Published online Aug 26, 2020. doi: 10.12998/wjcc.v8.i16.3450
Peer-review started: May 10, 2020
First decision: June 7, 2020
Revised: June 20, 2020
Accepted: July 14, 2020
Article in press: July 14, 2020
Published online: August 26, 2020
Processing time: 106 Days and 21.9 Hours
Peripheral lung cancer poses a substantial harm to human health, and it is easy to become exacerbated, potentially threatening the life and safety of patients
To assess the value of virtual bronchoscopic navigation (VBN) combined with transbronchial ultrasound-guided sheath-guided (EBUS-GS) exploration in the diagnosis of peripheral lung cancer.
A total of 236 patients with peripheral lung cancer (nodule diameter range, 8-30 mm; diagnosed using high-resolution computed tomography) were selected from three centers between October 2018 and December 2019. Patients who underwent EBUS-GS exploration alone were included in a control group, and those who received VBN in combination with EBUS-GS exploration were included in an observation group. The diagnostic rate and total operating time of different subgroups of the two groups were compared, and the time needed to determine the lesion was recorded.
There were no significant differences in diagnosis rate or total operation time between the two groups (P > 0.05), and the time needed to determine the lesion in the observation group was less than that of the control group (P < 0.05).
The combined use of VBN and EBUS-GS exploration technology has little effect on the diagnosis rate and total operation time of peripheral lung cancer, but it significantly shortens the time needed to determine the lesion and is a valuable diagnostic method.
Core tip: Transbronchial ultrasound-guided sheath-guided exploration and virtual bronchoscopic navigation improve the rate of diagnosis of pulmonary peripheral lesions. We discuss the role of virtual bronchoscopy navigation in combination with the transbronchial ultrasound-guided sheath-guided exploration technique in the diagnosis of peripheral lung cancer.
- Citation: Liu Y, Wang F, Zhang QC, Tong ZH. Value of virtual bronchoscopic navigation and transbronchial ultrasound-guided sheath-guided exploration in diagnosis of peripheral lung cancer. World J Clin Cases 2020; 8(16): 3450-3457
- URL: https://www.wjgnet.com/2307-8960/full/v8/i16/3450.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i16.3450
As computed tomography (CT) diagnostic techniques continue to improve, the detection rate of lung cancer has increased significantly[1-3]. Early histological diagnosis plays an important role in lung cancer patients[4]. Peripheral lung cancer occurs from the tertiary bronchus down, and lung cancer refers to cancers above the respiratory bronchioles, often adenocarcinoma. Early peripheral lung cancer has no significant symptoms, and when the symptoms are obvious, the cancer is in a late stage. Globally, lung cancer has a high incidence and mortality rate[5,6]. If patients with peripheral lung cancer have advanced metastasis, it is necessary to have a clear molecular diagnosis based on different targets to initiate individualized treatment and promote a therapeutic effect. The main inducing factors include smoking, occupational and environmental contact, ionizing radiation, chronic lung infection, heredity, and air pollution. The main clinical manifestations are chest pain and fever, and X-ray examination, multi-slice spiral CT examination, and CT target scanning are generally performed.
The main diagnostic methods at this stage are traditional transbronchial biopsy (TBB) under X-ray guidance, virtual bronchoscopy navigation (VBN), transthoracic aspiration biopsy, and endobronchial ultrasound with a guide sheath (EBUS-GS)[7]. During the course of diagnosis, the marginal signs, morphological size, CT appearance of adjacent tissues, and internal characteristics of the lesions are used as the basis for diagnosis. A CT target scan is needed if the patient has an atypical appearance. If the diagnosis cannot be confirmed, CT-guided percutaneous biopsy, short-term reexamination, or bronchoscopic lung biopsy is performed to avoid a missed diagnosis or misdiagnosis of early peripheral lung cancer. After the diagnosis is clear, molecular diagnosis and staging diagnosis should be performed to provide guidance for clinical treatment. A higher diagnostic rate is obtained by transthoracic aspiration biopsy, but this method easily causes pneumothorax and other complications[8]. EBUS-GS and VBN improve the rate of diagnosis of pulmonary peripheral lesions[9-11]. The present study thus examined the role of VBN in combination with the EBUS-GS exploration technique in the diagnosis of peripheral lung cancer.
