Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.382
Peer-review started: November 20, 2019
First decision: December 3, 2019
Revised: December 4, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: January 26, 2020
Processing time: 57 Days and 16 Hours
Influenza in children is a major cause of morbidity and mortality worldwide. Nervous system diseases are a factor relating to increased mortality rate. However, reports of how these underlying diseases contribute to the death of children with influenza are rare.
A 4-year-old-girl developed type A influenza-related encephalopathy (IAE) with seizures, acute disorder of consciousness, and intracranial hypertension (cerebrospinal fluid pressure: 250 mmH2O), and the Dandy-Walker variant was found by her first magnetic resonance imaging (MRI) when admission. Three days later, she suddenly presented anisocoria, acute pulmonary edema, and coma, and the later MRI found that she had compressed brainstem, oblongata “Z-like folding”, and swelling bilateral basal ganglia. After admission, the patient were tested for routine and special biomarkers and underwent neuroimaging and neuroelectrophysiology examinations as well as Oseltamivir and intravenous immunogloblin treatments. When predicting that unstable intracranial structures detected by MRI might have disastrous consequences in the progression of IAE, she was transferred into the pediatric intensive care unit and underwent continuous assessment of clinical condition while she did not have instability of basic vital signs; at the same time, her parents were fully informed about the risk and prognosis. Although she was ultimately dead from brain stem failure, the parents expressed understanding and did not trigger a doctor-patient conflict.
In case of finding an unstable intracranial structure, intensive care should be given to IAE patient and their clinical condition should be monitored continuously.
Core tip: Nervous system diseases can increase the mortality rate of influenza, but how these contribute to death is unclear. Here, we report a 4-year-old-girl with congenital brain malformation who progressed to death after getting type A influenza.
- Citation: Li SY, Li PQ, Xiao WQ, Liu HS, Yang SD. Brainstem folding in an influenza child with Dandy-Walker variant. World J Clin Cases 2020; 8(2): 382-389
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/382.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.382
Influenza in children is a major cause of morbidity and mortality worldwide. Annual epidemics in adults and children are associated with an estimated 3-5 million cases of severe illness, and about 290000-650000 deaths. Neurological complications associated with influenza include febrile seizures, acute childhood encephalitis/encephalopathy (IAE), etc[1,2]. Factors related to increased mortality rate include[3-8]: Children under 5 years old (children under 2 years old are prone to higher incidence of serious complications); persons with the following diseases or symptoms: Chronic respiratory diseases, cardiovascular diseases (excluding hypertension), kidney disease, liver disease, blood system diseases, nervous system and neuromuscular diseases, metabolic and endocrine diseases, inhibition of immune responses (including low immune function induced by the use of immunosuppressants or HIV infection); obesity (body mass index greater than 30); concomitant infection by Staphylococcus aureus or Streptococcus pneumoniae, etc. Rarely, we have met a 4-year-old influenza patient who eventually died of brain stem function failure caused by brain stem folding.
Fever with frequent convulsions for half a day.
A 4-year-old girl (weighing 17 kg) got type A influenza with a high fever onset, who did not have influenza vaccination, and rapidly developed frequent convulsions, which resulted in her emergency admission. After admission, the patient underwent clinical and biochemistry examinations (Table 1) as well as Oseltamivir and intravenous immunoglobulin treatments. On the second day of the illness, she developed an acute disorder of consciousness. Therefore, lumbar puncture was performed, and the cerebrospinal fluid (CSF) pressure was as high as 250 mmH2O (Table 1), then her first neuroimaging examination showed the Dandy-Walker variant (DWV) and pontocerebellar hypoplasia (PCH) (Figure 1). When predicting that unstable intracranial structures detected by magnetic resonance imaging (MRI) might have disastrous consequences in the progression of IAE, she was transferred into the pediatric intensive care unit immediately and underwent continuous assessment of clinical condition while she did not have instability of basic vital signs; at the same time, her parents were fully informed about the risk and prognosis.
