Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3841
Peer-review started: May 12, 2020
First decision: May 21, 2020
Revised: June 2, 2020
Accepted: July 30, 2020
Article in press: July 30, 2020
Published online: September 6, 2020
Processing time: 115 Days and 6.4 Hours
Epidermal growth factor receptor (EGFR) tyrosine-kinase inhibitors are widely used for the treatment of non-small-cell lung cancer with EGFR mutations. However, patients with rare, even compound EGFR mutations have different responses to EGFR-tyrosine-kinase inhibitors, which bring uncertainty to clinical treatment.
A 45-year-old female patient presented with a 3-mo history of cough and white sputum without chest pain. Chest computed tomography revealed lung space-occupying lesions and multiple lymphadenectasis. Bronchoscopy and pathology suggested lung adenocarcinoma. Compound variation of EGFR gene (exon 21 L858R/V834L) was detected in both tissue and circulating tumor deoxyribonucleic acid samples. As a result of next-generation sequencing and her family’s wishes, the patient was given oral treatment with icotinib hydrochloride (125 mg/d, tid) from March 21, 2019 and has achieved stable disease for the last 1 year.
Non-small cell lung adenocarcinoma with EGFR L858R/V834L was treated successfully with icotinib, and it may be a new medication treatment option.
Core tip: In non-small cell lung cancer, epidermal growth factor receptor (EGFR) L858R/V834L compound mutation is rare and targeted treatment of this mutation with icotinib has not been reported. In this report, the patient failed to achieve complete or partial response but achieved stable disease. The patient has been in remission for 1 year with no evidence of metastatic nodal disease, and there are signs of continuous remission. So far, the effect of this compound mutation on the efficacy of EGFR tyrosine kinase inhibitors is not clear. It is also unclear which EGFR tyrosine kinase inhibitor is most effective for this variation. This case suggests icotinib may be a possible clinical treatment.
- Citation: Zhai SS, Yu H, Gu TT, Li YX, Lei Y, Zhang HY, Zhen TH, Gao YG. Lung adenocarcinoma harboring rare epidermal growth factor receptor L858R and V834L mutations treated with icotinib: A case report. World J Clin Cases 2020; 8(17): 3841-3846
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3841.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3841
Non-small cell lung cancer (NSCLC) is one of the leading causes of cancer mortality. Epidermal growth factor receptor (EGFR) gene mutations in lung adenocarcinoma account for about 48% of patients. EGFR gene exon 19 single nucleotide polymorphism and deletion variation is a common type of mutation[1]. However, non-EGFR Del19/L858R rare mutations account for 23% of cases, mainly composed of exon 20ins, G719X, de novo T790M and L861Q combined with the classic mutation of the compound mutation. For the rare compound mutations of EGFR, there have been some drug studies. For example, first-generation EGFR tyrosine kinase inhibitors (TKIs) are effective for G719X, 21L861Q and the compound mutations carrying sensitive mutations, but more data are needed for efficacy against 20S768I[2,3]. The second-generation EGFR-TKIs have a wider spectrum. They are effective for G719X, S768I, L861Q and complex mutations, and studies have shown a significant increase in progression-free survival compared to first generation EGFR-TKIs[4]. The third-generation EGFR-TKIs have shown some therapeutic effect in small phase II studies, but this needs to be verified[5].
The molecularly targeted drug for lung cancer, icotinib hydrochloride (Kemena), is a small anticancer drug with completely independent intellectual property rights in China. The drug was approved by the China Food and Drug Administration on June 7, 2011 for the first-line treatment of patients with locally advanced or metastatic NSCLC with sensitive mutations in the EGFR gene. According to the phase III clinical trial and BRAIN studies, compared with the control group, progression-free survival and objective response rates were significantly prolonged in patients with EGFR mutation treated with icotinib, adverse reactions were significantly reduced and the safety was acceptable[6,7]. Here, we report a case of NSCLC with rare EGFR L858R/V834L compound mutation who was treated with icotinib. The treatment was effective, and there was no sign of resistance to icotinib.
