Published online Sep 26, 2020. doi: 10.12998/wjcc.v8.i18.4207
Peer-review started: April 14, 2020
First decision: July 25, 2020
Revised: July 30, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: September 26, 2020
Processing time: 160 Days and 19.9 Hours
Perivascular epithelioid cell tumor (PEComa) is a rare mesenchymal tumor that exhibits an epithelioid and spindle cell morphology. The tumor is characterized by immunoreactivity for melanocytic and myogenic markers but can be misdiagnosed as more common tumors with similar characteristics, including gastrointestinal stroma tumors or leiomyosarcomas. Recently, a subset of PEComas has been reported to harbor a transcription factor binding to TFE3 fusion. Herein, we report a rare case of TFE3-expressing malignant PEComa arising from the mesentery.
A 50-year-old woman presented with abdominal discomfort for 3 months. Results of laboratory tests were all within the normal ranges, and the patient had no notable medical history. Magnetic resonance imaging revealed a large tumor on the right side of the pelvic floor, which was originally suspected to be a primary ovarian tumor. However, during surgery, the tumor was revealed to have originated from the mesentery. Histologically, the tumor was composed of bundles of spindle cells and sheets of epithelioid cells. Extensive coagulative necrosis and numerous mitotic figures were observed. Immunohistochemistry revealed that the tumor cells were positive for smooth muscle actin, HMB-45, and TFE3 expression. Tumor involvement of the rectal serosa was identified, leading to a final diagnosis of malignant PEComa of the mesentery. Surgical resection was followed by adjuvant chemotherapy. No recurrence or metastasis was observed over a 6-month follow-up period.
Malignant PEComa of the mesentery is extremely rare and should be distinguished from morphological mimics through differential diagnosis and immunohistochemistry.
Core Tip: Primary mesenchymal tumors originating from the mesentery are rare and can only be diagnosed histologically. Broad differential diagnoses and immunohistochemical analyses are required along with consideration of perivascular epithelioid cell tumor among differential diagnoses.
- Citation: Kim NI, Lee JS, Choi YD, Ju UC, Nam JH. TFE3-expressing malignant perivascular epithelioid cell tumor of the mesentery: A case report and review of literature. World J Clin Cases 2020; 8(18): 4207-4214
- URL: https://www.wjgnet.com/2307-8960/full/v8/i18/4207.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i18.4207
Perivascular epithelioid cell tumor (PEComa) is an uncommon mesenchymal neoplasm. Bonetti et al[1,2] first described this rare tumor type in 1992, and Zamboni et al[3] subsequently coined the term “PEComa” in 1996. The World Health Organization recognized PEComa as a distinct tumor entity in 2002[4]. In 2005, Folpe et al[5] first reported four patients with PEComa of the mesentery. Including this first case series, to the best of our knowledge, only nine cases of primary PEComa of the mesentery have been reported to date[5-10]. Since PEComa is a rare disease, other more common tumors such as gastrointestinal stroma tumor or leiomyosarcoma that show similar morphological characteristics will first be considered in the context of a differential diagnosis.
Histologically, PEComas are composed of epithelioid and spindle cells with radial growth around blood vessels. The characteristic expression of myogenic and melanocytic markers supports a diagnosis of PEComa[11]. Recently, molecular studies identified a subset of PEComas with transcription factor binding to TFE3 rearrangement, suggesting that PEComas harboring TFE3 fusions may represent a distinct disease entity[12].
Herein, we describe a rare case of malignant PEComa originating from the mesentery and provide guidance for improving differential diagnosis of this tumor along with a review of the relevant literature.
A 50-year-old woman was admitted to the gynecology department of Chonnam National University Hospital with complaints of abdominal discomfort.
The patient exhibited symptoms 3 months prior to admission, and initial sonography performed in a primary health care center revealed an ovarian tumor of 8 cm × 6 cm × 6 cm at that time. The patient was then referred to a tertiary hospital for further evaluation and treatment.
The patient had no history of diabetes, hypertension, or other gynecological disease.
The patient had no significant personal or family medical history.
The patient did not exhibit weight loss, palpable lymphadenopathy, or organomegaly.