From October 2018 to December 2019, 236 patients with peripheral lung cancer (nodule diameter range, 8 mm-30 mm; diagnosed by high-resolution CT) were selected at Beijing Chaoyang Hospital of Capital Medical University, Henan Provincial People’s Hospital, and Zhoukou Central Hospital. Thirty-four patients who were subsequently diagnosed with benign lesions or lung metastases were excluded. This retrospective study included 202 patients who were diagnosed with primary lung cancer. Patients who underwent EBUS-GS exploration were included in a control group, and those who underwent VBN in combination with EBUS-GS exploration were included in an observation group. There were 101 patients in each group. The control group had 64 males and 37 females. The age ranged from 38-84 years, with a mean of 61.6 ± 3.2 years. There were 65 males and 36 females in the observation group. The age ranged from 39-83 years, with a mean of 62.3 ± 3.1 years.
The study was performed with the approval by the ethics committees of the hospitals involved. All of the patients signed an informed consent agreement under voluntary conditions. Patients with allergy to lidocaine or other local narcotic drugs, severe organic disease, or pregnancy were excluded. Comparison of the general data, including sex, age, and location and size of the lesions, between the two groups revealed no significant differences (P > 0.05, Table 1).
| Clinical data | Control group (n = 101) | Observation group (n = 101) | P value |
| Gender (n) | 0.884 | ||
| Male | 64 | 65 | |
| Female | 37 | 36 | |
| Age (yr) | 61.6 ± 3.2 | 62.3 ± 3.1 | 0.116 |
| Location of lesion | 0.916 | ||
| Lower lobe | 36 | 35 | |
| Medium lobe | 13 | 12 | |
| Upper lobe | 52 | 54 | |
| Disease size (mm) | 0.769 | ||
| ≤ 20 | 35 | 37 | |
| > 20-30 | 66 | 64 | |
| Computed tomography bronchial signs | 0.737 | ||
| Positive | 77 | 79 | |
| Negative | 24 | 22 | |
| Ultrasonic probe position | 0.162 | ||
| External to the lesion | 10 | 11 | |
| Near the lesion | 39 | 37 | |
| Within the lesion | 52 | 53 |
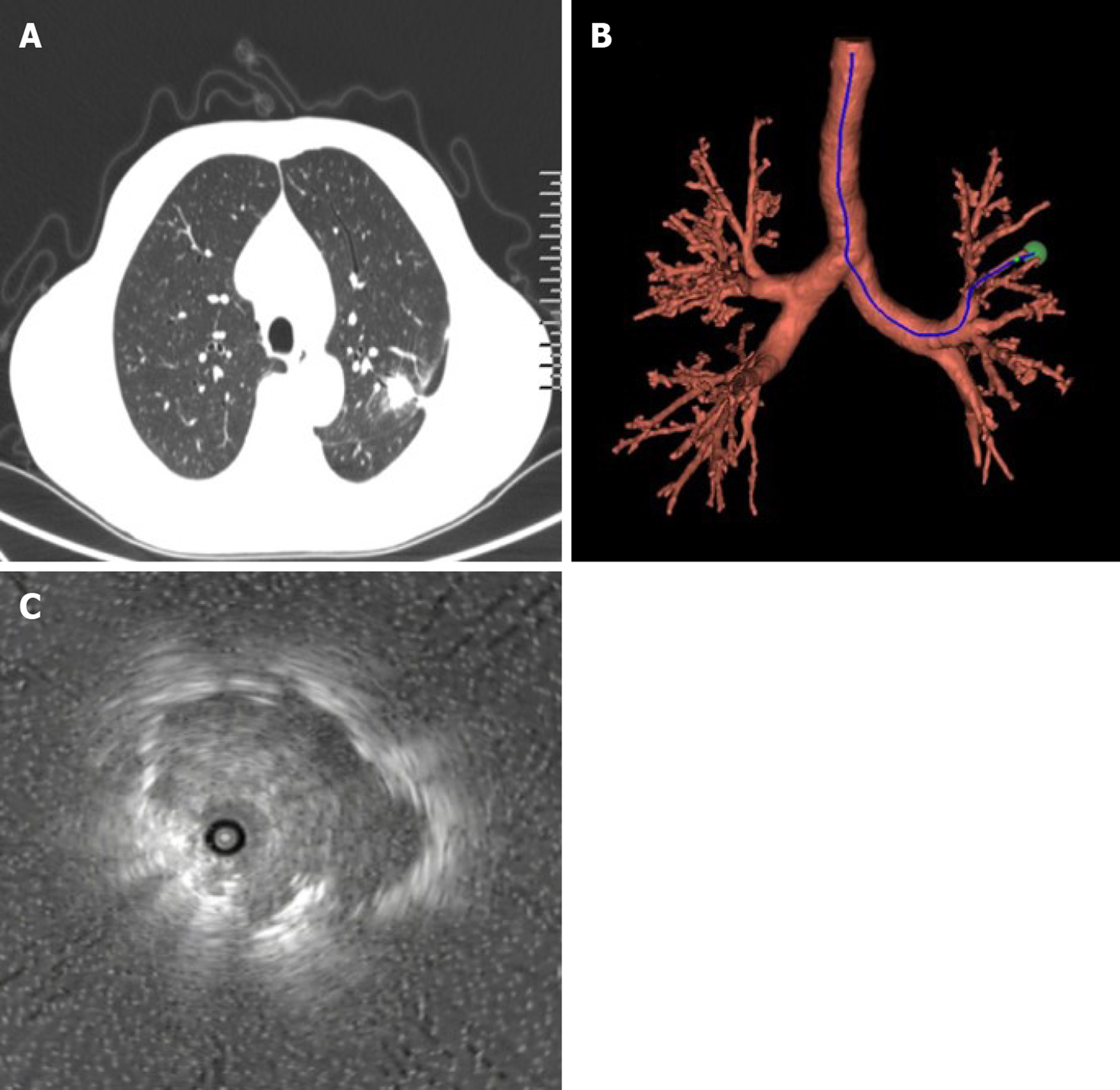