A 45-year-old woman presented to the outpatient department of our hospital complaining of hoarseness and a cough with sputum.
Since December 2018, the patient had had cough and sputum without obvious inducement, a small amount of white phlegm, no fever, no chest tightness, no shortness of breath and no headache.
Her past medical history was unremarkable.
No specific personal history of disease was recorded.
Her mental state was good. She had a hoarse voice. Superficial lymph nodes were not affected, heart and lung auscultation were normal, liver and spleen were not enlarged and neither lower limbs had edema.
Routine blood examination, liver and kidney function and electrolyte test indicators were, normal and carcinoembryonic antigen was 17.6 ng/mL (reference value < 5 ng/mL). Blood gases were normal. Laryngoscopy suggested vocal cord paralysis.
Lungs were examined by computed tomography (CT) (mediastinal window) on March 12, 2019. A soft tissue mass shadow was found in the left lower hilum of the lung; the boundary of the shadow was not clear, and it measured about 27.5mm × 26.8 mm; and the three-stage CT value was about 56/68/81 Hu. Multiple swollen lymph nodes were found in the left supraclavicular area and mediastinum, and the largest was about 11.6 mm × 27.2 mm and was located beside the aortic arch. A small amount of liquid density shadow was seen in the left thoracic cavity. CT examination (pulmonary window) revealed a mass shadow in the left lower lung dorsal segment, with coarse margin, pleural traction and dorsal bronchial occlusion. Multiple small nodules < 10 mm were found in both lungs, with clear boundaries; ground-glass nodules, 17.2 mm × 18.2 mm, were found in the right upper lung, some of which were high density. Magnetic resonance imaging of the head was normal, and abdominal CT was normal.
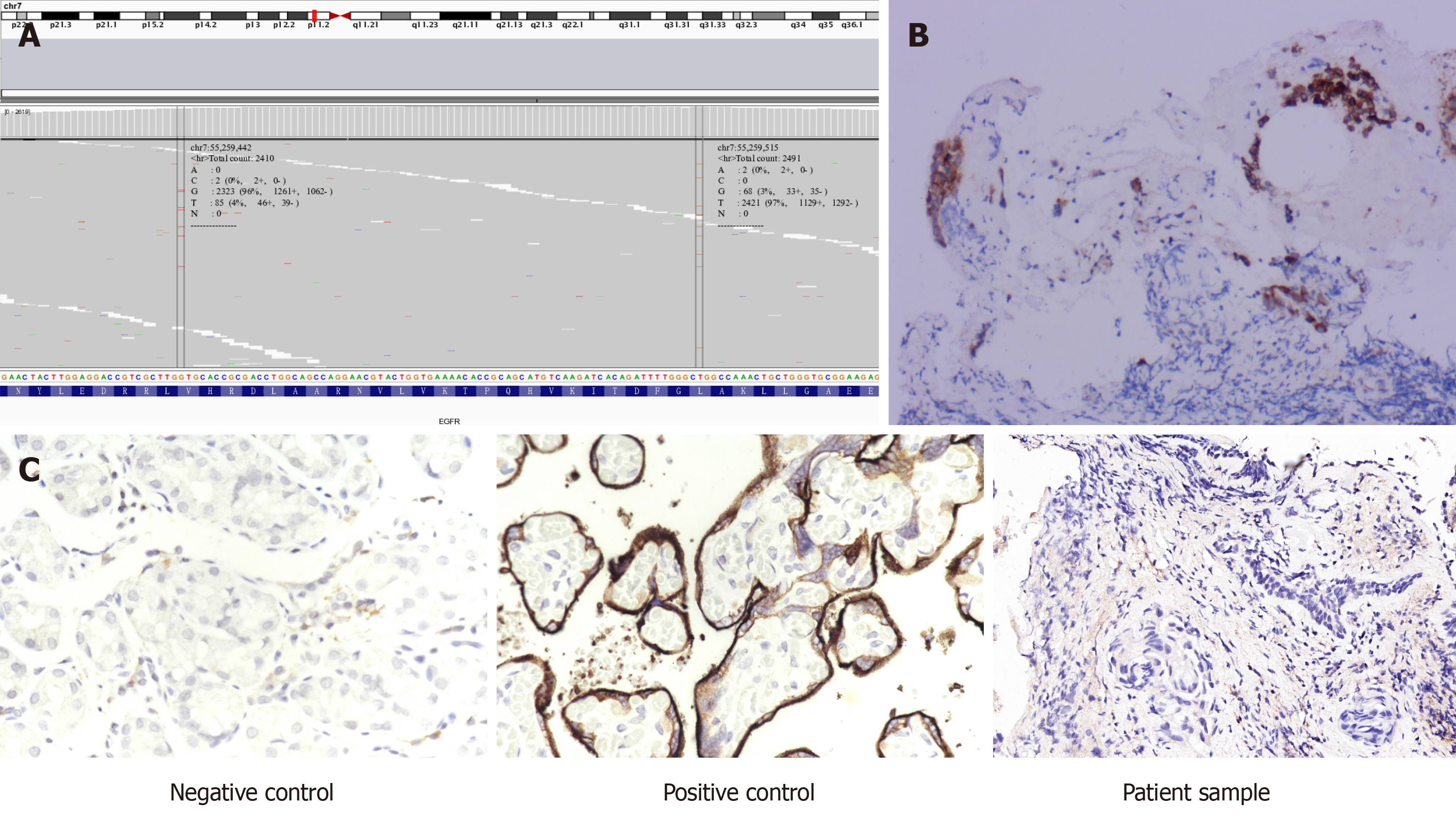
To determine potential therapeutic methods, with the patient’s consent, tissue samples from lung bronchoscopy and whole blood as a control were subjected to next-generation sequencing using by a 757-gene panel (Yucebio, Shenzhen, China). Compound variation of EGFR gene (exon 21 L858R/V834L) was detected in both tissue and circulating tumor deoxyribonucleic acid samples (Figure 1A). Immunohistochemistry showed that EGFR expression in tumor tissue was positive, and programmed death-ligand 1 expression was < 1% (total cholesterol < 1%) (Figures 1B and C).
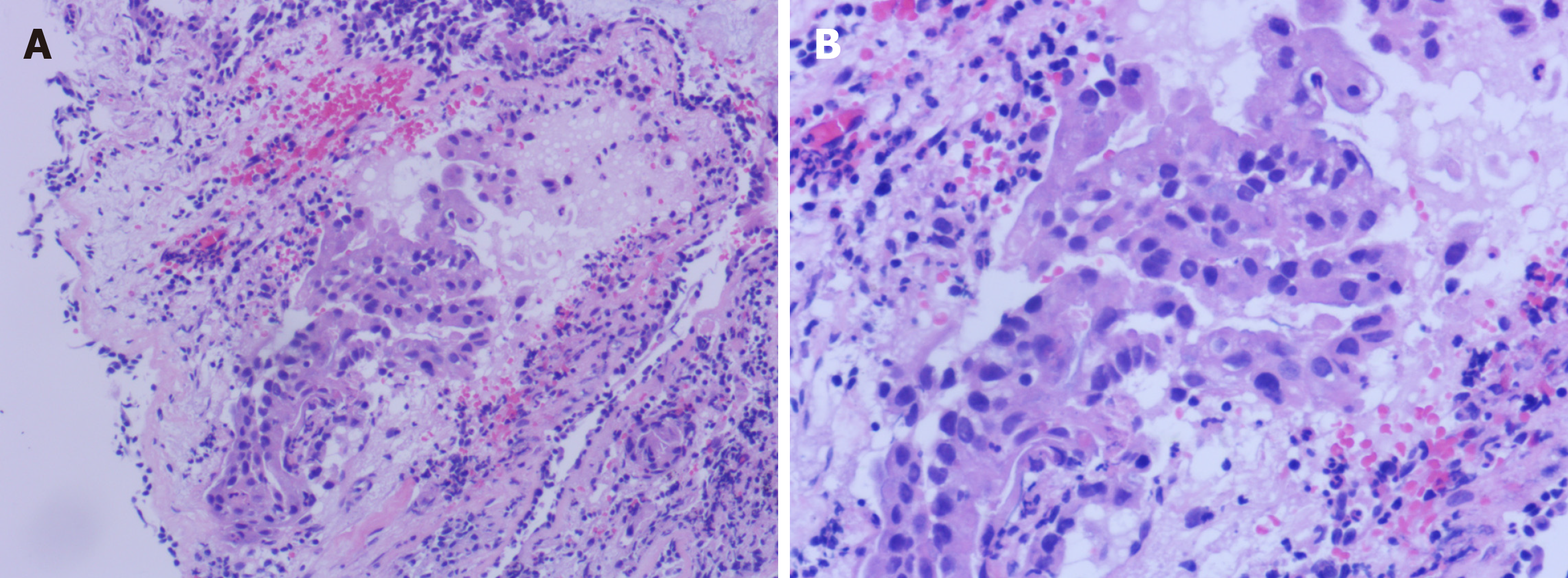
CT examination showed lung space occupying lesions and multiple lymphadenectasis. Bronchoscopy and pathology were performed in the outpatient department. The results were: CT4N3M1, stage IV lung adenocarcinoma (according to American Joint Cancer Committee, 8th edition), with both lungs, mediastinal and left periclavicular lymph nodes metastasis (Figure 2).
As a result of the tests and her family’s wishes, the patient was treated with oral icotinib hydrochloride (125 mg/d, tid) from March 21, 2019.
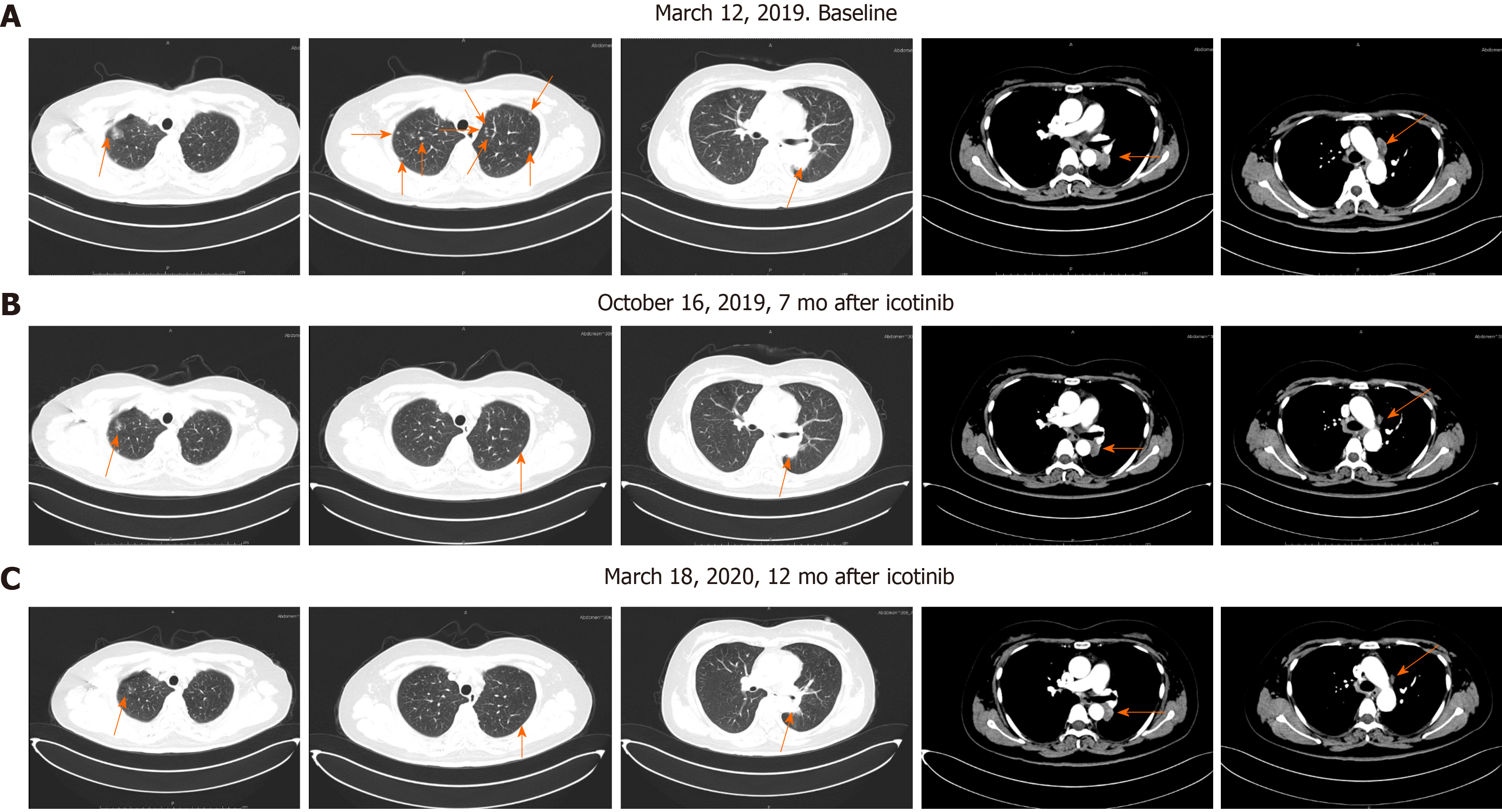
In the course of treatment, the patient’s condition gradually improved, hoarseness lessened, no adverse drug reaction occurred and carcinoembryonic antigen gradually decreased by 5.6 ng/mL. Lung CT examination showed that multiple small pulmonary nodules gradually reduced and disappeared, mediastinal lymph node metastasis decreased and periclavicular lymph node metastasis decreased and disappeared (Figure 3). No abnormality was found by head magnetic resonance imaging examination. At present, we continue to treat her with icotinib hydrochloride with regular follow-up in the outpatient clinic.
The somatic variant of EGFR V834L is a rare mutation. Only three cases have previously been found in lung adenocarcinoma, but little is known about its clinical significance. None of the cases reported targeted therapy of EGFR L858R/V834L complex mutation. In 2018, a study assessed the efficacy of first-generation of EGFR-TKI icotinib in patients with NSCLC carrying rare EGFR mutations and found that icotinib had clinical benefit for patients with rare, especially complex mutations[8]. In two previous reports containing cases of EGFR L858R/V834L, gefitinib and erlotinib were used for treatment of patients, and there was no targeted drug treatment for EGFR L858R/V834L patients[9,10]. Coincidentally, the cases with EGFR V834L mutation also had EGFR L858R mutation. In addition, in 2018, Li et al[11] found that a patient with NSCLC who progressed to multiline therapy carried an EGFR L858R/V834L complex mutation when the disease progressed slowly from 2012 to 2016. After 2016, the relapse time was shortened, and the driver mutation changed to EGFR 19 del. The authors speculated that this complex mutation might be related to a relatively inactive disease state[11].
