Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4380
Peer-review started: June 16, 2020
First decision: July 24, 2020
Revised: July 27, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: October 6, 2020
Processing time: 97 Days and 19 Hours
It is not known whether percutaneous radiofrequency ablation (PRFA) has the same treatment efficacy and fewer complications than laparoscopic resection in patients with small centrally located hepatocellular carcinoma (HCC).
To compare the effectiveness of PRFA with classical laparoscopic resection in patients with small HCC and document the safety parameters.
In this retrospective study, 85 patients treated with hepatic resection (HR) and 90 PRFA-treated patients were enrolled in our hospital from July 2016 to July 2019. Treatment outcomes, including major complications and survival data, were evaluated.
The results showed that minor differences existed in the baseline characteristics between the patients in the two groups. PRFA significantly increased cumulative recurrence-free survival (hazard ratio 1.048, 95%CI: 0.265–3.268) and overall survival (hazard ratio 0.126, 95%CI: 0.025–0.973); PRFA had a lower rate of major complications than HR (7.78% vs 20.0%, P < 0.05), and hospital stay was shorter in the PRFA group than in the HR group (7.8 ± 0.2 d vs 9.5 ± 0.3 d, P < 0.001).
Based on the data obtained, we conclude that PRFA was superior to HR and may reduce complications and hospital stay in patients with small HCC.
Core Tip: In this retrospective study, the effectiveness of percutaneous radiofrequency ablation (PRFA) with classical laparoscopic resection in patients with small hepatocellular carcinoma (HCC) was compared and the safety parameters determined. PRFA treatment reduced the incidence of complications compared with resection and significantly improved overall survival as well as recurrence-free survival. Therefore, PRFA was superior to hepatic resection and may reduce complications and mortality in patients with small HCC.
- Citation: Zhang YH, Su B, Sun P, Li RM, Peng XC, Cai J. Percutaneous radiofrequency ablation is superior to hepatic resection in patients with small hepatocellular carcinoma. World J Clin Cases 2020; 8(19): 4380-4387
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4380.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4380
Hepatocellular carcinoma (HCC) has a high mortality among all cancers worldwide[1]. Most patients with HCC have decreased liver function and require treatment to completely excise the lesion and effectively mitigate further damage to the liver[2]. Hepatic resection (HR) is recommended for patients with a single small HCC lesion up to 2 cm, which is a curative strategy and prevents recurrences. However, the operation takes a heavy toll on the patient’s body. Therefore, clinicians have employed other methods including percutaneous radiofrequency ablation (PRFA), percutaneous ethanol injection, and laparoscopic radiofrequency ablation (LRFA) therapy[3]. PRFA therapy is effective for controlling local tumours with improved survival and is the current standard for early-stage HCC requiring ablative treatments[4-11]. Although studies have demonstrated the superiority of LRFA to PRFA for patient survival[12], LRFA is more invasive than PRFA with higher risks of complications and requires general anaesthesia[13]. When percutaneous ablation treatments cannot be used, HR is a suitable alternative for the treatment of small HCC[14]. However, the most optimal treatment for patients with HCC has not been fully investigated. Thus, we aimed to compare the effectiveness and safety of PRFA with HR and investigate the recurrence, mortality, and survival rates in patients with HCC.
We enrolled 175 patients with small HCC in our hospital from July 2016 to July 2019, of whom 85 received HR and 90 were treated with PRFA. This study was approved by the ethics committee of Yangtze University (Jingzhou, China) and all patients provided informed written consent to participate in this study.
For HR, patients were placed under general anaesthesia, a 1 cm sub-umbilical incision was made, and a trocar with a diameter of 1 cm was inserted to determine the location of the tumour. The hepatic ligament was then removed and labelled on the surface of the liver 2 cm adjacent to the tumour. Finally, we completely resected the entire hepatic segment or lobe[15]. For PRFA we used computed tomography (CT) or magnetic resonance imaging (MRI) for ultrasonography guidance in real-time. We intercostally or subcostally inserted a 17-gauge cooled-tip electrode of 2–3 cm. The ablation procedures generally lasted 12 min with a 3 cm electrode and 6 min with a 2 cm electrode, and a power of 80 W–100 W was typically used. The lesions were assessed one and eight weeks after PRFA by CT or MRI. We defined complete ablation as hypoattenuation of the target area and the surrounding liver parenchyma, which was confirmed by radiology[12].