Patients in the control group underwent EBUS-GS examination and, based on the location of nodules on the chest CT images of the patients, assisted EBUS-GS to the location of the target lesion, and they were examined by endoscopic ultrasound. The target bronchi were reviewed via a biopsy hole guided by a sheath-wrapped ultrasound probe until the CT lesion size exhibited a similar abnormal echo. The probe was removed, and the casing was retained. The guide sheath was inserted into the lesion site. Five biopsy tissue samples were removed from each patient and sent to the pathology department. If there was no abnormal hypoechoic appearance, the surgeon determined the sampling method. The location of the lesion was washed with 20 mL of saline or brushing, and blind examination was performed around the location of the lesion. The ultrasonic image was used as the basis to judge the specific location of the ultrasound probe in the lesion. The EBUS-GS examinations of the observation group required VBN auxiliary access to the target lesion. Before the surgery, the patients received a 64-row spiral CT scan of the chest, and the data were transferred to a CD-ROM. The digital images were entered into the navigation system to communicate medical data, which formed a VB image of the peripheral lung lesions. The operator set the starting point and end point, and the software selected the path to the target lesion. The quantile points on the bronchial path were marked to ensure that the guide bronchoscope would penetrate into the peripheral lung lesions. At the time of examination, abnormal lesion size, image, and location were found, and biopsy was required. The diagnostic rate and total operating time of different subgroups of patients in the two groups were recorded, and the time needed to determine the lesion was recorded (Figure 1).
SPSS19.0 software was used for the statistical processing of the experimental data, and the results are expressed as mean ± SD for measurement data. The t-test, percentage for count data, and chi-square test were used to analyze the data. P < 0.05 was considered statistically significant.