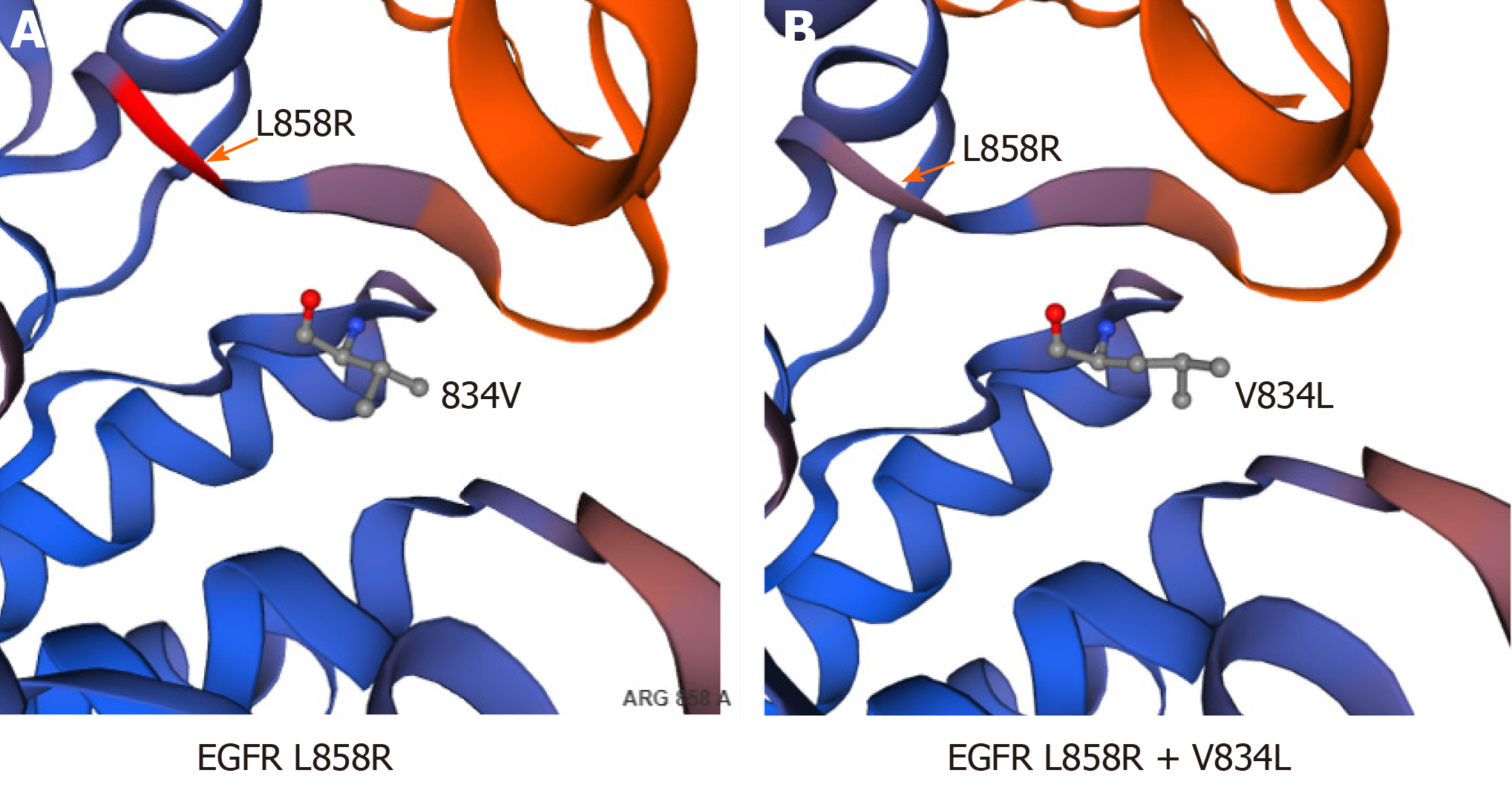
In our case, the patient carried a somatic compound mutation of EGFR V834L/L858R, and there are no specific reports of drug treatment of such patients at present. After treatment with icotinib, multiple nodular lesions continued to be alleviated, and the clinical efficacy was evaluated by achievement of stable disease, suggesting that icotinib has some therapeutic effect on NSCLC with EGFR V834L/L858R compound mutation. The wild-type amino acid in EGFR position 834, valine, and mutation-type amino acid leucine are both hydrophobic, and leucine only has an additional C-H group compared to valine, which may have little effect on the protein structure (Figure 4A and B). According to the structure of EGFR protein and considering that positions 834 and 858 are close in the spatial structure of the protein (Figure 4), they may have some effect on the drug binding of some EGFR-TKIs[12]. Therefore, it is necessary to carry out targeted drug therapy for NSCLC patients with this rare complex mutation.