During the 2.0 ± 0.5-year follow-up period, the patients were followed up by CT or MRI examinations every 3–4 mo in the first two years after PRFA treatment. We also measured liver function and α-fetoprotein levels. Previously published definitions and guidelines were used to define patient outcome and oncologic response[16]. CT or MRI during the follow-up period showing any tumour growth along the ablated or resected locations were considered recurrences and were managed accordingly depending on liver function and tumour characteristics.
All data were analysed by SPSS 20.0. We compared the continuous data of the two groups using the Student’s t-test and the categorical data were examined by the χ2-test. Univariate logistic regression and multivariate Cox proportional-hazards regression were used to analyse the variables that significantly affected the recurrence or survival rates. The recurrence-free and overall survival were examined by Kaplan–Meier plot. Statistical significance was set at P < 0.05.
Table 1 compares the baseline characteristics of the study participants in the HR and PRFA groups. We observed that a higher proportion of patients who received HR had liver cirrhosis and multiple tumours (C2) and exhibited higher TNM stages compared with patients who received PRFA. These data were consistent with the results of liver function tests such as decreased albumin levels. Furthermore, we also found that the PRFA group showed lower AFP levels, which is a tumour marker for HCC. Additionally, there were no differences in the distribution or location of HCC tumours between the two groups. Patients who received PRFA had a significantly lower occurrence of complications compared with the HR group, which was paired with reduced hospitalisation duration.
| HR (n = 85) | PRFA (n = 90) | P value | |
| Gender (M/F) | 47 (55.3)/38 (44.7) | 52 (57.8)/38 (42.2) | 0.740 |
| Age (yr) | 63.5 ± 7.6 | 62.8 ± 8.5 | 0.414 |
| Cirrhosis aetiology | 0.915 | ||
| HCV | 58 (68.2) | 59 (65.6) | |
| HBV | 12 (14.1) | 13 (14.4) | |
| Other | 15 (17.7) | 18 (20) | |
| Platelet count (103/mm3) | 125 ± 58 | 118 ± 62 | 0.442 |
| Total bilirubin (mg/dL) | 1.05 ± 0.49 | 1.08 ± 0.51 | 0.692 |
| PT (INR) | 1.13 ± 0.06 | 1.14 ± 0.18 | 0.627 |
| Albumin (g/dL) | 3.87 ± 0.32 | 4.02 ± 0.40 | 0.007 |
| AFP (ng/dL) | 82.68 ± 7.85 | 80.24 ± 7.24 | 0.034 |
| Tumour size (cm) | 1.82 ± 0.24 | 1.76 ± 0.32 | 0.164 |
| Number of tumours | 0.012 | ||
| 1 | 63 (74.1) | 82 (91.2) | |
| 2 | 12 (14.1) | 4 (4.4) | |
| ≥ 3 | 10 (11.8) | 4 (4.4) | |
| TNM stage | < 0.001 | ||
| I | 61 (71.8) | 84 (93.3) | |
| II | 24 (28.2) | 6 (6.7) | |
| Subcapsular tumour | 35 (41.2) | 48 (53.3) | 0.107 |
| Complications | 17 (20) | 7 (7.78) | 0.033 |
| Postoperative haemorrhage | 4 (4.71) | 1 (1.11) | 0.331 |
| Bile leak | 2 (2.35) | 1 (1.11) | 0.960 |
| Subphrenic collection/abscess | 3 (3.53) | 1 (1.11) | 0.573 |
| Infected ascites | 4 (4.71) | 2 (2.22) | 0.626 |
| Liver failure | 1 (1.18) | 0 (0) | 0.977 |
| Pleural effusion | 3 (3.53) | 2 (2.22) | 0.948 |
| Hospital mortality | 0 | 0 | - |
| Days of hospital stay during initial therapy | 9.5 ± 0.3 | 7.8 ± 0.2 | < 0.001 |
Our univariate and multivariate analyses revealed that levels of serum albumin and AFP, the number of tumours (especially C2 tumours), and hospital duration in the PRFA group significantly affected the recurrence-free survival (Table 2). Similarly, the PRFA procedure, serum albumin and AFP levels, and hospital duration predicted overall survival of patients with HCC (Table 3).