Comparison of the diagnostic rates of different subgroups of patients in the two groups revealed no significant differences according to the location of the lesion (P > 0.05), the size of the lesion (P > 0.05), or the location of the probe (P > 0.05, Table 2).
| Group | Control (n = 101) | Observation (n = 101) | χ2 | P value |
| Focus position | ||||
| Upper left | 24 | 23 | 0.015 | 0.901 |
| Lower left | 26 | 25 | 0.014 | 0.907 |
| Upper right | 21 | 21 | 0.002 | 0.965 |
| Lower right | 15 | 16 | 0.052 | 0.819 |
| Lesion size | 0.007 | 0.933 | ||
| 8-20 mm | 39 | 38 | ||
| 21-30 mm | 47 | 47 | ||
| Probe position | ||||
| Internal | 51 | 52 | 0.063 | 0.802 |
| External | 2 | 2 | 0 | 0.991 |
| Approaching | 33 | 31 | 0.066 | 0.797 |
| Total | 86 | 85 | ||
The total operation time between the two groups was not statistically significant (P > 0.05), but the time needed to determine the lesion in the observation group was significantly less than that in the control group (P < 0.05; Table 3).
| Group | Control (n = 101) | Observation (n = 101) | t | P value |
| General operating time (min) | 25.4 ± 4.9 | 26.1 ± 4.8 | 1.026 | 0.306 |
| Time needed to determine the lesion (min) | 8.7 ± 1.1 | 4.9 ± 1.0 | 24.549 | 0 |
Peripheral lung cancer is also called “lung field type” compared with central lung cancer. The growth of the tumor in central lung cancer is located in the middle bronchus and above, while peripheral lung cancer is located below the segment bronchus. The early symptoms of peripheral lung cancer are not visible, so it is difficult to detect the disease in time. Once the symptoms appear, most patients are near the late stage. However, the earlier that treatment can be initiated, the better the effect of the treatment will be for the patient.
The American College of Chest Physicians guidelines reported in 2013 that patients with peripheral lung malignancies > 20 mm had a 63% success rate but it was only 34% in another study[12]. The published diagnostic rate also reveals huge changes, and the timeliness of diagnosis directly affects the treatment and prognosis of patients. Peripheral solitary pulmonary nodules (SPN) are the usual manifestations of early peripheral lung cancer. The lesions are single and isolated, and the gaseous lung tissue completely surrounds these lesions[13]. If the diameter is below 30 mm, there are no symptoms, such as pleural effusion, hilar enlargement, or atelectasis. Because the lesion is small, it does not have a great impact on the structure and function of the lung tissue. There are no obvious clinical symptoms, which are difficult to detect and are usually detected at the time of physical examination. With improvements of the medical level, low-dose spiral CT has been popularized, and the detection rate of SPN has improved. However, pathological examination is needed to confirm its nature. Interventional pulmonary disease has become an essential technique in the diagnosis of lung cancer. Only 18%-75% of patients with peripheral lung cancer were diagnosed using conventional bronchoscopy, and only 11%-42% of patients with tumors less than 200 mm were diagnosed.
VB assesses complex variations and structures of bronchial trees, such as bronchial stenosis, central-type airway malignancy, and airway malformation, and it plays a guiding role in bronchial biopsy of peripheral-type lesions. VBN evolved on the basis of CT techniques to reconstruct the bronchial tree. The examiner uses non-invasive methods to see the intratracheal images, and the bronchoscope reaches the target bronchus on this basis. The VBN reconstructs the CT data in the DICOM format directly into the navigation software computer, which automatically builds the three-dimensional bronchial tree. The automatic processing is completed by calibrating the target area of the lung, relying on the navigation software, which creates the biopsy path that guides the preoperative planning and selection of lung target sites. VBN has navigation and positioning functions, and the virtual view can be easily advanced and withdrawn according to the requirements. It can directly enter the lesion under its guidance during surgery.
The EBUS-GS probe uses ultrasonic waves that penetrate and are absorbed, reflected, and scattered by different tissues, and these waves form the acoustic spectrum image of the tissue structure, which is more characteristic. Because of the unique structure of bronchial trees, the clinical application of EBUS-GS leads to the “visualization” of peripheral lung lesions, which greatly improves the accuracy of biopsy tissue sampling, thus reducing the blindness of routine blind examinations and promoting the improvement of the positive diagnosis rate of peripheral lung cancer[14]. It can also allow to judge the blood supply, estimate the blood vessel size, and calculate the distance between vessels. By observing the different positions on the abnormal sonogram, we can avoid the most abundant blood vessel positions individually, which reduces the incidence of bleeding, improves the predictability of bleeding complications, and promotes the accuracy and safety of peripulmonary biopsy[15-17].