Results of laboratory tests, including carcinoembryonic antigen and cancer antigen 125 levels, and Risk of Ovarian Malignancy Algorithm score were all in the normal range.
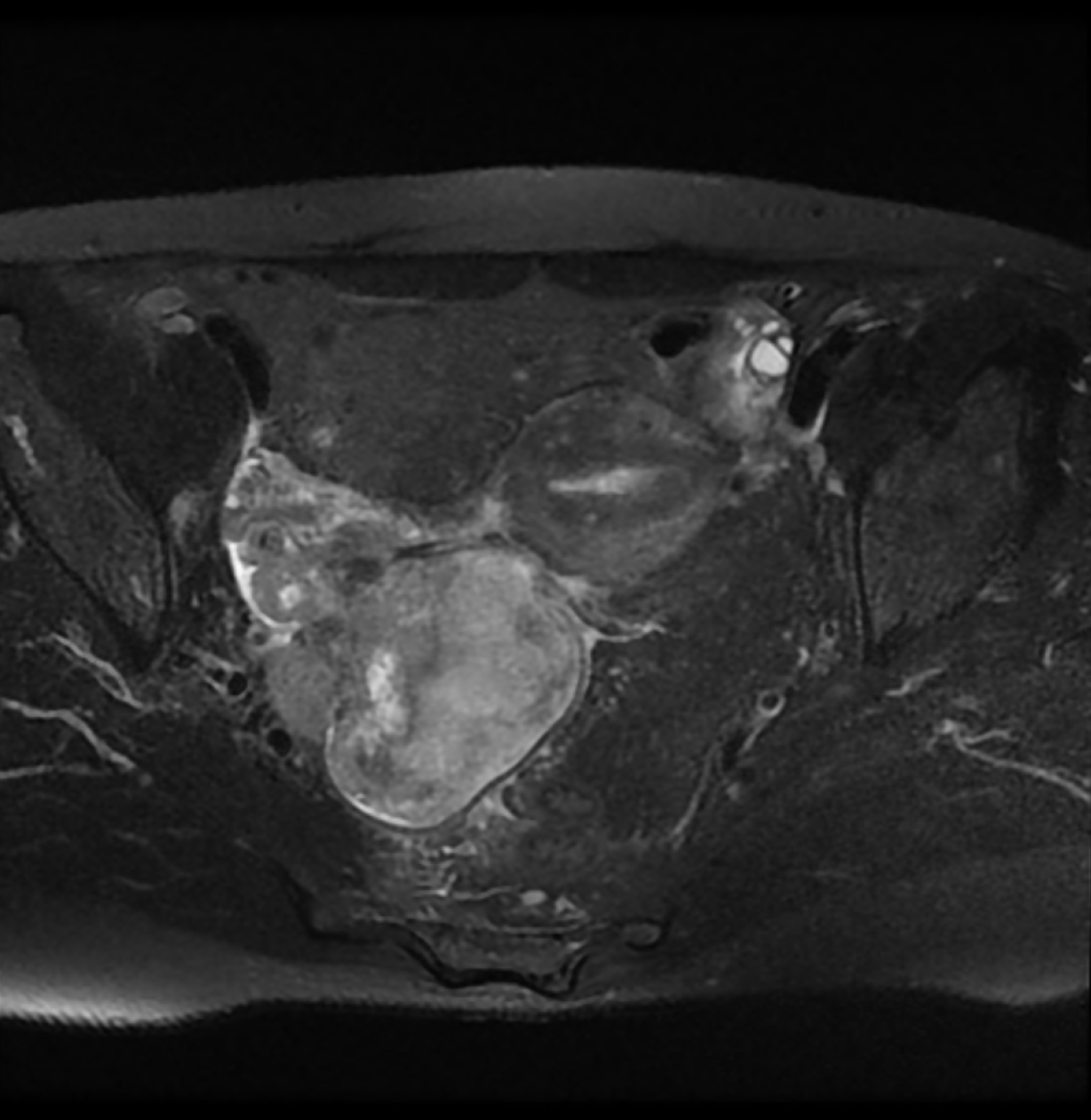
Magnetic resonance imaging revealed a large heterogeneous mass (8 cm × 6.5 cm × 7 cm) occupying the pelvic cavity (Figure 1). Based on this finding and location, the preoperative diagnosis was malignant ovarian tumor. A chest computed tomography (CT) scan showed no evidence of distant metastasis to the thorax.
The final diagnosis was malignant PEComa of the mesentery with rectal involvement.
The patient underwent total hysterectomy with bilateral salpingo-oophorectomy and pelvic lymph node dissection. A large mass was identified on the right side of the pelvic cavity, which was attached to the mesentery. The right ovary and fallopian tube were grossly normal. The rectum was also partially resected owing to suspicion of tumor involvement in the serosa.
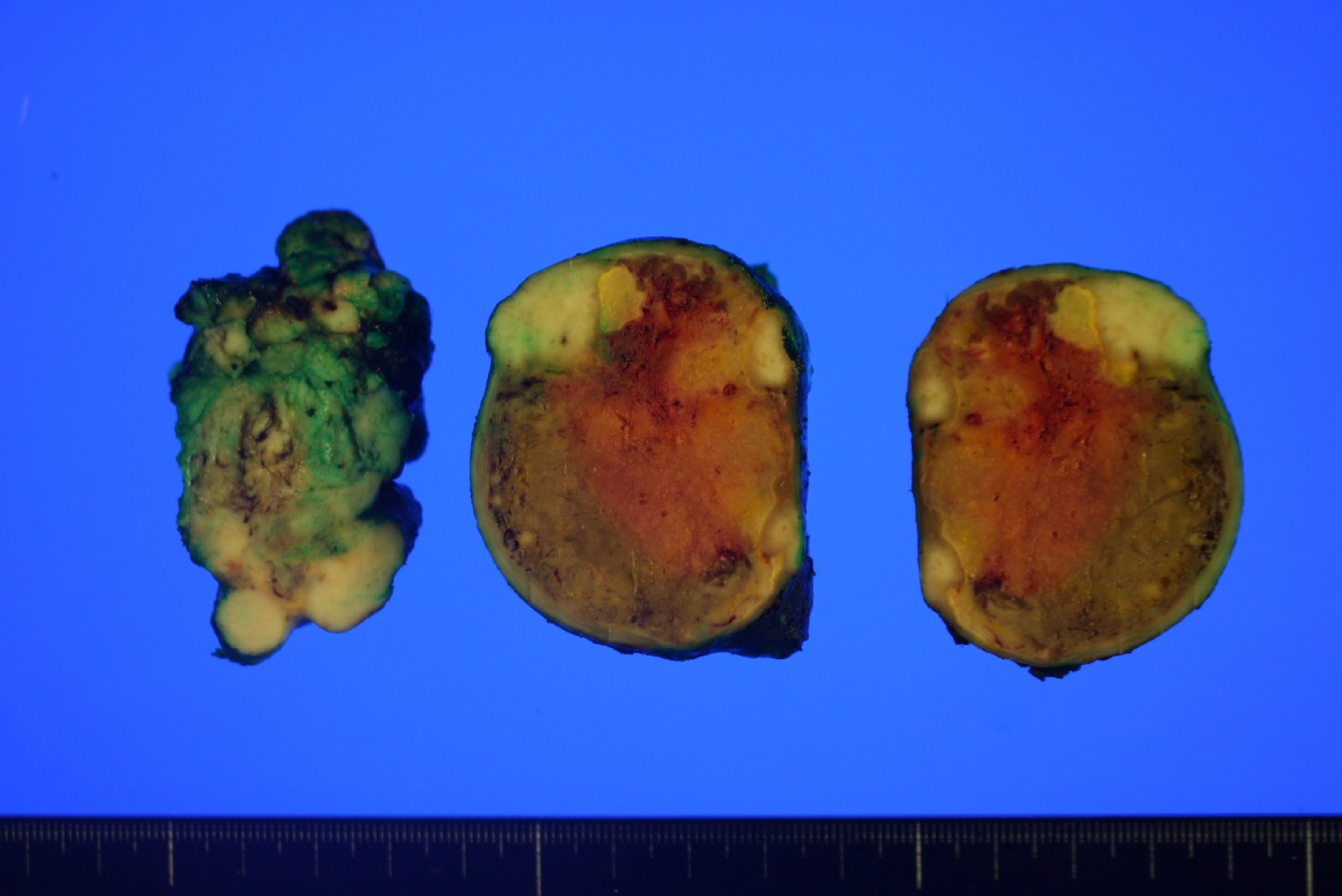
Upon macroscopic examination, the lesion appeared as a white-to-tan solid mass. A cross section of the tumor revealed that the mass was lobulated, did not have a fibrous capsule, and had a focal area of necrosis (Figure 2).