| Characteristic | |
| Etiology of pharyngeal swab PCR | FA |
| Routine blood test | |
| White blood cells (×109/L) | 9.6 |
| Neutrophils (×109/L) | 7.1 |
| Lymphocytes (×109/L) | 1.63 |
| Monocytes (×109/L) | 0.86 |
| Hemoglobin (g/L) | 100 |
| Platelets (× 109/L) | 288 |
| CSF test after admission | |
| CSF pressure (mmH2O) | 250 |
| White blood cells (×109/L) | 1 |
| C-reactive protein (mg/mL) | 0.05 |
| Chloride (mEq/L) | 126 |
| Glucose (mmol/L) | 4.05 |
| MP (g/L) | 0.12 |
| Lactate dehydrogenase (U/L) | 8 |
| Electrolyte | |
| Na (mEq/L) | 133 |
| K (mEq/L) | 3.7 |
| Blood glucose | 6.3 |
| Lactate (mmol/L) | 1.4 |
| Biochemistry | |
| ALT (U/L) | 122 |
| AST (U/L) | 363 |
| ALT/AST | 0.34 |
| Lactate dehydrogenase (U/L) | 1129 |
| Creatinine kinase (U/L) | 4083 |
| Creatinine kinase MB (U/L) | 169 |
| Ammonia (μM/L) | 27.7 |
| Immune indexes | |
| IgG (U/mL) | 21.5 |
| IgA (U/mL) | 0.8 |
| IgM (U/mL) | 1.99 |
| C3 (U/mL) | 1.16 |
| C4 (U/mL) | 0.26 |
| IgE (U/mL) | 7 |
| Serum indexes | |
| AQP-4 Ab (ng/mL) | 388.154 |
| Human MOG Ab (ng/mL) | 5.21 |
| NMDAR Ab (ng/mL) | 471.03 |
| Caspase-3/7 (mg/mL) | 11.09 |
| MDA (ng/mL) | 665.99 |
| Blood coagulation | |
| Prothrombin time (s) | 12.3 |
| Activated prothrombin time (s) | 31 |
| INR | 0.92 |
| Fibrinogen (mg/L) | 5.79 |
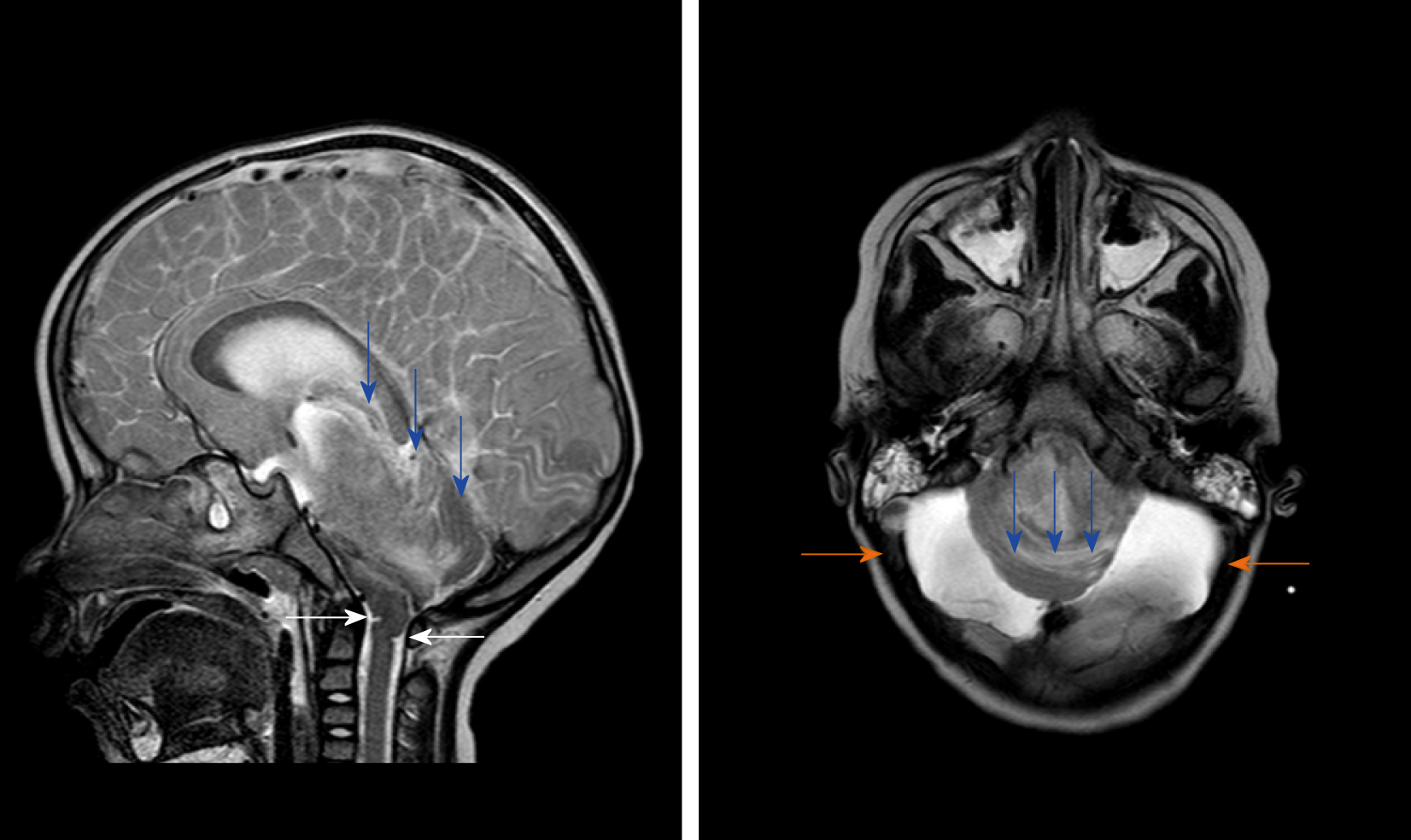
On the fifth day of the disease, she suddenly developed anisocoria and disappearance of light reflection, irregular breathing rhythm, acute pulmonary edema, and deep coma. After this, her brain MRI (Figure 2) showed that the brainstem was significantly displaced by pressure, and the medulla oblongata exhibited “Z-like compressive folding”; cerebellar incision was observed; some ventricles disappeared; and there were multiple intracranial lesions. Before she was died of brain stem functional failure, her neuroelectrophysiology examination showed