The results of this study showed no significant difference in the diagnosis rate between the two groups (P > 0.05). VBN in combination with EBUS-GS exploration did not affect the diagnosis rate, and there was no significant difference in total operation time between the two groups (P > 0.05).
Because EBUS-GS did not guide the localization of the lesion, the lesion could not be penetrated in 11%–21% of the patients even in combination with X-ray surveillance, which caused EBUS-GS to fail. At this stage, there are two controlled studies on whether VBN increases the diagnosis rate of peripheral pulmonary lesions (PPLs). Ishida et al[18] reported that the combined application of VBN and EBUS-GS improves the diagnosis rate (80.4% vs 67.0%) and shortened the time (24 min vs 26.2 min). Asano and other researchers demonstrated that VBN did not improve the overall diagnosis rate of PPLs (67.1% vs 59.9%), but a subgroup analysis revealed that VBN located some areas that were not visible under the X-ray, which was increased in the lung field. The combined use of VBN and EBUS-GS in a retrospective analysis showed a 63.35–84.4% diagnostic rate for PPLs and 44.4%–75.9% for ≤ 2 lesions. The use of VBN in navigation was not combined with X-ray in this study, which is consistent with the above findings for the diagnosis rate and total diagnosis rate of lesions ≤ 2 cm.
In summary, the combined use of VBN and EBUS-GS exploration technology has little effect on the diagnostic rate and total operating time in peripheral lung cancer, but it significantly shortens the time needed to determine the lesion. It is thus a valuable diagnostic method that can improve clinical research and lead to increased use[19,20].
Early peripheral lung cancer has no significant symptoms, and when the symptoms are obvious, the cancer is in a late stage.
It is necessary to have a clear molecular diagnosis based on different targets to initiate individualized treatment and promote a therapeutic effect.
To explore a new technique in the diagnosis of peripheral lung cancer.
Patients in the control group underwent transbronchial ultrasound-guided sheath-guided (EBUS-GS) exploration, and the observation group received virtual bronchoscopic navigation (VBN) in combination with EBUS-GS exploration.
There were no significant differences in diagnosis rate or total operation time between the two groups (P > 0.05), and the time needed to determine the lesion in the observation group was less than that of the control group (P < 0.05).
The combined use of VBN and EBUS-GS exploration technology has little effect on the diagnostic rate and total operating time in peripheral lung cancer.
The new technology provides a new idea for the early diagnosis of lung cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Abed M, Kawai HF S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Song G, Qiu T, Xuan Y, Zhao Y, Jiao W. [Clinical Application of Vectorial Localization of Peripheral Pulmonary Nodules Guided by Electromagnetic Navigation Bronchoscopy in Thoracic Surgery]. Zhongguo Fei Ai Za Zhi. 2019;22:709-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Wang J, Zhao Y, Chen Q, Zhang P, Xie W, Feng J, Cao J. Diagnostic value of rapid on-site evaluation during transbronchial biopsy for peripheral lung cancer. Jpn J Clin Oncol. 2019;49:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, Weenink C, Yousaf-Khan U, Horeweg N, van 't Westeinde S, Prokop M, Mali WP, Mohamed Hoesein FAA, van Ooijen PMA, Aerts JGJV, den Bakker MA, Thunnissen E, Verschakelen J, Vliegenthart R, Walter JE, Ten Haaf K, Groen HJM, Oudkerk M. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 2344] [Article Influence: 390.7] [Reference Citation Analysis (0)] |
| 4. | Yamakama H, Baba M, Shiba M, Urabe N, Fujisawa T, Yamaguchi Y, Oiwa T. [Application of transbronchial brushing for histological diagnosis of lung cancer]. Nihon Kyobu Shikkan Gakkai Zasshi. 1989;27:64-70. [PubMed] |