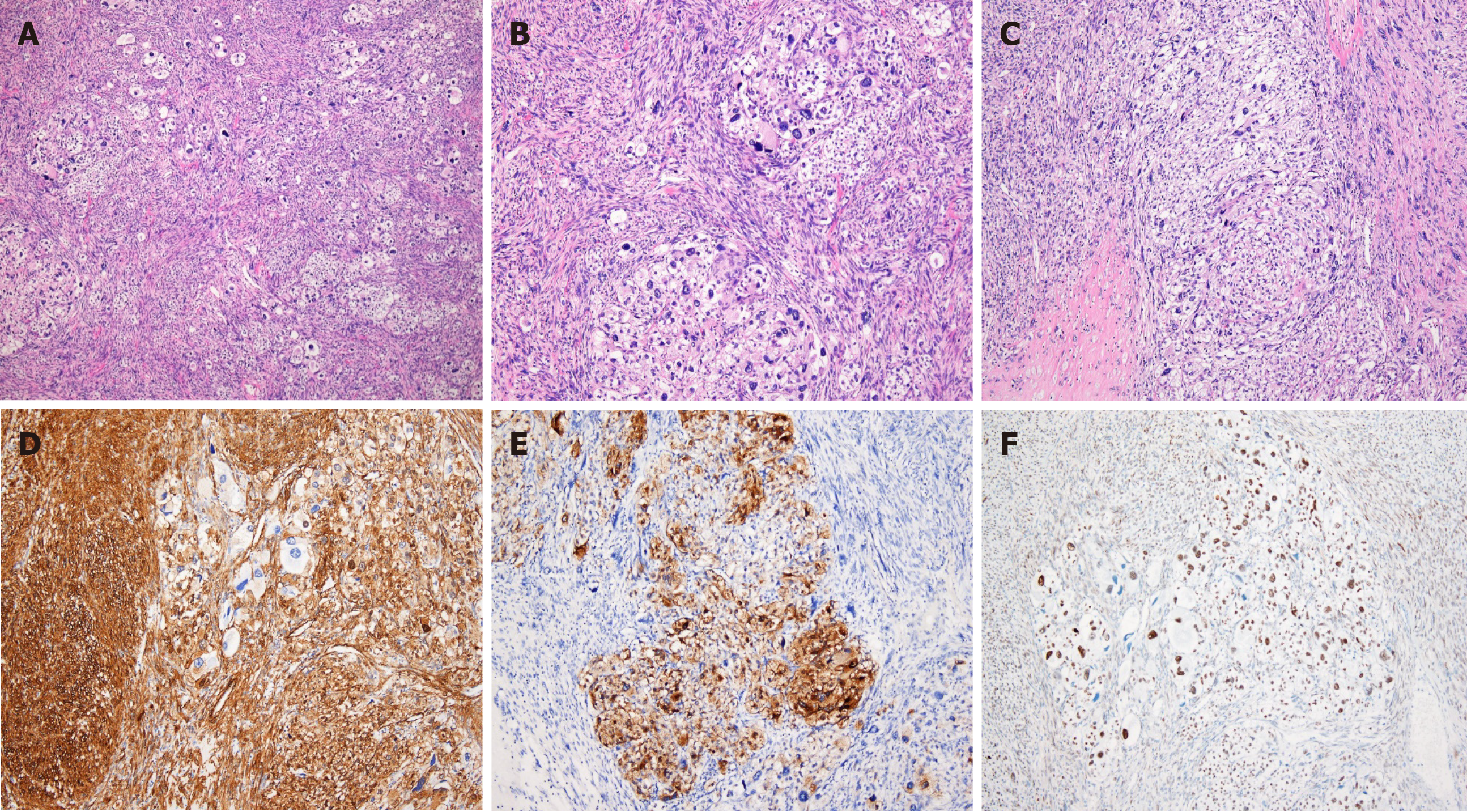
Histologically, the tumor appeared as a high-grade spindle cell sarcoma with involvement of the rectal serosa. No metastases were found in the regional lymph nodes. The tumor was composed of fascicles of spindle cells with an infiltrative growth pattern. The majority of the tumor cells exhibited marked atypia with dispersed chromatin and macronucleoli. Extensive coagulative necrosis was observed with substantial mitotic activity detected reaching up to 40 mitoses per 10 high-power fields. Epithelioid foci were also identified. Nests of large epithelioid cells were surrounded by a delicate vasculature in a radial manner. The tumor cells displayed large, vesicular nuclei with prominent nucleoli and an abundant eosinophilic granular cytoplasm with striking pleomorphism (Figure 3).