electric silence (Figure 3). Although she was ultimately died within three weeks after onset, the parents expressed understanding and did not trigger a doctor-patient conflict.
She had backward neurodevelopment, and could not stand alone until being over 1 year old and walk alone until being over 2 years old. However, the cause had not been diagnosed until this admission.
Unremarkable.
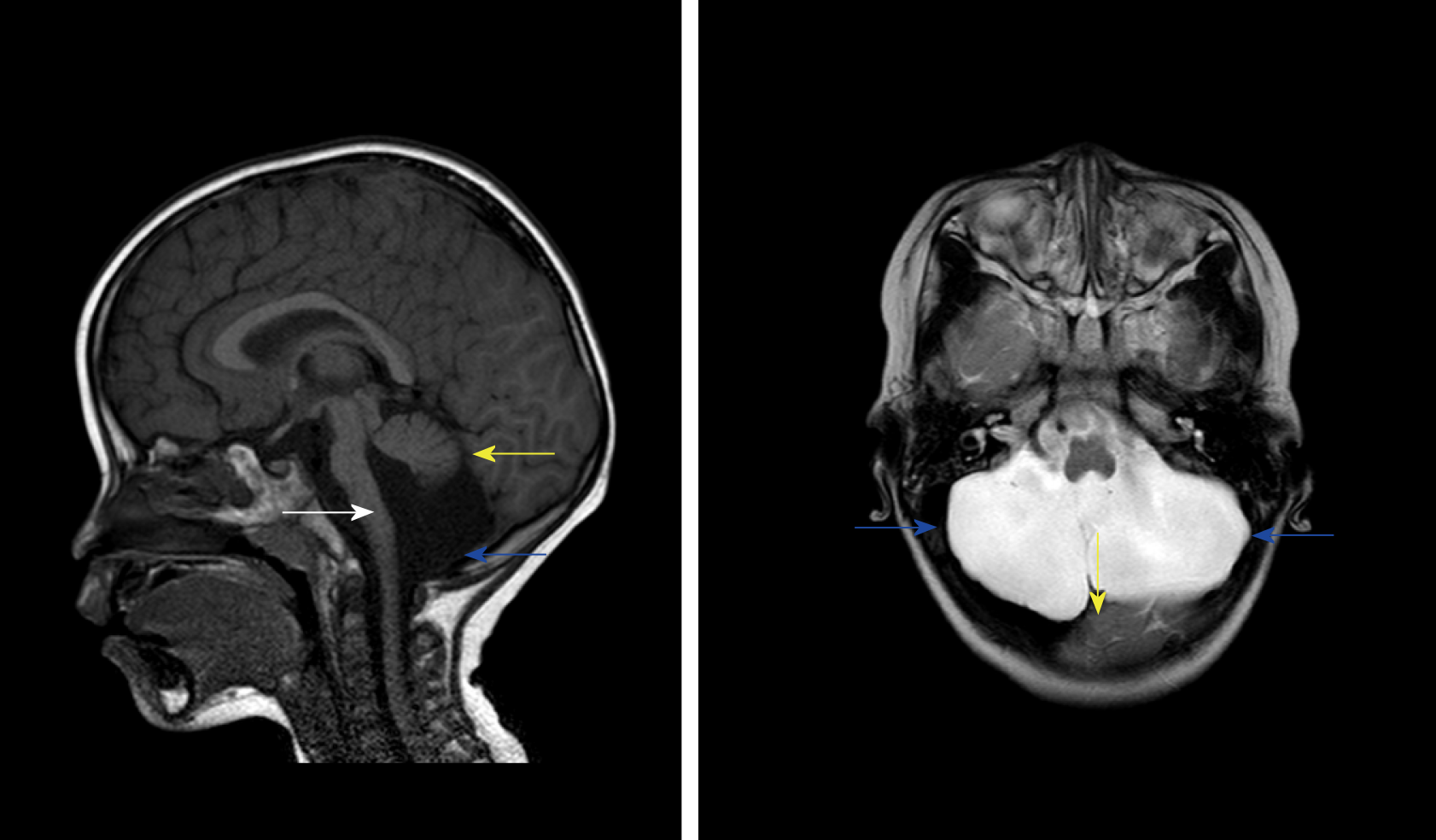
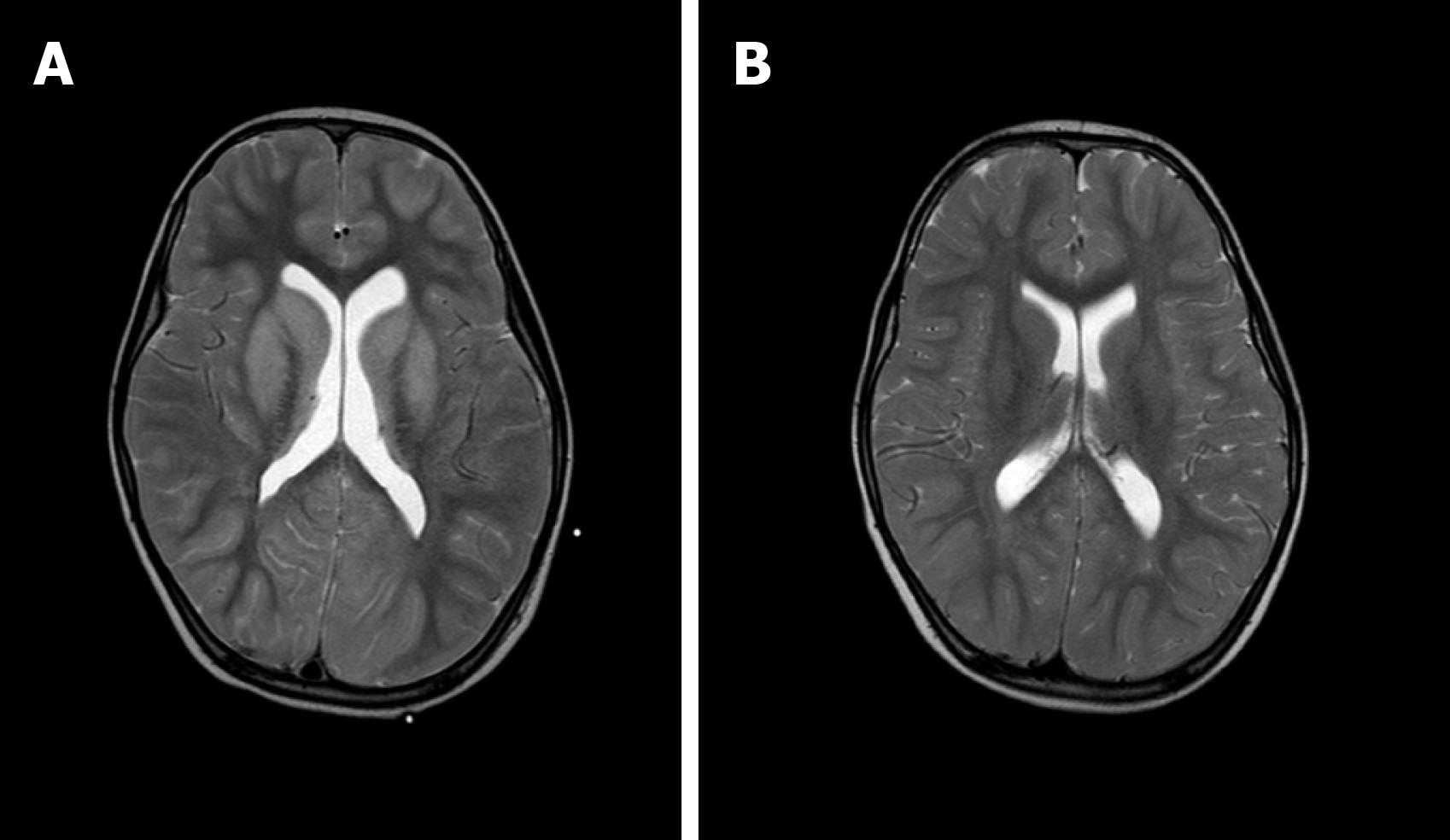
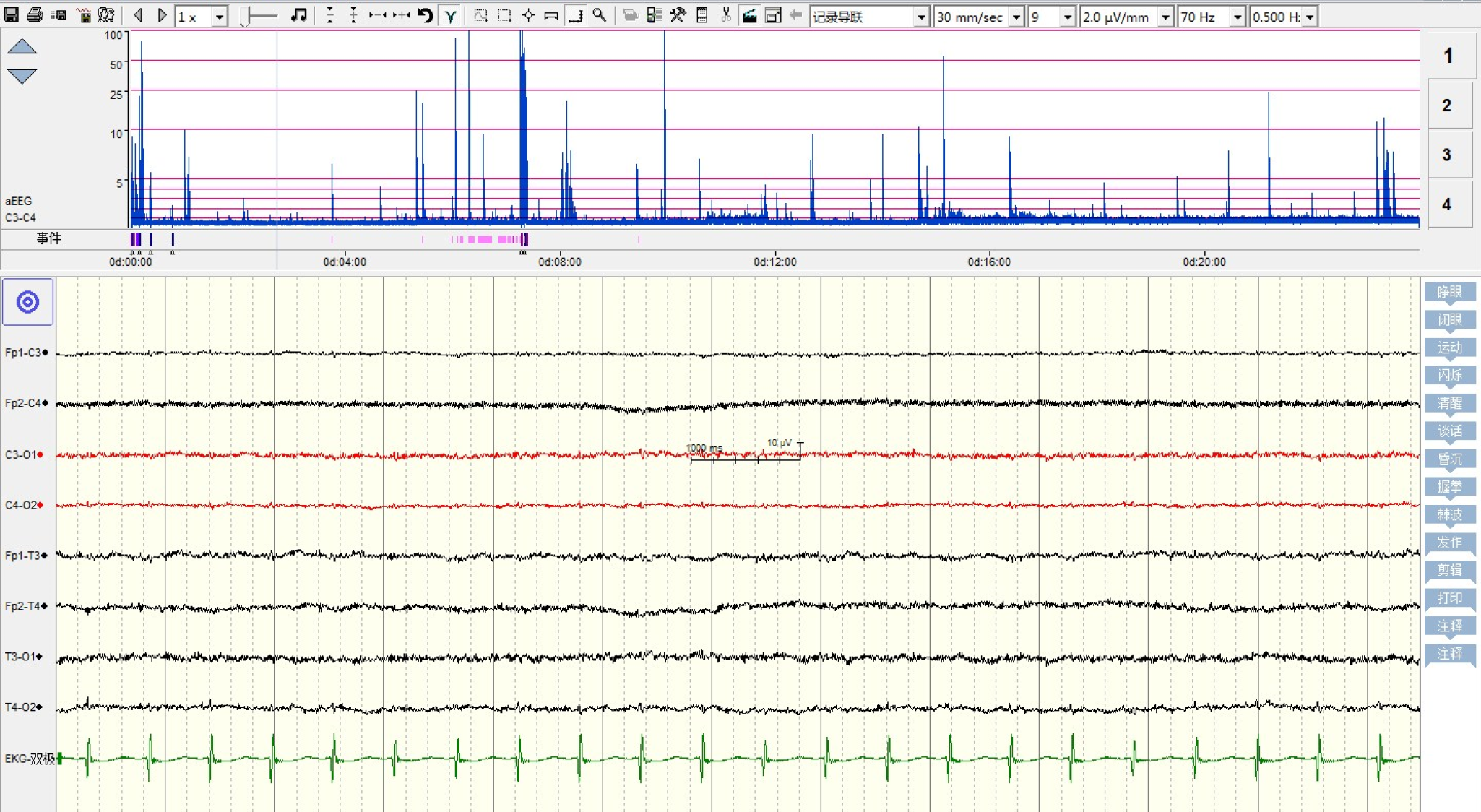
The patient was diagnosed with influenza A by real-time reverse transcription polymerase chain reaction. Clinical biochemistry showed that blood ALT, AST, LDH, and CK were higher than those in the flu-survivor group[9], AQP-4 Ab in blood was positive (388.154 ng/mL), and CSF-pressure was as high as 250 mmH2O with normal biochemical and routine indicators of CSF. On the second day after the onset, which was 3 d before brainstem lesions, she underwent the first brain MRI and was found to have DWV[10] (Figure 1). Sagittal T1WI MRI showed posterior fossa cystic lesions, cyst-like dilatation of the fourth ventricle and communication with the cistern magnum (blue arrow), and hypoplasia of the inferior vermis of cerebellum. The developed superior vermis was elevated upwardly and rotated by posterior fossa cystic lesions (yellow arrow), which caused the death. The pons was smaller, and the medulla was not compressed (white arrow). Sagittal T2WI MRI (Figure 2) showed that the gyri were swelling, the sulcus became superficial (arrow), the supratentorial cranial pressure was increased, the pressure gradient pushed the supratentorial structure down (blue arrow), resulting in compression of the posterior fossa cystic lesion and volume decrease , and the medulla moved down and folded into a “Z-like” shape (white arrow). Axial T2WI MRI showed that the supratentorial structure pushed down the curtain, the pons and medulla were obviously compressed by the hernia tissue (blue arrow), and the surrounding CSF gap disappeared. Posterior fossa cystic lesions shrank in volume under compression, with residual bilateral cystic areas (orange arrow). Figure 3 shows axial T2WI MRI of the basal ganglia after and before brainstem folding. After brain stem folding, swelling of the bilateral putamen, cauda nucleus, frontal lobe, parietal lobe, and occipital cortex, increased T2WI signals, and slightly enlarged bilateral lateral ventricle can be seen (Figure 3B). Three days before brain stem folding, no swelling was observed in the bilateral putamen, caudate nucleus, frontal lobe, parietal lobe, or occipital cortex, with normal T2WI signals, and no expansion was observed in the bilateral lateral ventricles. Before the patient died, continuous electroencephalograph monitoring at bedside revealed electric silence (Figure 4).
