Published online Aug 26, 2020. doi: 10.12998/wjcc.v8.i16.3377
Peer-review started: April 6, 2020
First decision: April 29, 2020
Revised: May 5, 2020
Accepted: July 15, 2020
Article in press: July 15, 2020
Published online: August 26, 2020
Processing time: 140 Days and 19.7 Hours
Minimal hepatic encephalopathy (MHE) is a critical neurocognitive complication of decompensated liver cirrhosis and portosystemic shunting, which results in a wide range of cognitive deficits including impairments in working attention, psychomotor speed, and executive function. Current guidelines have recommended paper-and-pencil psychometric tests for the diagnosis of MHE. Most high-risk cirrhotic patients are required to be examined; however, paper-and-pencil psychometric tests are neither convenient nor rapid to perform in the clinic. Recently, novel computerized psychometric tests, including the inhibitory control test, EncephalApp Stroop App, and critical flicker frequency, have been proven to be rapid, effective, and convenient methods for screening MHE in clinical practice and for identifying high-risk cirrhotic patients for further validation using rigid neuropsychometric examinations. However, diagnostic accuracy of these tests is influenced by educational background, age, and cultural differences. This review summarizes clinical evidence of the application of novel computerized psychometric tests for screening MHE.
Core tip: This review summarizes clinical evidence of computerized psychometric tests for screening minimal hepatic encephalopathy (MHE). Computerized psychometric tests are rapid, effective, and convenient methods for screening MHE in clinical practice and for identifying high-risk cirrhotic patients for further validation, which helps facilitate early diagnosis and treatment of MHE. However, calibration and validation of these tests are required to reach more convincing conclusions before their widespread clinical application in cirrhotic patients at risk for MHE.
- Citation: Luo M, Mu R, Liu JF, Bai FH. Novel computerized psychometric tests as primary screening tools for the diagnosis of minimal hepatic encephalopathy. World J Clin Cases 2020; 8(16): 3377-3389
- URL: https://www.wjgnet.com/2307-8960/full/v8/i16/3377.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i16.3377
Hepatic encephalopathy (HE) is a serious neuropsychiatric syndrome in patients with decompensated liver diseases and/or portosystemic shunting[1]. It involves impairments in both executive and cognitive function, ranging from mild psychometric abnormalities to a coma. Based on the severity of HE symptoms, the syndrome is categorized into overt HE (OHE) and covert HE. Covert HE includes minimal HE (MHE) and WestHaven grade I HE[1]. Patients with OHE present with neuropsychiatric symptoms including asterixis, dyspraxia, and even a coma. In contrast, patients with MHE present with a spectrum of trivial cognitive deficits in attention span, psychomotor speed, and working memory without obvious neurological symptoms[2]. The prevalence of MHE has been reported in up to 50% of cirrhotic patients, and MHE impairs daily function, affects health-related quality of life, and induces a high risk of progression to OHE[3-6].
Although the early diagnosis of MHE has been shown to be beneficial for further treatment and follow-up, diagnosing MHE is hampered by the lack of standard and reliable diagnostic tests in clinical practice. The American Association for the Study of Liver Diseases updated guidelines in 2014 recommends the most commonly used paper-and-pencil psychometric test, psychometric hepatic encephalopathy score (PHES), for the diagnosis of MHE[1]. Although the PHES have been validated in clinical studies, it requires physicians for administration and interpretation; thus, it cannot be rapidly and conveniently performed in a clinic. A survey by the American Association for the Study of Liver Diseases showed that most physicians have realized the necessity of MHE testing in high-risk cirrhotic patients; however, only a minority of cirrhotic patients are routinely tested for MHE[7]. Therefore, simple and rapid tests that can be performed by clinic assistants would increase the rates of MHE testing in the initial clinical assessment and follow-up.
