register-linkage study. Eur J Cancer Care (Engl). 2019;28:e12983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | National Institute for Health and Care Excellence. TA539 August 2018. Lutetium (177Lu) oxodotreotide for treating unresectable or metastatic neuroendocrine tumors. [Last accessed September 03, 2019] Available from: https://www.nice.org.uk/guidance/ta539. |
| 35. | Scottish Medicines Consortium. Lutetium (177Lu) oxodotreotide 370MBq/mL solution for infusion. June 2018. [Last accessed September 04, 2019] Available from: https://www.scottishmedicines.org.uk/media/3557/Lutetium-177 Lu-oxodotreotide-lutathera-final-june-2018-for-website.pdf. |
| 36. | Tandvårds-och läkemedelsförmånsverket. Underlag för beslut i landstingen Lutathera (lutetium (177Lu)oxodotreotid). [Last accessed September 04, 2019] Available from: https://www.tlv.se/download/18.500ea4181641067957a50823/1529492321767/bes180614_lutathera_underlag.pdf. |
| 37. | Satapathy S, Mittal BR. 177Lu-DOTATATE peptide receptor radionuclide therapy versus Everolimus in advanced pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Nucl Med Commun. 2019;40:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Bergsma H, van Lom K, Raaijmakers MHGP, Konijnenberg M, Kam BLBLR, Teunissen JJM, de Herder WW, Krenning EP, Kwekkeboom DJ. Persistent Hematologic Dysfunction after Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE: Incidence, Course, and Predicting Factors in Patients with Gastroenteropancreatic Neuroendocrine Tumors. J Nucl Med. 2018;59:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Sunitinib European Summary of Product Characteristics. [Last accessed August 29, 2019] Available from: https://www.ema.europa.eu/en/documents/product-information/sutent-epar-product-information_en.pdf. |
| 40. | Signorovitch J, Swallow E, Kantor E, Wang X, Klimovsky J, Haas T, Devine B, Metrakos P. Everolimus and sunitinib for advanced pancreatic neuroendocrine tumors: a matching-adjusted indirect comparison. Exp Hematol Oncol. 2013;2:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Ishak KJ, Rael M, Hicks M, Mittal S, Eatock M, Valle JW. Relative effectiveness of sunitinib versus everolimus in advanced pancreatic neuroendocrine tumors: an updated matching-adjusted indirect comparison. J Comp Eff Res. 2018;7:947-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | European Commission 2018. The Swedish pension system and pension projections until 2070. [Last accessed September 02, 2019] Available from: https://ec.europa.eu/info/sites/info/files/economy-finance/final_country_fiche_se.pdf. |