Immunohistochemistry revealed that the tumor expressed HMB45, smooth muscle actin, and TFE3, but was negative for Melan A, Desmin, CD117, CD34, CD68, Myogenin, S100, MDM2, and CDK4 (Figure 3). Thus, histopathological and immunohistochemical examinations confirmed the diagnosis of malignant PEComa.
Subsequently, whole-body positron emission tomography/CT scan were performed to check for possible metastases; however, no abnormality was found. Following surgery, the patient was treated with sirolimus chemotherapy.
No recurrence or metastasis was observed during a 6-month follow-up period.
PEComa is a rare mesenchymal tumor composed of histologically and immunohistochemically distinct perivascular epithelioid cells. The tumor cells exhibit an epithelioid and spindle morphology and express melanocytic and myogenic markers[11]. Although the histogenesis and the normal counterpart of PEComa remain unknown, the term “PEComa” is widely accepted to include angiomyolipoma of the kidney, clear-cell “sugar” tumor or lymphangioleiomyomatosis of the lung, and other tumors originating from various anatomical sites[13,14].
PEComas have been described in various organs; however, the majority are gynecological and gastrointestinal in origin[5,15-17]. Mesenteric PEComas are extremely rare, with only nine cases reported to date. A literature review revealed that mesenteric PEComas affect women more often than men (male:female = 20:80), regardless of age[5-10]. Seven out of ten reported cases, including the present case, were considered to be malignant PEComa exhibiting worrisome features, with only one case showing lymph node involvement. The majority of cases diagnosed as malignant PEComa were treated by surgical resection followed by adjuvant chemotherapy; however, the tumors recurred within 6-22 months in two cases[5-10] (Table 1).
| No. | Ref. | Age/sex | Diagnosis | Size (cm) | Nuclear grade | Cellularity | MF / 50 HPF | Invasion | Necrosis | LN status | Morphology | Treatment | Outcome |
| 1 | Folpe et al[5] | 67/F | PEComa with UMP | 13 | High | Moderate | 0 | No | No | Uninvolved | Epithelioid | SE only | NED at 84 months |
| 2 | 97/F | PEComa | 4 | Intermediate | Moderate | 0 | No | No | Uninvolved | Epithelioid | SE only | NED at 38 months | |
| 3 | 80/F | Malignant PEComa | 9.5 | High | High | > 50 | Vascular invasion | Yes | Uninvolved | Epithelioid and spindle | SE only | NED at 19 months | |
| 4 | 46/F | Malignant PEComa | 12 | Intermediate | Moderate | 5 | Vascular invasion | Yes | Uninvolved | Epithelioid | SE+CT | Recur and liver metastases at 22 months; die at 27 months | |
| 5 | Gross et al[6] | 5.5/M | Malignant PEComa | 5 | High | Moderate | NA | Surrounding tissue invasion | No | Uninvolved | Spindle | SE only | NED at 24 months |
| 6 | Lai et al[7] | 59/M | Malignant PEComa | 11 | High | High | 3 | Vascular invasion | Yes | Uninvolved | Epithelioid and spindle | SE+CT | Recur at 6 months; alive |
| 7 | Fu et al[8] | 38/F | Malignant PEComa | 10 | Intermediate | Moderate | 2 | Surrounding tissue invasion | Yes | Involved | SE+CT | NED at 6 months | |
| 8 | Shi et al[9] | 48/F | Malignant PEComa | 14 | High | High | Elevated | No | Yes | Uninvolved | Epithelioid | SE+CT | NED at 60 months |
| 9 | Wejman et al[10] | 67/F | PEComa | 3.5 | Mild | Moderate | 1-2 | No | No | Uninvolved | Epithelioid and spindle | SE+CT | NA |
| 10 | Present case | 50/F | Malignant PEComa | 8 | High | High | 40 | Surrounding tissue invasion | Yes | Uninvolved | Epithelioid and spindle | SE+CT | NED at 6 months |