| Variables | Univariate logistic regression | Cox proportional-hazards regression | |
| P value | Hazard ratio (95%CI) | P value | |
| Albumin (g/dL) | 0.018 | 0.325 (0.109–0.875) | 0.020 |
| AFP: normal vs abnormal | 0.037 | 1.658 (1.135–3.258) | 0.023 |
| Number of tumours | |||
| 1 | 1.000 | 0.023 | |
| 2 | 5.784 (1.387–20.268) | 0.015 | |
| 3 | 7.458 (0.896–87.257) | 0.056 | |
| TNM stage | < 0.001 | ||
| I | |||
| II | |||
| Days of hospital stay during initial therapy | 0.028 | 1.058 (1.005–1.224) | 0.027 |
| HR vs PRFA | 0.043 | 1.045 (0.325–2.838) | 0.039 |
| Variables | Univariate logistic regression | Cox proportional-hazards regression | |
| P value | Hazard ratio (95%CI) | P value | |
| Albumin (g/dL) | < 0.001 | 0.058 (0.008 – 0.425) | 0.003 |
| AFP: normal vs abnormal | 0.0346 | 1.647 (1.057 – 3.269) | 0.018 |
| Days of hospital stay during initial therapy | 0.013 | 1.325 (1.057 – 1.523) | 0.006 |
| HR vs PRFA | 0.026 | 0.114 (0.015 – 0.846) | 0.035 |
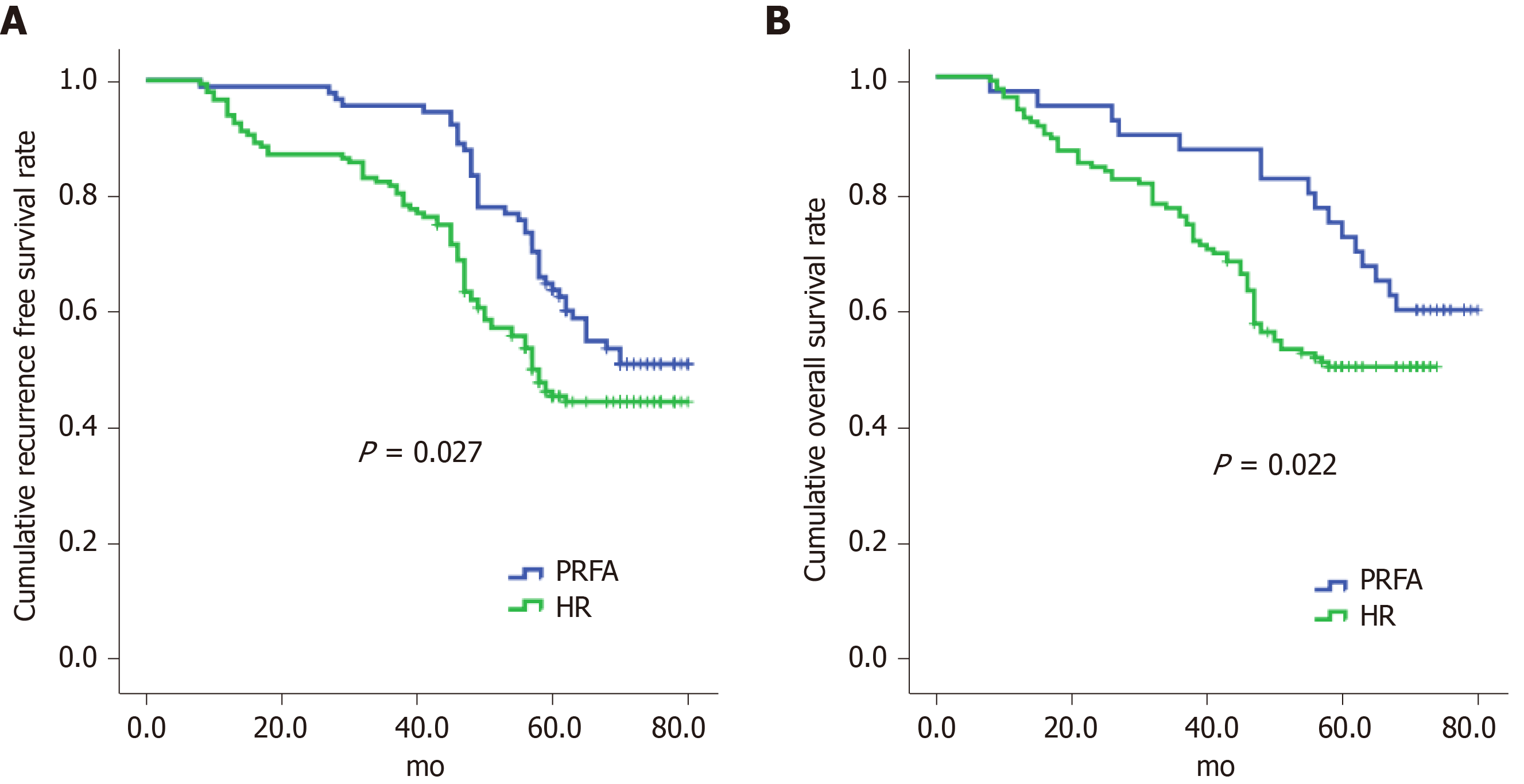
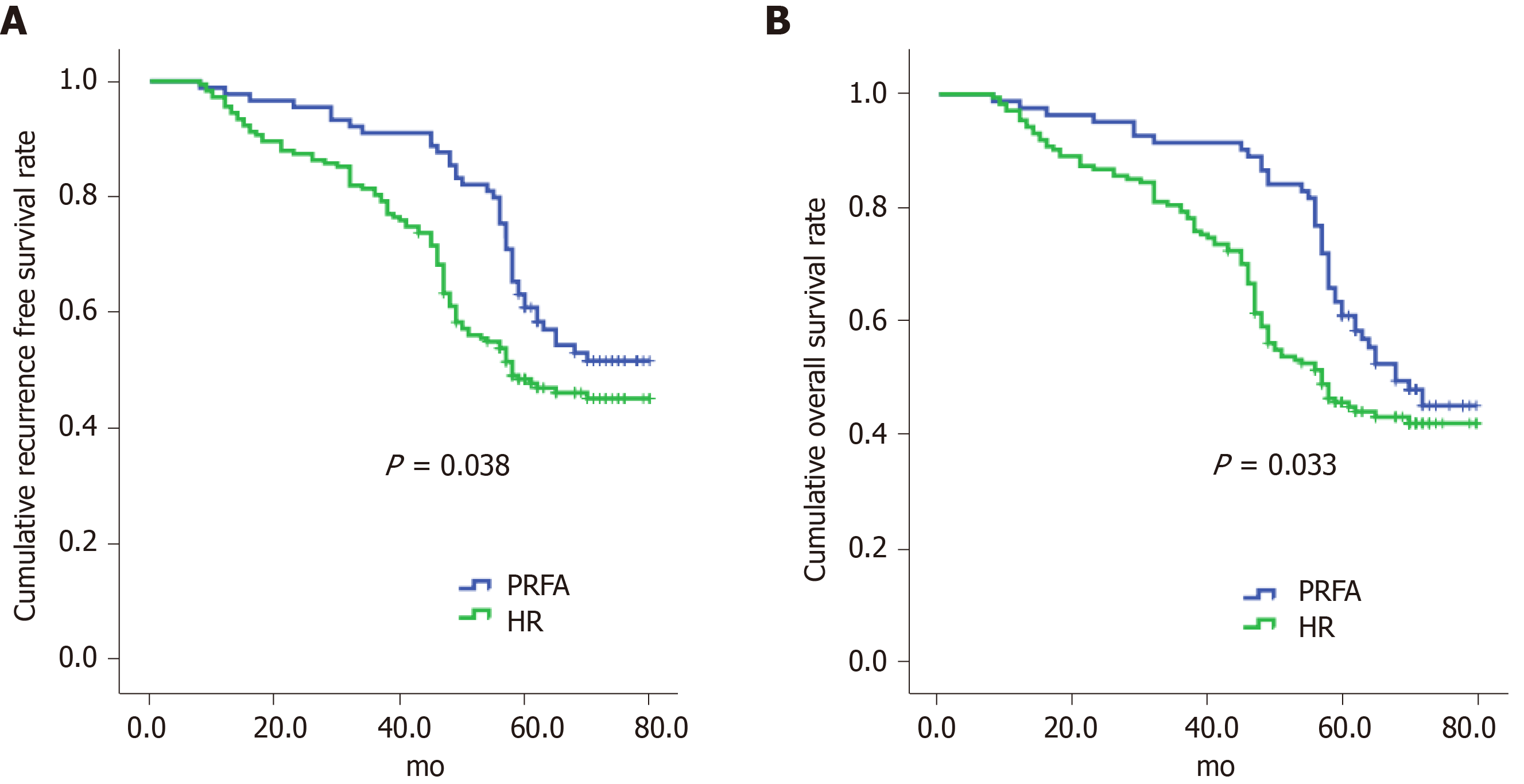
PRFA significantly increased cumulative recurrence-free survival (hazard ratio 1.048, 95%CI: 0.265–3.268) and overall survival (hazard ratio 0.126, 95%CI: 0.025–0.973) compared with HR (Figure 1) and was a significant predictor of both outcomes (Figure 2).