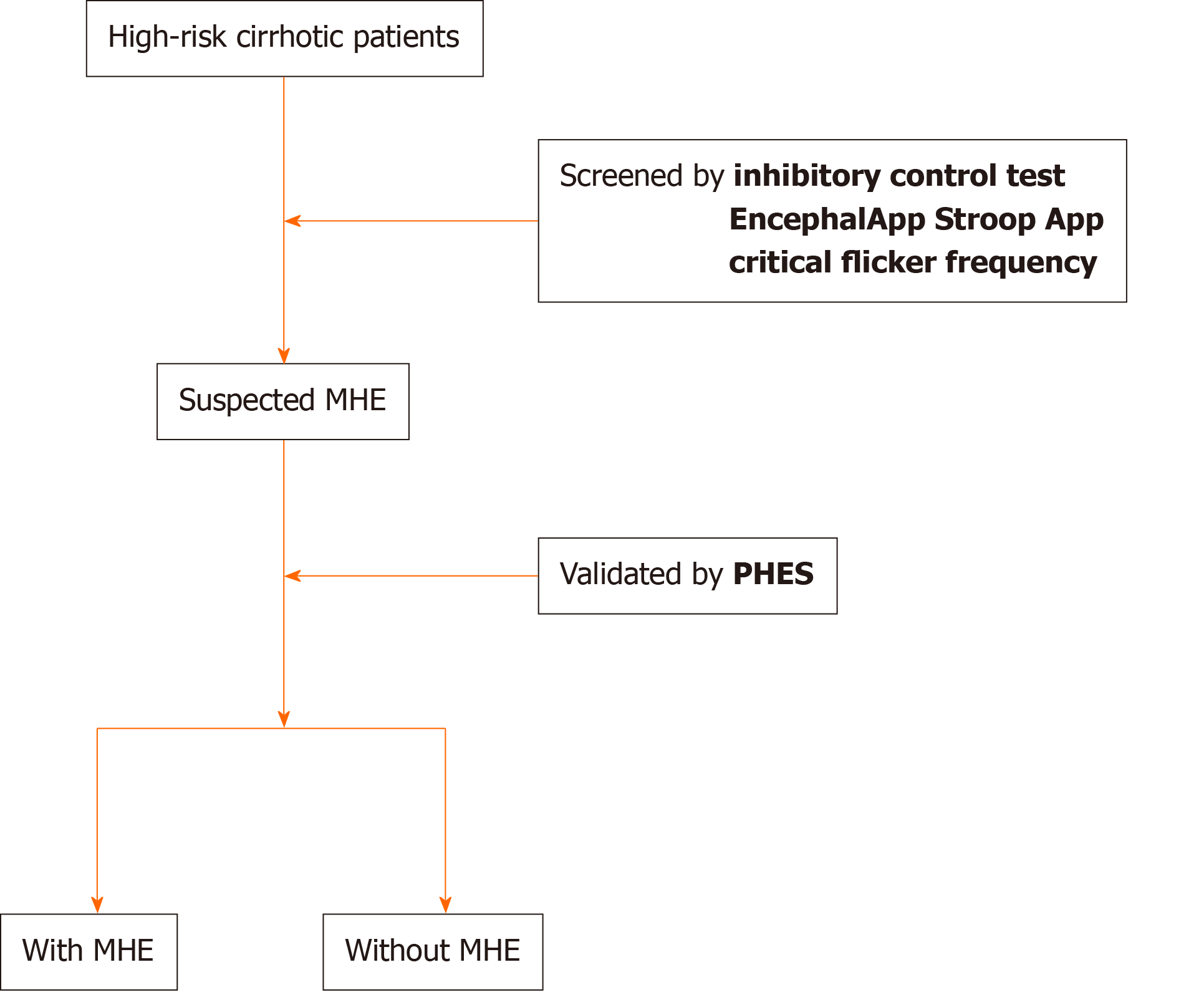
Psychometric tests can be computerized for simplification and performed in a clinic within just a few minutes. Recent studies showed that novel computerized psychometric tests have been used to screen MHE in cirrhotic patients, including the inhibitory control test (ICT), EncephalApp Stroop App (EncephalApp), and critical flicker frequency (CFF)[6,8-17]. These tests have been verified to be rapid, simple, and reliable methods for screening MHE and for predicting the development of OHE compared with paper-and-pencil psychometric tests[6,8-17] (Table 1). High-risk cirrhotic patients are initially screened for MHE using rapid, convenient, and highly sensitive computerized psychometric tests, and then the findings are validated by more detailed neuropsychometric tests (Figure 1). Here, we review the clinical evidence and advances of novel computerized psychometric tests for screening MHE.