| 5. | Cao X, Liu M, Zhai F, Li N, Li F, Bao C, Liu Y, Chen G. Comparative evaluation of image registration methods with different interest regions in lung cancer radiotherapy. BMC Med Imaging. 2019;19:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Yang L, Dou Y, Sui Z, Cheng H, Liu X, Wang Q, Gao P, Qu Y, Xu M. Upregulated miRNA-182-5p expression in tumor tissue and peripheral blood samples from patients with non-small cell lung cancer is associated with downregulated Caspase 2 expression. Exp Ther Med. 2020;19:603-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Okachi S, Imai N, Imaizumi K, Iwano S, Ando M, Hase T, Aso H, Morise M, Wakahara K, Ito S, Hashimoto N, Sato M, Kondo M, Hasegawa Y. Factors Affecting the Diagnostic Yield of Transbronchial Biopsy Using Endobronchial Ultrasonography with a Guide Sheath in Peripheral Lung Cancer. Intern Med. 2016;55:1705-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | McLean AEB, Barnes DJ, Troy LK. Diagnosing Lung Cancer: The Complexities of Obtaining a Tissue Diagnosis in the Era of Minimally Invasive and Personalised Medicine. J Clin Med. 2018;7:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Usuda J. [Virtual Bronchoscopic Navigation (VBN) and Electromagnetic Navigation System]. Kyobu Geka. 2018;71:843-849. [PubMed] |
| 10. | Bo L, Li C, Pan L, Wang H, Li S, Li Q, Bai C, Zeng Y, Nan Y, Wang Y, Huang H, Zhou R, Zhou H, Liu W, Sun J, Liu Z, Jin F. Diagnosing a solitary pulmonary nodule using multiple bronchoscopic guided technologies: A prospective randomized study. Lung Cancer. 2019;129:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Li S, Yan W, Chen M, Sun L, Wu Q, Chen K. [Diagnostic Utility of Virtual Bronchoscopic Navigation Assisted Endobronchial Ultrasonography with Guide Sheath for Peripheral Pulmonary Lesions]. Zhongguo Fei Ai Za Zhi. 2019;22:125-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Haidong H, Yunye N, Wei Z, Zarogoulidis P, Hohenforst-Schmidt W, Man YG, Yuguang Y, Yuchao D, Chong B. Multiple guided technologies based on radial probe endobronchial ultrasound for the diagnosis of solitary peripheral pulmonary lesions: a single-center study. J Cancer. 2017;8:3514-3521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Khan T, Usman Y, Abdo T, Chaudry F, Keddissi JI, Youness HA. Diagnosis and management of peripheral lung nodule. Ann Transl Med. 2019;7:348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Yasokawa N, Yorizumi N, Kurose K, Abe M, Oga T. Detachment and recovery of an X-ray opaque tip of a disposable guide sheath kit: A rare complication of endobronchial ultrasound-guided transbronchial biopsy. Respir Med Case Rep. 2020;29:101030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Chen ZB, Jin YP, Yu YM, Zhu DP, Ma HY, Chen L, Zhang Y, Ding QL, Deng ZC. [A study of the diagnostic value of endobronchial ultrasound guide sheath transbronchial lung biopsy combined with virtual bronchoscopic navigation in peripheral pulmonary lesions]. Zhonghua Jie He He Hu Xi Za Zhi. 2016;39:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Ikezawa Y, Shinagawa N, Sukoh N, Morimoto M, Kikuchi H, Watanabe M, Nakano K, Oizumi S, Nishimura M. Usefulness of Endobronchial Ultrasonography With a Guide Sheath and Virtual Bronchoscopic Navigation for Ground-Glass Opacity Lesions. Ann Thorac Surg. 2017;103:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Fang F, Pan L, Bo LY, Li WP, Fu EQ, Li CC, Jin FG. [Diagnostic value of endobronchial ultrasonography with guide-sheath combined with virtual bronchoscopy navigation in peripheral lung cancer]. Zhonghua Jie He He Hu Xi Za Zhi. 2018;41:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration. 2014;88:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Oshige M, Shirakawa T, Nakamura M, Mineshita M, Kurimoto N, Miyazawa T, Becker HD. Clinical application of virtual bronchoscopic navigation system for peripheral lung lesions. J Bronchology Interv Pulmonol. 2011;18:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Umeda Y, Demura Y, Anzai M, Matsuoka H, Araya T, Nishitsuji M, Nishi K, Tsuchida T, Sumida Y, Morikawa M, Ameshima S, Ishizaki T, Kasahara K, Ishizuka T. (18)F-FDG uptake predicts diagnostic yield of transbronchial biopsy in peripheral lung cancer. Lung Cancer. 2014;85:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |