Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.276
Peer-review started: August 28, 2019
First decision: November 19, 2019
Revised: November 27, 2019
Accepted: December 13, 2019
Article in press: December 13, 2019
Published online: January 26, 2020
Processing time: 141 Days and 18.4 Hours
Diarrhoea is a frequent symptom in children with cancer, and occurs due to a composite effect of underlying disease and immunosuppression consequent to therapy, malnutrition, and non-infective aetiologies such as mucositis. In a large proportion of cases, the aetiology of diarrhoea remains unknown but is often attributed to multiple pathogens including parasites.
To identify and describe the pathogens causing diarrhoea in Bangladeshi children with cancer.
Two cross-sectional pilot studies were conducted involving paediatric oncology patients with diarrhoea. Stool samples were collected from children who were hospitalised with or without being treated with chemotherapy during the study period, and had diarrhoea at any stage during their admission. In the first study, stool samples were tested by conventional microbiological methods and by polymerase chain reaction for parasites, and by immunoassays for Clostridium difficile. In the second study, conventional microbiology was conducted for bacteria and parasites including an enzyme-linked immunosorbent assay for Cryptosporidium antigen, and in a subset, immunoassays for Clostridium difficile.
In the first study Giardia lamblia was detected in 68.5% of samples, Entamoeba histolytica in 13%, Cryptosporidium in 5.6%, non-toxigenic C. difficile in 22.4%, and other bacteria in 5.2%. In the second study, E. histolytica was detected in 10% of samples, Cryptosporidium in 4.3%, G. lamblia in 1.4%, C. difficile in 5.1%, and other bacteria in 5.7% of samples.
These pilot data suggest that parasites are important aetiologies of diarrhoea in Bangladeshi children with malignancy. While molecular diagnostic tools detect an array of stool pathogens with greater sensitivity, conventional diagnostic methods are also useful.
Core tip: In these two pilot studies, pathogens responsible for causing diarrhoea in Bangladeshi children with cancer were explored. In both studies, there were an abundance of parasites including Giardia lamblia, Entamoeba histolytica, and Cryptosporidium, as well as some bacteria, notably non-toxigenic Clostridium difficile.
- Citation: Karim S, Begum F, Islam A, Tarafdar MA, Begum M, Islam MJ, Malik B, Ahsan MS, Khatami A, Rashid H. Pathogens causing diarrhoea among Bangladeshi children with malignancy: Results from two pilot studies. World J Clin Cases 2020; 8(2): 276-283
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/276.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.276
Diarrhoea is a frequent symptom in children with cancer[1], and occurs due to a composite effect of underlying disease and immunosuppression consequent to therapy, malnutrition, and non-infective aetiologies such as mucositis[2]. In a large proportion of cases, the aetiology of diarrhoea remains unknown but is often attributed to multiple pathogens including parasites[3,4].
In immunocompromised individuals, intestinal parasitic infections may run a severe course, at times leading to fatality[5]. However despite this, there are limited data on the epidemiology of such infections among children with malignancy in South Asia. In urban slums of Bangladesh, about five diarrhoeal episodes per year are reported among otherwise healthy infants[6], and in a typical year, a tertiary hospital admits more than 3600 children for diarrhoea, a significant proportion of which are caused by intestinal protozoa[7,8]. As the leading cause, Giardia lamblia has been shown to account for about 15% of identified pathogens causing diarrhoea in young children in urban slums of Bangladesh, while Cryptosporidium and Entamoeba histolytica each account for about 4%[6]. However, the profile of pathogens causing diarrhoea among Bangladeshi children with malignancy is not yet described.
To this end, we presented the results of two pilot studies describing the frequency of pathogens identified during episodes of diarrhoea among paediatric oncology patients admitted to a tertiary referral hospital in Bangladesh. The role of cheaper and more widely available conventional microbiological tests (as opposed to molecular diagnostics) in detecting those pathogens was also investigated.
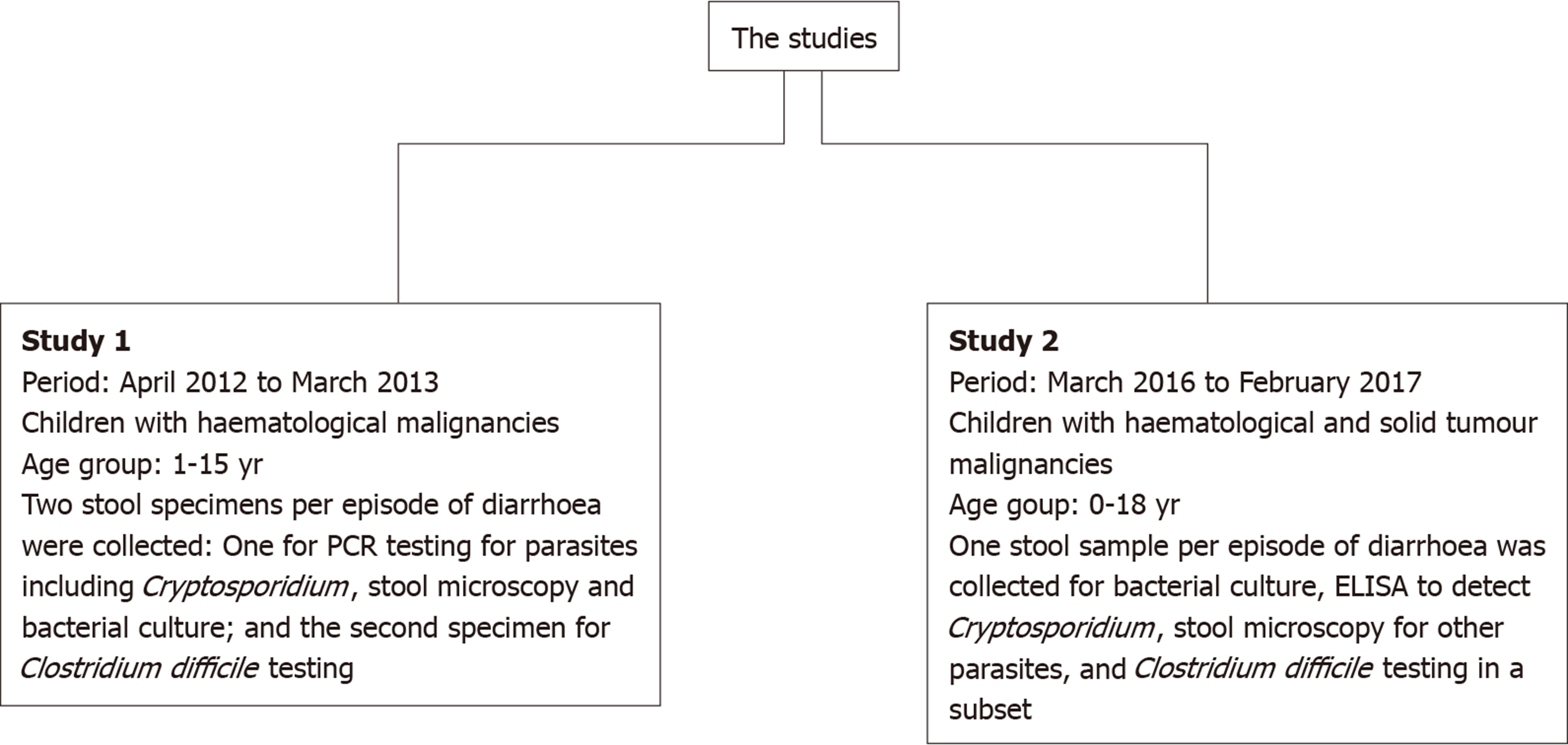
Two pilot cross-sectional studies were conducted at Bangabandhu Sheikh Mujib Medical University Hospital (Dhaka, Bangladesh): The first one from April 2012 to March 2013, and the second from March 2016 to February 2017. Both studies involved hospitalised children with malignancies who developed diarrhoea, defined as an alteration in normal bowel pattern with the passage of three or more consecutive unformed stools within a 24 h period, during their admission. The two study designs differed slightly as summarised in Figure 1. Children with cancer who were hospitalised with or without being treated with chemotherapy during the study period and had diarrhoea at any stage during admission and whose parents/guardian provided consent to participate were eligible for inclusion. For the included children, a separate data form was used each year for collecting demographic and clinical data including age, gender, type and stage of cancer, phase of treatment, and hydration and circulatory status.
In the first study, following recruitment, a fresh stool sample was collected into a pre-labelled container for microscopy for parasites, cysts, and ova and aerobic culture on selective media for enteric bacterial pathogens using standard protocols. In addition, multiplexed, real time, polymerase chain reaction (PCR) for Cryptosporidium spp, E. histolytica, and G. lamblia was conducted on 54 of the total 58 samples using a commercial assay as described elsewhere[9]. A second stool sample was collected for identification of Clostridium difficile toxin and glutamate dehydrogenase by enzyme immunoassays (EIAs) using TOX A/B IITM and C. DIFF CHEKTM-60 (TechLab®, Blacksburg, VA, United States). Some methodological details were presented at the 46th Congress of the International Society of Paediatric Oncology in Toronto, Canada 22nd–25th October, 2014, and the results of this study have been published in brief as conference proceedings[10].
In the second study, a single stool sample was collected in a pre-labelled container for microscopy for parasites, cysts, and ova and aerobic culture on selective media for enteric bacterial pathogens using standard protocols, as well as an enzyme-linked immunosorbent assay (ELISA) for Cryptosporidium using the Cryptosporidium Ag ELISA kit (DRG Diagnostics GmbH, Marburg, Germany). In a random subset (n = 39), C. difficile antigen and toxin were also investigated using TOX A/B IITM and C. DIFF CHEKTM-60 immunoassays.
On the first day of diarrhoea, blood samples were obtained for complete blood count and serum creatinine and electrolytes, as part of the routine diagnostic workup. In both studies, blood tests were conducted at the Paediatric Haematology and Oncology Laboratory of Bangabandhu Sheikh Mujib Medical University; while in the first study, stool microbiology, ELISA, and molecular tests were carried out at the Microbiology laboratory of the International Centre for Diarrhoeal Disease Research (Dhaka, Bangladesh), in the second study, stool microbiology and ELISA were carried out at the Microbiology Laboratory of Bangabandhu Sheikh Mujib Medical University.
Data were collated on a master Excel spread sheet before importing to Statistical Package for Social Sciences software (IBM SPSS Statistics for Windows, version 25.0; IBM Corp., Armonk, NY, United States). Categorical data were expressed as number and proportion while continuous data were expressed as range with measures of central tendency and/or dispersion. Some patients had more than one episode of diarrhoea and hospitalisation, and each presentation was counted separately towards the final denominator.
During a 12-mo period from April 2012 to March 2013, a total of 58 diarrhoeal episodes were experienced by 51 patients. The demographic characteristics of children included in the study are outlined in Table 1. Of note, more than 50% of the children with diarrhoea included in the study were aged < 60 mo. Pathogens detected are listed in Table 1. There was an abundance of G. lamblia (68.5%), and non-toxigenic C. difficile was detected in 13 episodes (22.4%).