In recent years, clinicians have aimed for effective, precise, and minimally invasive treatments for patients with HCC, and PRFA and laparoscopic surgery have gradually become the primary recommended treatments[17]. Compared with traditional open cholecystectomy, laparoscopic surgery is advantageous due to less trauma and bleeding and shorter recovery times with comparable survival and recurrence rates[18]. PRFA is a newly developed local treatment that relies on heat to induce necrosis of the tumour and surrounding tissues and has been demonstrated to achieve the same clinical effect as open surgery for patients with single small HCC up to 3 cm in size[19,20]. PRFA can be easily performed and is repeatable with little damage to liver function[21]. However, the best choice of therapy for patients with HCC requires further study.
In this study, we found that hospitalization duration was significantly shorter and complications were less frequent in the PRFA group than in the HR group, and this was consistent with the results of other studies[22,23]. Also there were higher recurrence rates in patients treated by HR compared with PRFA. This could be due to the higher TNM stages of HCC tumours in patients treated with HR. Furthermore, these patients were more likely to have multiple tumours. PRFA did not significantly affect recurrence-free survival and was consistent with a previous study, although it did improve overall survival[24]. However, PRFA may reduce HCC recurrence, which would lead to reduced patient mortality. Our data indicated that PRFA was a contributing and prognostic factor for improving overall survival, liver function, and tumour characteristics. Furthermore, local progression of HCC, intra-segmental recurrences, and recurrences less than 12 mo after treatment were more frequent after HR, which was not attributable to a selection bias. Studies have reported that HCC lesions less than 2 cm in diameter may harbour highly proliferative tumour cells, thus it is critical to locate micro invasions or microsatellites.
In conclusion, PRFA was superior to HR for the survival of small HCC patients, especially those with peripheral tumours. In addition, it safeguarded liver function and reduced the complication and recurrence rates compared with HR. Therefore, we recommend PFRA as the standard treatment for patients with HCC.
Based on the data obtained, we conclude that PRFA was superior to hepatic resection and may reduce complications and hospital stay in patients with small HCC. Therefore, increased clinical application of PFRA will prove PFRA as the standard treatment for patients with small HCC.
It is not known whether percutaneous radiofrequency ablation (PRFA) has the same treatment efficacy and fewer complications than laparoscopic resection in patients with small centrally located hepatocellular carcinoma (HCC).
This retrospective study aimed to compare the effectiveness of PRFA with classical laparoscopic resection in patients with small HCC and document the safety parameters, to provide an experimental basis for the clinical treatment of small HCC.
To determine whether PRFA has the same effect as surgical resection with fewer complications in patients with small HCC, in order to provide more specific options for HCC treatment.
In this retrospective study, 85 patients treated with hepatic resection and 90 PRFA-treated patients were enrolled in our hospital from July 2016 to July 2019, Treatment outcomes, including major complications and survival data, were determined.
The results showed that minor differences existed in the baseline characteristics between the patients in the two groups. PRFA significantly increased cumulative recurrence-free survival (hazard ratio 1.048, 95%CI: 0.265–3.268) and overall survival (hazard ratio 0.126, 95%CI: 0.025–0.973); PRFA had a lower rate of major complications than HR (7.78 vs 20.0%, P < 0.05), and the hospital stay was also shorter in the PRFA group than in the HR group (7.8 ± 0.2 d vs 9.5 ± 0.3 d, P < 0.001).
Based on the data obtained, we conclude that PRFA was superior to hepatic resection and may reduce complications and hospital stay in patients with small HCC.
The clinical application of PFRA should be increased to prove PFRA as the standard treatment for patients with small HCC.
| 1. | Zhang J, Qi YP, Ma N, Lu F, Gong WF, Chen B, Ma L, Zhong JH, Xiang BD, Li LQ. Overexpression of Epcam and CD133 Correlates with Poor Prognosis in Dual-phenotype Hepatocellular Carcinoma. J Cancer. 2020;11:3400-3406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1284] [Article Influence: 183.4] [Reference Citation Analysis (3)] |
| 3. | Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, Yang T, Yan Z, Lei Z, Si A, Wan X, Zhang H, Gao C, Cheng Z, Pawlik TM, Wang H, Lau WY, Wu M, Shen F. Long-term Effects of Repeat Hepatectomy vs Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Nishikawa H, Kimura T, Kita R, Osaki Y. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia. 2013;29:558-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Donadon M, Solbiati L, Dawson L, Barry A, Sapisochin G, Greig PD, Shiina S, Fontana A, Torzilli G. Hepatocellular Carcinoma: The Role of Interventional Oncology. Liver Cancer. 2016;6:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Toshikuni N, Tsutsumi M, Takuma Y, Arisawa T. Real-time image fusion for successful percutaneous radiofrequency ablation of hepatocellular carcinoma. J Ultrasound Med. 2014;33:2005-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Izumi N. Recent advances of radiofrequency ablation for early hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26 Suppl 1:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: An updated review. World J Gastrointest Pharmacol Ther. 2016;7:477-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-77; quiz 578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 590] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 10. | Osaki Y, Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol Res. 2015;45:59-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, Gansen DN, de Groen PC, Lazaridis KN, Narayanan Menon KV, Larusso NF, Alberts SR, Gores GJ, Fleming CJ, Slettedahl SW, Harmsen WS, Therneau TM, Wiseman GA, Andrews JC, Roberts LR. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Eun HS, Lee BS, Kwon IS, Yun GY, Lee ES, Joo JS, Sung JK, Moon HS, Kang SH, Kim JS, Shin HJ, Kim TK, Chun K, Kim SH. Advantages of Laparoscopic Radiofrequency Ablation Over Percutaneous Radiofrequency Ablation in Hepatocellular Carcinoma. Dig Dis Sci. 2017;62:2586-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Takahashi H, Akyuz M, Aksoy E, Karabulut K, Berber E. Local recurrence after laparoscopic radiofrequency ablation of malignant liver tumors: Results of a contemporary series. J Surg Oncol. 2017;115:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Santambrogio R, Bruno S, Kluger MD, Costa M, Salceda J, Belli A, Laurent A, Barabino M, Opocher E, Azoulay D, Cherqui D. Laparoscopic ablation therapies or hepatic resection in cirrhotic patients with small hepatocellular carcinoma. Dig Liver Dis. 2016;48:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Molina V, Sampson-Dávila J, Ferrer J, Fondevila C, Díaz Del Gobbo R, Calatayud D, Bruix J, García-Valdecasas JC, Fuster J. Benefits of laparoscopic liver resection in patients with hepatocellular carcinoma and portal hypertension: a case-matched study. Surg Endosc. 2018;32:2345-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Guo WX, Sun JX, Cheng YQ, Shi J, Li N, Xue J, Wu MC, Chen Y, Cheng SQ. Percutaneous radiofrequency ablation versus partial hepatectomy for small centrally located hepatocellular carcinoma. World J Surg. 2013;37:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Lai C, Jin RA, Liang X, Cai XJ. Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J Zhejiang Univ Sci B. 2016;17:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Chen J, Li H, Liu F, Li B, Wei Y. Surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma for various resection extent. Medicine (Baltimore). 2017;96:e6460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Choi EJ, Choi YM, Kim HJ, Ok HG, Chang EJ, Kim HY, Yoon JU, Kim KH, Byeon GJ. The Effects of Thoracic Epidural Analgesia during Percutaneous Radiofrequency Ablation for Hepatocellular Carcinoma. Pain Res Manag. 2018;2018:4354912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Kim HY, Park JW. Clinical trials of combined molecular targeted therapy and locoregional therapy in hepatocellular carcinoma: past, present, and future. Liver Cancer. 2014;3:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Jeong Y, Yoon SM, Han S, Shim JH, Kim KM, Lim YS, Lee HC, Kim SY, Park JH, Lee SW, Ahn SD, Choi EK, Kim JH. Propensity Score Matching Analysis of Changes in Alpha-Fetoprotein Levels after Combined Radiotherapy and Transarterial Chemoembolization for Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. PLoS One. 2015;10:e0135298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology. 2018;287:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 23. | Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 613] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 24. | Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, Henmi S, Hatamaru K, Ishikawa T, Saito S, Nasu A, Kita R, Kimura T, Arimoto A, Osaki Y. Comparison of percutaneous radiofrequency thermal ablation and surgical resection for small hepatocellular carcinoma. BMC Gastroenterol. 2011;11:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Iannitti D, Kao JT S-Editor: Wang DM L-Editor: Webster JR P-Editor: Xing YX