Microscopically, these tumors consist of bundles of spindle cells and sheets of epithelioid cells located around the blood vessels, and the tumor cells show striking pleomorphism with elevated mitotic activity. Such tumors with unusual locations and histopathological features may pose a diagnostic challenge, and differential diagnoses can include leiomyosarcoma, clear cell sarcoma of the soft tissue, alveolar soft part sarcoma (ASPS), malignant melanoma, gastrointestinal stromal tumor, and dedifferentiated liposarcoma.
Leiomyosarcomas should be considered according to the location and histological features of the tumor. PEComas and leiomyosarcomas are morphologically similar as they are both composed of spindle and/or epithelioid cells displaying varying degrees of atypia with positive reactivity for smooth muscle markers. However, leiomyosarcomas are consistently negative for melanocytic markers[18,19].
Clear cell sarcoma of the soft tissue and malignant melanoma may also exhibit similar histological features to PEComa, along with positive immunohistochemical staining of melanocytic markers[20]. However, clear cell sarcomas of the soft tissue display cellular nests separated by a fibrocollagenous network and strong S100 expression, whereas PEComas typically show a delicate vascular rich stroma and do not express S100. ASPS is a rare malignant soft tissue neoplasm with a strong predilection to develop in young adults and adolescents. The tumor shares both histological and immunohistochemical features with PEComa. ASPS is composed of large epithelioid cells with abundant cytoplasm, arranged in nests or sheets that are separated by a delicate vascular network of capillaries[21]. However, ASPS can be differentiated from PEComa by immunohistochemical staining based on negative staining for melanocytic markers, which aids in differentiating from PEComa[20]. The differential diagnosis of PEComa from gastrointestinal stromal tumors and dedifferentiated liposarcomas is based on the absence of CD117, DOG-1, MDM2, and CDK4 expression.
The tumor in the present case showed positive immunoreactivity for melanocytic and myogenic markers and was negative for S100, Desmin, CD34, CD117, MDM2, and PAX-8. Further investigation of TFE3 immunoreactivity and its nuclear expression led to the ultimate diagnosis of malignant PEComa of the mesentery.
TFE3 is a member of the microphthalmia-associated transcription factor family, and its expression is upregulated in ASPS and translocation-associated renal cell carcinomas[22]. Since Folpe et al[5] first described TFE3 expression in PEComa, molecular analyses have identified a subgroup of PEComas with TFE3 gene rearrangement, suggesting that PEComas harboring TFE3 fusions may represent a distinct disease[12,23-28].
The majority of PEComas are composed of epithelioid and spindle cells, exhibiting positive myogenic expression and the presence of spindle cell components. In addition, PEComas are typically associated with tuberous sclerosis, whereas the subsect of PEComas with TFE3 translocation have different histological features, exhibiting a predominantly epithelioid nested or alveolar architecture without spindle cell components, and immunohistochemistry reveals a lack of myogenic markers[29,30]. Compared with the conventional type, TFE3-translocated PEComas typically have been reported in younger patients without a history of tuberous sclerosis complex[12,31-33]. However, Williamson et al[32] reported a TFE3-rearranged PEComa showing both an epithelioid and spindle morphology with expression of smooth muscle actin, which are consistent with the findings of the present case.