| Particulars | Study 11 | Study 22 |
| Male:Female | 32:19 | 47:19 |
| Age in mo, range (mean) | 13-180 (70.8) | 11-216 (73.2) |
| Age group | ||
| ≤ 60 mo | 32 (55.2) | 41 (58.6) |
| 61-120 mo | 16 (27.6) | 15 (21.4) |
| > 120 mo | 10 (17.2) | 14 (20) |
| Diagnosis | ||
| ALL | 37 (63.8) | 40 (57.1) |
| NHL | 17 (29.4) | 10 (14.3) |
| AML | 4 (7.9) | 7 (10) |
| Others | 1 (1.4) | |
| Solid tumours | 12 (17.1) | |
| Phase of treatment | ||
| Induction | 37 (63.8) | 43 (61.4) |
| Consolidation | 14 (24.1) | 10 (14.3) |
| Maintenance | 6 (10.3) | 12 (17.1) |
| Not applicable | 1 (1.7) | 5 (7.1) |
| ANC category | ||
| < 500/mm3 | 47 (81) | 42 (60) |
| ≥ 500/mm3 | 11 (19) | 28 (40) |
| Number of bowel motions/d | ||
| ≥ 10 | 29 (50) | 10 (14.2) |
| 6-9 | 19 (32.8) | 40 (57.1) |
| ≤ 5 | 10 (17.2) | 20 (28.6) |
| Pathogens detected in stool samples | ||
| Giardia lamblia | 37 (68.5)2 | 1 (1.4) |
| Entamoeba histolytica | 7 (13)2 | 7 (10) |
| Cryptosporidium | 3 (5.6)2 | 3 (4.3) |
| Clostridium difficile | 13 (22.4) | 2 (5.1)2 |
| Campylobacter jejuni | 2 (3.4) | 1 (1.4) |
| Salmonella spp | 1 (1.7) | 1 (1.4) |
| Shigella sonnei | 0 (0) | 1 (1.4) |
| Vibrio cholerae | 0 (0) | 1 (1.4) |
In all but two episodes (96.6%), the children had a history of receiving antibiotic therapy or prophylaxis, on average 3.9 d (range 1-16) prior to or during the episode of diarrhoea. Antibiotics received included prophylaxis with oral trimethoprim-sulfamethoxazole (25.9%) or levofloxacin (19%), and treatment with cefepime plus amikacin (19%) or meropenem plus vancomycin (13.8%).
All three children with Cryptosporidium infection were male, aged 3.5, 4.5, and 6 years; two of them had acute lymphoblastic leukaemia (ALL) and the other had non-Hodgkin’s lymphoma. All three had severe neutropenia, with absolute neutrophil counts (ANCs) of 150, 20, and 180 per µL. Two patients had multiple parasitic co-infections: one with all three tested parasites and the other with G. lamblia and Cryptosporidium (Table 2). One of these children had severe dehydration.
| Patients | Age in yr | Gender | Primary diagnosis | Phase of treatment | Preceding hospital stay in d1 | Frequency of bowel motions/d | Fever | Mucositis | Stool microscopy | ANC |
| Study 1 | ||||||||||
| Patient 1 | 4.5 | Male | NHL | Induction | 15 | 16 | Present | Present | 11-20 pus cells per HPF | 150 |
| Patient 2 | 3.5 | Male | ALL | Maintenance | 0 | 6-9 | Present | Absent | > 50 pus cells per HPF | 20 |
| Patient 3 | 6 | Male | ALL | Maintenance | 0 | 3 | Present | Absent | > 10 per HPF | 180 |
| Study 2 | ||||||||||
| Patient 1 | 4 | Male | RMS | Induction | 7 | > 10 | Absent | Absent | Normal | 790 |
| Patient 2 | 5 | Female | PNET | Induction | 0 | 6-9 | Present | Present | Pus cells | 20 |
| Patient 3 | 2.5 | Male | ALL | Induction | 22 | 6-9 | Present | Absent | Normal | 40 |
During a 12-mo period from March 2016 to February 2017, a total of 70 diarrhoeal episodes were experienced by 66 patients. The demographic characteristics of children included in the study and the pathogens detected are outlined in Table 1. Of note, about 60% of children with diarrhoea included in the study were aged < 60 mo and the majority of pathogens detected were parasites.
Two out of three children with Cryptosporidium infection were male, aged 2.5 and 4 years, and the other was female, aged 5 years. One had rhabdomyosarcoma, another had a primitive neuroectodermal tumour, and the third patient had ALL. Two had severe neutropenia with ANCs of 20 and 40, and the other had ANC of 790 per µL (Table 2).
These two pilot studies show that parasites, notably G. lamblia, are responsible for a large proportion of diarrhoeal aetiologies among children with malignancy in Bangladesh. A greater number of potential pathogens were detected with PCR compared to ELISA and conventional microbiological methods, as demonstrated in other studies[11]; however, the latter is still found to be useful.
Apart from an exceptionally high detection rate of giardiasis in the first study, the epidemiological profile of parasites was similar to that found among otherwise healthy Bangladeshi children with diarrhoea[7]. G. lamblia was detected at a significantly higher rate than among otherwise healthy Bangladeshi children 15.2%[6], and in children with cancer in other countries with a similar socioeconomic profile such as Mexico (28.7%)[12]. These differences may be attributed to both the study population (children with malignancy vs otherwise healthy children) as well as study methodologies (use of PCR in the current study, compared to conventional microscopic detection in the Mexican study). This could also be because of selection bias, as some children with diarrhoea or episodes of diarrhoea may have been missed.
Interestingly, the Cryptosporidium burden reported in these pilot studies (4%-5%) is similar to what has been reported in children with cancer in neighbouring countries; e.g., 3.8% in Iran, 4% in Turkey, 2% in Malaysia, 1.3% in India, and 9.6% in Egypt[4,13-16]. The slight variation in these rates is likely because of disparities in testing practice, diagnostic methods used, age groups included, and study designs[1,11]. Conversely, an Australian study that investigated 149 stool samples from 60 paediatric oncology patients with diarrhoea found none to be positive for Cryptosporidium. Contamination of drinking water may be the source of many Cryptosporidium infections in Bangladesh, whereas in Australia exposure to contaminated recreational water (e.g., swimming pools) is the most common source of infection[17]. A comparative study involving Jordanian children demonstrated that compared to children without cancer, paediatric oncology patients had higher prevalence of Cryptosporidium infection (5.1% vs 14.4%, P ≤ 0.05)[18]. These data suggest that the aetiological role of Cryptosporidium is dependent on cancer as an underlying co-morbidity, as well socio-economic and geographic variables among others.
In our setting, in the first dedicated pilot study, 22.4% children were found to be positive for C. difficile in their stool with an absence of toxin positivity based on EIA; while in the second study 5.1% (only in 39 subjects tested) were positive for C. difficile (none were toxin positive). In comparison, among Dutch immunocompromised children admitted to a tertiary hospital, the prevalence of C. difficile detected by culture and cytotoxin tissue culture assay was 27.4%, with over half toxin-positive[19]. In contrast, the prevalence of toxigenic C. difficile among symptomatic paediatric oncology patients was found to be 8.7% in a prospective Australian study (based on culture and EIA for toxin A and cytopathic assay for toxin B), with an additional 4% with non-toxigenic C. difficile[20]. Interestingly, in this study, the prevalence of toxigenic and non-toxigenic C. difficile was higher among asymptomatic children (19% and 6.7% respectively) indicating that toxigenic C. difficile may be part of children’s indigenous gastrointestinal flora, particularly in young infants, as observed in other studies[20,21]. The prevalence of toxigenic C. difficile colonisation may also be higher in children with underlying malignancy. The colonisation rate of C. difficile among asymptomatic Iranian children with cancer was 25% by stool culture, 92% of which were toxicogenic based on cytopathic effect on HeLa cells[22]. Although no studies of Bangladeshi children with malignancy exist, among otherwise healthy Bangladeshi children hospitalised with diarrhoea, 1.6% were infected with C. difficile diagnosed by cell cytotoxin assay in 1993-1994[23].
Despite high rates of colonisation, with even toxigenic strains of C. difficile among asymptomatic children with malignancy, it is important to have an accurate estimate of the prevalence in our population since it has been shown that colonisation with a toxigenic strain is predictive of subsequent C. difficile infection[24]. Further studies using PCR to detect presence of C. difficile toxins would be useful given the limited sensitivity of EIAs used in the current studies.
There were several limitations to these studies. First, the sample sizes were small, the study methodologies were different across the two studies, diagnostic tools used were not uniform, the age groups differed, and in the second study C. difficile was tested in only a small subset of patients; hence the findings are not generalisable. However, despite these shortfalls, these two are the first ever studies in Bangladeshi children with cancer to provide data on the infectious aetiologies of diarrhoea in this population and inspire further research. In conclusion, this study confirms that parasites constitute a significant burden in Bangladeshi children with malignancy who present with diarrhoea. While molecular diagnostic tools detect an array of stool pathogens with greater sensitivity, conventional laboratory diagnostic methods are also useful.
Diarrhoea is a frequently occurring symptom among children with cancer. In a large proportion of cases, the aetiology of diarrhoea remains unknown but often multiple pathogens are attributed.
There is little or no information about pathogens responsible for diarrhoea among children with cancer in Bangladesh, a country where diarrhoeal diseases are endemic.
To describe pathogens causing diarrhoea in Bangladeshi children with cancer.
Two cross-sectional pilot studies were carried out involving hospitalised paediatric oncology patients with diarrhoea. Stool samples were tested by conventional microscopy and culture techniques and by polymerase chain reaction for parasites and bacteria, as well as immunoassays for Clostridium difficile, and enzyme-linked immunosorbent assay for Cryptosporidium antigen.
In the first study Giardia lamblia was detected in around 69% of samples, Entamoeba histolytica in 13%, Cryptosporidium in 6%, non-toxigenic C. difficile in 22% and other bacteria in 5%. In the second study, Entamoeba histolytica was detected in 10% of samples, Cryptosporidium in 4%, G. lamblia in 1%, non-toxigenic C. difficile in 5% and other bacteria in 6% of samples.
These pilot data suggest that parasites are important aetiologies of diarrhoea among Bangladeshi children with malignancy.
In a resource poor setting such as Bangladesh, while molecular diagnostic tools allow detection of an array of stool pathogens with greater frequency, conventional laboratory diagnostic methods are still useful.
The authors would like to thank Professor (Brigadier General) Md. Nizam Uddin, Principal, Rangpur Army Medical College, Bangladesh for his helpful comments on the manuscript.
| 1. | O'Connor O, Cooke RP, Cunliffe NA, Pizer B. Clinical value of stool culture in paediatric oncology patients: hospital evaluation and UK survey of practice. J Hosp Infect. 2017;95:123-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Davila M, Bresalier RS. Gastrointestinal complications of oncologic therapy. Nat Clin Pract Gastroenterol Hepatol. 2008;5:682-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Esteghamati A, Khanaliha K, Bokharaei-Salim F, Sayyahfar S, Ghaderipour M. Prevalence of Intestinal Parasitic Infection in Cancer, Organ Transplant and Primary Immunodeficiency Patients in Tehran, Iran. Asian Pac J Cancer Prev. 2019;20:495-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | El-Mahallawy HA, El-Din NH, Salah F, El-Arousy M, El-Naga SA. Epidemiologic profile of symptomatic gastroenteritis in pediatric oncology patients receiving chemotherapy. Pediatr Blood Cancer. 2004;42:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 319] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Mondal D, Minak J, Alam M, Liu Y, Dai J, Korpe P, Liu L, Haque R, Petri WA. Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis. 2012;54:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Haque R, Mondal D, Karim A, Molla IH, Rahim A, Faruque AS, Ahmad N, Kirkpatrick BD, Houpt E, Snider C, Petri WA. Prospective case-control study of the association between common enteric protozoal parasites and diarrhea in Bangladesh. Clin Infect Dis. 2009;48:1191-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Korpe PS, Haque R, Gilchrist C, Valencia C, Niu F, Lu M, Ma JZ, Petri SE, Reichman D, Kabir M, Duggal P, Petri WA. Natural History of Cryptosporidiosis in a Longitudinal Study of Slum-Dwelling Bangladeshi Children: Association with Severe Malnutrition. PLoS Negl Trop Dis. 2016;10:e0004564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Haque R, Roy S, Siddique A, Mondal U, Rahman SM, Mondal D, Houpt E, Petri WA. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am J Trop Med Hyg. 2007;76:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Begum F, Islam A, Haque R, Chowdhury Y, Mia A, Yasmin F. Clostridium difficile-associated diarrhea in children with haematological malignancy (EP 402). In: 46th Congress of The International Society of Paediatric Oncology (SIOP) 2014 Toronto, Canada, 22nd -25th October, 2014 SIOP Abstracts. Pediatr Blood Cancer. 2014;61 Suppl 2:S343. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | de Boer RF, Ott A, Kesztyüs B, Kooistra-Smid AM. Improved detection of five major gastrointestinal pathogens by use of a molecular screening approach. J Clin Microbiol. 2010;48:4140-4146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Martínez Pérez A, Justiniani Cedeño NE. [incidence of intestinal parasites in pediatric patients with hematologic neoplasms from 1 to 15 years of age]. Rev Alerg Mex. 1999;46:26-29. [PubMed] |
| 13. | Berahmat R, Mahami-Oskouei M, Rezamand A, Spotin A, Aminisani N, Ghoyounchi R, Madadi S. Cryptosporidium infection in children with cancer undergoing chemotherapy: how important is the prevention of opportunistic parasitic infections in patients with malignancies? Parasitol Res. 2017;116:2507-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Aksoy U, Erbay A, Akisu C, Apa H, Ozkoç S, Oztürk S. Intestinal parasites in children with neoplasms. Turk J Pediatr. 2003;45:129-132. [PubMed] |
| 15. | Menon BS, Abdullah MS, Mahamud F, Singh B. Intestinal parasites in Malaysian children with cancer. J Trop Pediatr. 1999;45:241-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Sreedharan A, Jayshree RS, Sridhar H. Cryptosporidiosis among cancer patients: an observation. J Diarrhoeal Dis Res. 1996;14:211-213. [PubMed] |
| 17. | Burgner D, Pikos N, Eagles G, McCarthy A, Stevens M. Epidemiology of Cryptosporidium parvum in symptomatic paediatric oncology patients. J Paediatr Child Health. 1999;35:300-302. [PubMed] |
| 18. | Hijjawi N, Zahedi A, Kazaleh M, Ryan U. Prevalence of Cryptosporidium species and subtypes in paediatric oncology and non-oncology patients with diarrhoea in Jordan. Infect Genet Evol. 2017;55:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Wolfhagen MJ, Meijer K, Fluit AC, Torensma R, Bruinsma RA, Fleer A, Verhoef J. Clinical significance of Clostridium difficile and its toxins in faeces of immunocompromised children. Gut. 1994;35:1608-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Burgner D, Siarakas S, Eagles G, McCarthy A, Bradbury R, Stevens M. A prospective study of Clostridium difficile infection and colonization in pediatric oncology patients. Pediatr Infect Dis J. 1997;16:1131-1134. [PubMed] |
| 21. | Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr. 2010;51:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Armin S, Shamsian S, Drakhshanfar H. Colonization with Clostridium difficile in Children with Cancer. Iran J Pediatr. 2013;23:473-476. [PubMed] |
| 23. | Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol. 1999;37:3458-3464. [PubMed] |
| 24. | Al-Rawahi GN, Al-Najjar A, McDonald R, Deyell RJ, Golding GR, Brant R, Tilley P, Thomas E, Rassekh SR, O'Gorman A, Wong P, Turnham L, Dobson S. Pediatric oncology and stem cell transplant patients with healthcare-associated Clostridium difficile infection were already colonized on admission. Pediatr Blood Cancer. 2019;66:e27604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Bangladesh
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kai K S-Editor: Zhang L L-Editor: Filipodia E-Editor: Ma YJ