In this case of NSCLC with EGFR V834L/L858R complex mutation, icotinib achieved good clinical efficacy. However, this treatment needs to be validated in a larger population.
| 1. | Gou LY, Wu YL. Prevalence of driver mutations in non-small-cell lung cancers in the People's Republic of China. Lung Cancer (Auckl). 2014;5:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Zhang B, Wang S, Qian J, Yang W, Qian F, Lu J, Zhang Y, Qiao R, Han B. Complex epidermal growth factor receptor mutations and their responses to tyrosine kinase inhibitors in previously untreated advanced lung adenocarcinomas. Cancer. 2018;124:2399-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, Yamamoto N, Yu CJ, Ou SH, Zhou C, Massey D, Zazulina V, Wu YL. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16:830-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 792] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 4. | Shen YC, Tseng GC, Tu CY, Chen WC, Liao WC, Chen WC, Li CH, Chen HJ, Hsia TC. Comparing the effects of afatinib with gefitinib or Erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer. 2017;110:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Nick T, Michael T, Pan ZY, Jerome G, Michael S, Rebeca M andVladimir H. Clinical comparison of ABP 215 and bevacizumab in patients with NSCLC: Pharmacokinetic results and justification for extrapolation across bevacizumab indications. Journal of Clinical Oncology. 2017;35:9050. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Shi Y, Zhang L, Liu X, Zhou C, Zhang L, Zhang S, Wang D, Li Q, Qin S, Hu C, Zhang Y, Chen J, Cheng Y, Feng J, Zhang H, Song Y, Wu YL, Xu N, Zhou J, Luo R, Bai C, Jin Y, Liu W, Wei Z, Tan F, Wang Y, Ding L, Dai H, Jiao S, Wang J, Liang L, Zhang W, Sun Y. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 7. | Yang JJ, Zhou C, Huang Y, Feng J, Lu S, Song Y, Huang C, Wu G, Zhang L, Cheng Y, Hu C, Chen G, Zhang L, Liu X, Yan HH, Tan FL, Zhong W, Wu YL. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017;5:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Zhou SY, Hu XS, Wang Y, Li JL, Zhou LQ, Hao XZ, Liu YT and Shi YK. Effectiveness of icotinib against non-small-cell lung cancer with uncommon EGFR mutation. Journal of Clinical Oncology. 2018;e21083-e21083. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Wu SG, Chang YL, Hsu YC, Wu JY, Yang CH, Yu CJ, Tsai MF, Shih JY, Yang PC. Good response to gefitinib in lung adenocarcinoma of complex epidermal growth factor receptor (EGFR) mutations with the classical mutation pattern. Oncologist. 2008;13:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Lgnatius SH, Ali SM, Bogart J, Stephen L. Graziano, Michael DM, Jeffrey SR, Vincent AM and Alexa BS. Characterization of 1,233 NSCLCs with non-del19/L858R EGFR mutations (EGFRm) using comprehensive genomic profiling (CGP). Journal of Clinical Oncology. 2018;36:9040. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Li J, Zhang L, Wu Y, Yang W, Yang Z, Zhang H. Clinical Outcome and Molecular Analysis of a Chinese Patient With Lung Adenocarcinoma Harboring Rare EGFR Mutation V834L. J Thorac Oncol. 2018;13:e189-e191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Yasuda H, Park E, Yun CH, Sng NJ, Lucena-Araujo AR, Yeo WL, Huberman MS, Cohen DW, Nakayama S, Ishioka K, Yamaguchi N, Hanna M, Oxnard GR, Lathan CS, Moran T, Sequist LV, Chaft JE, Riely GJ, Arcila ME, Soo RA, Meyerson M, Eck MJ, Kobayashi SS, Costa DB. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5:216ra177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 459] [Article Influence: 38.3] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang KQ S-Editor: Zhang L L-Editor: Filipodia P-Editor: Xing YX