The patient’s final diagnosis was DWV (ICD-10: Q03.1001) with influenza-associated encephalopathy (ICD-10: J10.8002+G94.8*) and secondary central brain herniation (ICD-10: G93.501) (brain stem shift) leading to brainstem functional failure.
After admission, the patient was given Oseltamivir and intravenous immunoglobulin treatment in accordance with the “Protocol for diagnosis and treatment of influenza (2018 revised version)”[11] issued by the General Office of the National Health and Health Commission. And she was given advanced life support in time when her condition changed.
The patient died of acute brain stem function failure.
Nervous system diseases and no flu shots can increase the mortality rate and influenza-related complications[12-16]. The above two points were gathered on our patient. The imbalance between supratentorial and infratentorial pressure can lead to the occurrence of central brain herniation. The common causes of pressure increase include cerebral edema secondary to infection or trauma, stroke, etc. When suffering from type A influenza, the patient rapidly developed convulsive status and acute disorder of consciousness with normal biochemical and routine indicators of CSF, but CSF pressure was as high as 250 mmH2O, which could be clinically diagnosed as influenza-associated encephalopathy with intracranial hypertension, and severe intracranial hypertension related with IAE has been reported[17]. In our patient with DWV and PCH, IAE rapidly developed and manifested as diffuse edema of the cerebral cortex and basal ganglia, which created a positive pressure gradient over the tentorium and increased the risk of cerebral herniation. Therefore, it could be speculated that the sudden shrink of her cyst was associated with IAE with intracranial hypertension, especially the progressive swelling of the basal ganglia and above structure (Figure 3). The child’s DWV and PCH had not been diagnosed before. When she was diagnosed with IAE with intracranial hypertension and the unstable intracranial structure had been found, her competent doctor transferred her into the pediatric intensive care unit immediately and gave her continuous assessment while she did not have instability of basic vital signs; at the same time, her parents were fully informed about the risk and prognosis. These positive measures have prevented the doctor-patient conflict caused by the sudden deterioration and death of the child. Although the guidelines for Oseltamivir was followed, it did not help to avoid death[18].
As necrotic cells increased, necrosis-associated biomarker levels were reflected in blood, including LDH[19] and creatine kinase isoenzymes (CK-BB, etc.)[20]. The AQP-4 antibody could mediate neurological diseases by being associated with brain edema[21], which suggests that the immune mechanism might be involved in the pathogenesis of this case. The higher plasma levels of ALT, AST, LDH, CK, and AQP-4 antibody could contribute to an increased risk of death.
This case highlights a rare but severe complication of influenza in young children, and reminds that if influenza children with unstable intracranial structural abnormalities without flu vaccination developed neurological symptoms, the occurrence of influenza-related encephalopathy should be considered. Once confirmed, more active dynamical observation of changes in intracranial structures, more positive assessment and monitoring should be given, and the parents should be fully informed about the risk and prognosis.
| 1. | Yildizdaş D, Kendirli T, Arslanköylü AE, Horoz OO, Incecik F, Ince E, Ciftçi E. Neurological complications of pandemic influenza (H1N1) in children. Eur J Pediatr. 2011;170:779-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Maricich SM, Neul JL, Lotze TE, Cazacu AC, Uyeki TM, Demmler GJ, Clark GD. Neurologic complications associated with influenza A in children during the 2003-2004 influenza season in Houston, Texas. Pediatrics. 2004;114:e626-e633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Kuo HW, Schmid D, Liu YL, Lachner P, Allerberger F. Influenza-related excess mortality, Austria 2001 till 2009. Wien Klin Wochenschr. 2011;123:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Sachedina N, Donaldson LJ. Paediatric mortality related to pandemic influenza A H1N1 infection in England: an observational population-based study. Lancet. 2010;376:1846-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016;16:481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Jochems SP, Marcon F, Carniel BF, Holloway M, Mitsi E, Smith E, Gritzfeld JF, Solórzano C, Reiné J, Pojar S, Nikolaou E, German EL, Hyder-Wright A, Hill H, Hales C, de Steenhuijsen Piters WAA, Bogaert D, Adler H, Zaidi S, Connor V, Gordon SB, Rylance J, Nakaya HI, Ferreira DM. Inflammation induced by influenza virus impairs human innate immune control of pneumococcus. Nat Immunol. 2018;19:1299-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 7. | Tekin S, Keske S, Alan S, Batirel A, Karakoc C, Tasdelen-Fisgin N, Simsek-Yavuz S, Isler B, Aydin M, Kapmaz M, Yilmaz-Karadag F, Ergonul O. Predictors of fatality in influenza A virus subtype infections among inpatients in the 2015-2016 season. Int J Infect Dis. 2019;81:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Santos-Sancho JM, López-de Andrés A, Jimenez-Trujillo I, Hernández-Barrera V, Carrasco-Garrido P, Astasio-Arbiza P, Jimenez-Garcia R. Adherence and factors associated with influenza vaccination among subjects with asthma in Spain. Infection. 2013;41:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Xie ZH, Lin YR, Chen YH. Analysis of clinical characteristics of severe and critically ill influenza A (H1N1)]. Zhonghua Weizhongbing Jijiu Yixue Zazhi. 2019;1154-1157. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Schmidt MJ, Jawinski S, Wigger A, Kramer M. Imaging diagnosis--Dandy Walker malformation. Vet Radiol Ultrasound. 2008;49:264-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | National Health Commission of the People’s Republic of China. Protocol for diagnosis and treatment of influenza (2018 revised version). Zhonghua Linchuang Ganranbing Zazhi. 2019;12:1-5. [DOI] [Full Text] |
| 12. | Shah SI, Caprio M, Hendricks-Munoz K. Administration of inactivated trivalent influenza vaccine to parents of high-risk infants in the neonatal intensive care unit. Pediatrics. 2007;120:e617-e621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Jamotte A, Chong CF, Manton A, Macabeo B, Toumi M. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health. 2016;16:630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Abu-Rish EY, Elayeh ER, Mousa LA, Butanji YK, Albsoul-Younes AM. Knowledge, awareness and practices towards seasonal influenza and its vaccine: implications for future vaccination campaigns in Jordan. Fam Pract. 2016;33:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Azziz Baumgartner E, Dao CN, Nasreen S, Bhuiyan MU, Mah-E-Muneer S, Al Mamun A, Sharker MA, Zaman RU, Cheng PY, Klimov AI, Widdowson MA, Uyeki TM, Luby SP, Mounts A, Bresee J. Seasonality, timing, and climate drivers of influenza activity worldwide. J Infect Dis. 2012;206:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza-United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;1057-1062 Available from: URL: https://www.cdc.gov/mmwr/pre- view/mmwrhtml/mm5933a1.htm. |
| 17. | Citerio G, Sala F, Patruno A, Gori A, Grioni D, Rossi M, Giussani C, Grimaldi M. Influenza A (H1N1) encephalitis with severe intracranial hypertension. Minerva Anestesiol. 2010;76:459-462. [PubMed] |
| 18. | Wolkewitz M, Schumacher M. Neuraminidase Inhibitors and Hospital Mortality in British Patients with H1N1 Influenza A: A Re-Analysis of Observational Data. PLoS One. 2016;11:e0160430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Franke RP, Fuhrmann R, Mrowietz C, Rickert D, Hiebl B, Jung F. Reduced diagnostic value of lactate dehydrogenase (LDH) in the presence of radiographic contrast media. Clin Hemorheol Microcirc. 2010;45:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Uesugi T, Ikai I, Satoh S, Yagi T, Kanazawa A, Takeyama O, Nishitai R, Okabe H, Katsura N, Terajima H, Takahashi R, Yamaoka Y. Influence of humoral immunoreaction on hepatic nonparenchymal cells in ex situ xenoperfused rat livers. J Surg Res. 2001;99:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 21. | Hinson SR, Lennon VA, Pittock SJ. Autoimmune AQP4 channelopathies and neuromyelitis optica spectrum disorders. Handb Clin Neurol. 2016;133:377-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang C S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Xing YX