PEComa is a neoplasm of uncertain malignant potential. Although the majority of PEComas show a benign course, some are potentially malignant. However, there are no established criteria or markers to predict the clinical behavior of the tumor. Folpe et al[5], as well as Bleeker et al[34], proposed criteria for the classification of PEComas with worrisome features, including a tumor size > 5 cm, infiltrative growth pattern, high nuclear grade, cellularity, necrosis, vascular invasion, and a mitotic rate > 1/50 high-power fields. The tumor in the present study was 7 cm in size, had extensive coagulative necrosis, and showed a mitotic rate of 40/10 high-power fields. Therefore, the tumor met the aforementioned criteria for high risk of malignancy. Furthermore, the tumor involved the rectal serosa but no lymph node metastasis was observed. Therefore, a diagnosis of malignant PEComa was made based on the histopathological and immunohistochemical features.
Since PEComa is a very rare neoplasm, there are currently no effective therapies or management strategies. Surgical resection is the most common approach for curative treatment, and adjuvant chemotherapy is recommended for patients with tumors exhibiting malignant features. This unusual case of malignant PEComa of the mesentery further expands this field to help inform the diagnosis and treatment of this rare entity.
PEComas are rare mesenchymal neoplasms and should be thoroughly distinguished from their morphological mimics. Analysis of histopathological features and immunohistochemistry are essential for a differential diagnosis. The present case further emphasizes the importance of differential diagnosis based on the correct use of immunohistochemistry along with close surveillance of this unique tumor entity. Further studies are required to gain insight into the pathogenesis of PEComa and its clinical behavior.
We are grateful to the patient for allowing us to use her medical records in our case report.
| 1. | Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992;16:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 317] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Bonetti F, Pea M, Martignoni G, Doglioni C, Zamboni G, Capelli P, Rimondi P, Andrion A. Clear cell ("sugar") tumor of the lung is a lesion strictly related to angiomyolipoma--the concept of a family of lesions characterized by the presence of the perivascular epithelioid cells (PEC). Pathology. 1994;26:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 208] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, Pederzoli P, Bonetti F. Clear cell "sugar" tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996;20:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 277] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of Soft Tissue and Bone. 3rd ed. Lyon: IARC Press, 2002: 221-222. |
| 5. | Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 663] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 6. | Gross E, Vernea F, Weintraub M, Koplewitz BZ. Perivascular epithelioid cell tumor of the ascending colon mesentery in a child: case report and review of the literature. J Pediatr Surg. 2010;45:830-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Lai CL, Hsu KF, Yu JC, Chen CJ, Hsieh CB, Chan DC, Li HS, Hsu HM. Malignant perivascular epithelioid cell tumor of the mesentery: a case report and literature review. Onkologie. 2012;35:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Fu X, Jiang JH, Gu X, Li Z. Malignant perivascular epithelioid cell tumor of mesentery with lymph node involvement: a case report and review of literature. Diagn Pathol. 2013;8:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Shi Y, Geng J, Xie H, Wang B. Malignant perivascular epithelioid cell tumor arising in the mesentery: A case report. Oncol Lett. 2015;9:2189-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Wejman J, Nowak K, Gielniewska L, Komorowska M, Dąbrowski W. PEComa of the mesentery coexisting with colon cancer: a case report. Diagn Pathol. 2015;10:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015;19:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Argani P, Aulmann S, Illei PB, Netto GJ, Ro J, Cho HY, Dogan S, Ladanyi M, Martignoni G, Goldblum JR, Weiss SW. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010;34:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 343] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 13. | Tazelaar HD, Batts KP, Srigley JR. Primary extrapulmonary sugar tumor (PEST): a report of four cases. Mod Pathol. 2001;14:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 324] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 15. | Doyle LA, Hornick JL, Fletcher CD. PEComa of the gastrointestinal tract: clinicopathologic study of 35 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2013;37:1769-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Xu J, Yan Y, Xiang X, Jiang P, Hu X, Yang W. Gastric Perivascular Epithelioid Cell Tumor (PEComa). Am J Clin Pathol. 2019;152:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Shi HY, Wei LX, Sun L, Guo AT. Clinicopathologic analysis of 4 perivascular epithelioid cell tumors (PEComas) of the gastrointestinal tract. Int J Surg Pathol. 2010;18:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Miettinen M. Smooth muscle tumors of soft tissue and non-uterine viscera: biology and prognosis. Mod Pathol. 2014;27 Suppl 1:S17-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | George S, Serrano C, Hensley ML, Ray-Coquard I. Soft Tissue and Uterine Leiomyosarcoma. J Clin Oncol. 2018;36:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 20. | James AW, Dry SM. Diagnostically Challenging Epithelioid Soft Tissue Tumors. Surg Pathol Clin. 2015;8:309-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Jaber OI, Kirby PA. Alveolar Soft Part Sarcoma. Arch Pathol Lab Med. 2015;139:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Kenerson H, Folpe AL, Takayama TK, Yeung RS. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol. 2007;38:1361-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Kuiper RP, Schepens M, Thijssen J, Schoenmakers EF, van Kessel AG. Regulation of the MiTF/TFE bHLH-LZ transcription factors through restricted spatial expression and alternative splicing of functional domains. Nucleic Acids Res. 2004;32:2315-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Agaram NP, Sung YS, Zhang L, Chen CL, Chen HW, Singer S, Dickson MA, Berger MF, Antonescu CR. Dichotomy of Genetic Abnormalities in PEComas With Therapeutic Implications. Am J Surg Pathol. 2015;39:813-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 25. | Schoolmeester JK, Dao LN, Sukov WR, Wang L, Park KJ, Murali R, Hameed MR, Soslow RA. TFE3 translocation-associated perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: morphology, immunophenotype, differential diagnosis. Am J Surg Pathol. 2015;39:394-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Shen Q, Rao Q, Xia QY, Yu B, Shi QL, Zhang RS, Zhou XJ. Perivascular epithelioid cell tumor (PEComa) with TFE3 gene rearrangement: clinicopathological, immunohistochemical, and molecular features. Virchows Arch. 2014;465:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Cho HY, Chung DH, Khurana H, Zhai QJ, Ro JY. The role of TFE3 in PEComa. Histopathology. 2008;53:236-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Malinowska I, Kwiatkowski DJ, Weiss S, Martignoni G, Netto G, Argani P. Perivascular epithelioid cell tumors (PEComas) harboring TFE3 gene rearrangements lack the TSC2 alterations characteristic of conventional PEComas: further evidence for a biological distinction. Am J Surg Pathol. 2012;36:783-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Dickson BC, Brooks JS, Pasha TL, Zhang PJ. TFE3 expression in tumors of the microphthalmia-associated transcription factor (MiTF) family. Int J Surg Pathol. 2011;19:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Righi A, Dimosthenous K, Rosai J. PEComa: another member of the MiT tumor family? Int J Surg Pathol. 2008;16:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Wang XT, Xia QY, Ni H, Wang ZY, Ye SB, Li R, Wang X, Lv JH, Shi SS, Ma HH, Lu ZF, Shen Q, Zhou XJ, Rao Q. Xp11 neoplasm with melanocytic differentiation of the prostate harbouring the novel NONO-TFE3 gene fusion: report of a unique case expanding the gene fusion spectrum. Histopathology. 2016;69:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Williamson SR, Bunde PJ, Montironi R, Lopez-Beltran A, Zhang S, Wang M, Maclennan GT, Cheng L. Malignant perivascular epithelioid cell neoplasm (PEComa) of the urinary bladder with TFE3 gene rearrangement: clinicopathologic, immunohistochemical, and molecular features. Am J Surg Pathol. 2013;37:1619-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Chen XF, Yeong J, Chang KTE, Lim AST, Kuick CH, Lim TH, Sudhanshi J, Selvarajan S, Gan VHL, Khor LY. TFE3-Expressing Epithelioid Rich Perivascular Epithelioid Cell Neoplasm (PEComa) of the Bladder with Unusual Benign Course. Ann Clin Lab Sci. 2018;48:110-115. [PubMed] |
| 34. | Bleeker JS, Quevedo JF, Folpe AL. "Malignant" perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Sarcoma. 2012;2012:541626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yu B S-Editor: Yan JP L-Editor: A P-Editor: Li JH