| Ref. | Location | Number of cirrhotic patients | Gold standard | Optimal cut-off | AUROC (sensitivity/specificity) |
| Inhibitory control test | |||||
| Bajaj et al[6] | United States | 50 | SPT | > 5 lures | 0.96 (90%/90%) |
| Bajaj et al[8] | United States | 135 | SPT | > 5 lures | 0.90 (87%/77%) |
| Gupta et al[9] | India | 200 | PHES | > 14 lures | 0.86 (93%/78%) |
| Sharma et al[27] | India | 50 | PHES | > 17 lures | 0.70 (89%/56%) |
| Taneja et al[10] | India | 102 | PHES | > 14 lures | 0.75 (78%/65%) |
| Duarte-Rojo et al[40] | United States | 437 | PHES | > 5 lures | 0.561 (45%/67%) |
| Pawar et al[24] | India | 180 | PHES | > 14 lures | 0.72 (79%/66%) |
| EncephalApp Stroop App | |||||
| Bajaj et al[14] | United States | 125 | PHES | > 274.9 s | 0.84 (88%/78%) |
| Bajaj et al[13] | United States | 167 | SPT | > 190 s | 0.88 (80%/81%) |
| Allampati et al[12] | United States | 437 | PHES/ICT | > 195.9 s | PHES: 0.80 (80%/61%) |
| ICT: 0.80 (72%/54%) | |||||
| Duarte-Rojo et al[40] | United States | 437 | PHES | > 195.9 s | 0.59 (70%/48%) |
| Critical flicker frequency | |||||
| Kircheis et al[46] | Germany | 92 | Vienna Test System | < 39 Hz | 0.72 (55%/100%) |
| Romero-Gómez et al[15] | Spain | 114 | PHES | < 38 Hz | 0.79 (72%/77%) |
| Sharma et al[49] | India | 110 | NCT-A/B | < 39 Hz | 0.80 (65%/91%) |
| Metwally et al[17] | Egypt | 86 | PHES | < 39 Hz | 0.94 (91%/92%) |
| Coşkun et al[48] | Turkey | 70 | PHES | < 39 Hz | 0.71 (39%/82%) |
| StroopEffect-2 | |||||
| Suresh et al[54] | India | 37 | RAKIT | > 180.4 s | 0.97 (100%/89.6%) |
| eNCT | |||||
| Wuensch et al[55] | Germany | 112 | PHES | 10″ (18″) | 0.92 (82%/85%) |
| CDR assessment battery | |||||
| Mardini et al[57] | United Kingdom | 89 | PHES | -5 to 15 | 0.917 (86.4%/81%) |
| CNSVS | |||||
| Prakash et al[60] | United States | 100 | PHES | > 2 SD | 0.74 (85%/64%) |
| ImPACT | |||||
| Tsushima et al[62] | United States | 90 | SPT | > 2 SD | 0.78 (62.5%/94.7%) |
Inhibitory control is also widely known as response inhibition. It is an executive function, and self-control is one of the considerable aspects of inhibitory control. Executive functions of the prefrontal cortex, caudate nucleus, and subthalamic nucleus are found to be associated with inhibitory control cognition, and inhibitory control is impaired in addiction, schizophrenia, and traumatic brain injury[18-21]. ICT is a neuropsychological test that evaluates subjects’ ability to override their natural and habitual behavioral responses to stimulus in order to select more appropriate behaviors to achieve their goals[22].
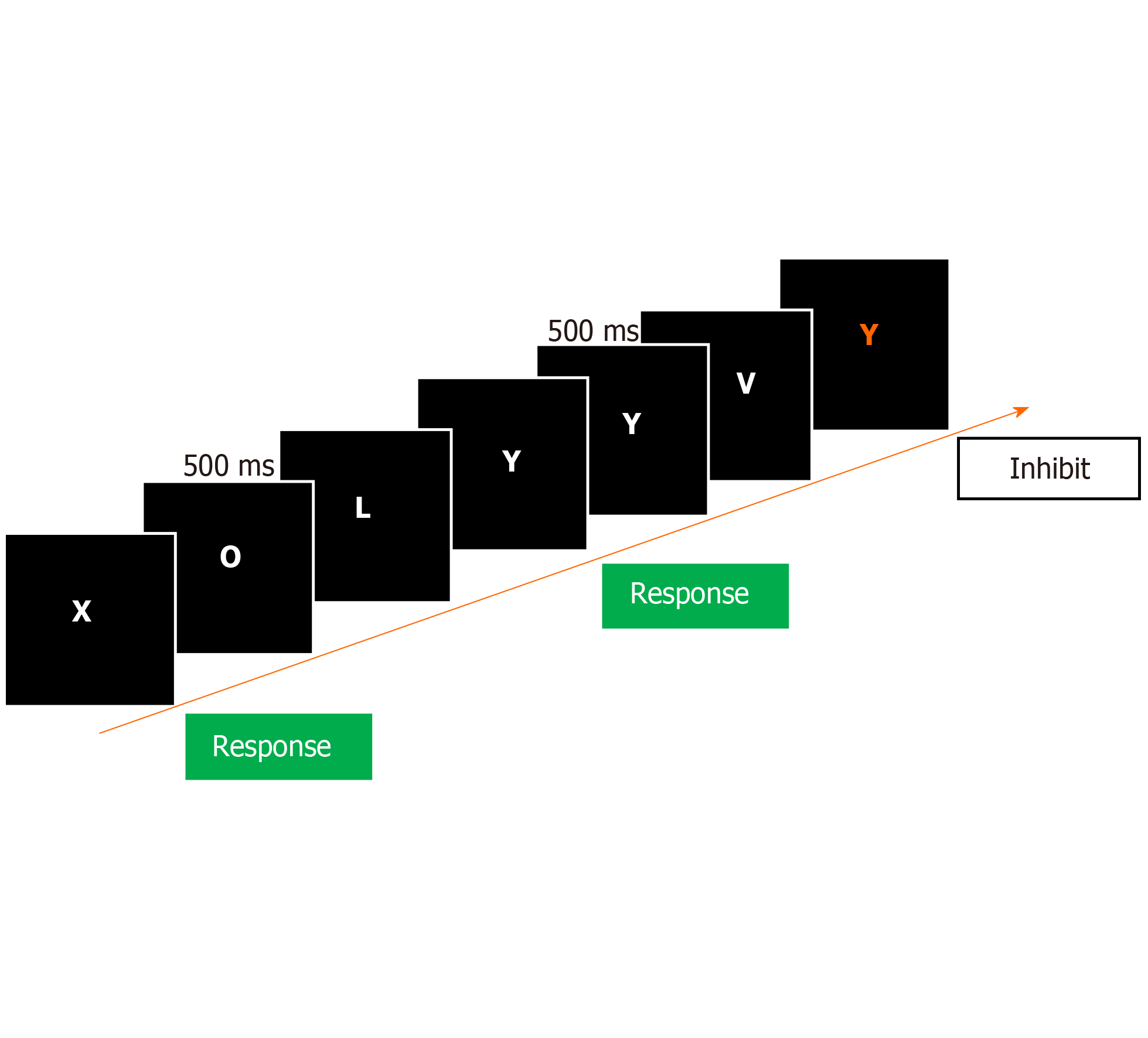
The ICT can be performed by medical assistants after the training session using laptop computers with automatic analyzed systems, which greatly improves the applicability and convenience of this test in the clinical setting. A running stream of letters within which the letters X and Y are distributed is randomly running across a computer screen at 500 ms intervals. The subject is instructed to only respond upon seeing alternating X and Y (called targets) and to stay quiet as seeing nonalternating X and Y (called lures) (Figure 2)[6]. Six test sessions including a total of 40 lures, 212 targets, and 1728 random letters are generated in the training in approximately 15 min. It takes about 15 min to complete the ICT assessment. Response rates and reaction times of lures and targets are automatically calculated upon completion of the test. The results are reported in the form of a number and percentage respectively of incorrect lure responses, correct lure inhibitions, correct target responses, and incorrect target misses[11]. ICT lures measure inhibition ability, and target accuracy reflects attention ability[11]. A good psychometric performance will be indicated by a lower lure response, higher target response, and shorter reaction times of lures and targets. Therefore, the ICT evaluates response inhibition, and assesses a subject’s ability to inhibit lure responses and correctly respond to targets. A significant correlation was found between lures and targets with psychometric impairment in PHES components, which provides discrete measures yet assisting perspectives into impairment in MHE[8].
The ICT has been used to screen MHE in the United States, Germany, Italy, and India (Table 1)[6,8-11,23,24]. In the United States, with a cut-off value of ≥ 5 lures/person, Bajaj et al[6] found that the ICT had a 90% sensitivity and specificity for screening MHE, and the area under the receiver operator characteristic (AUROC) was 0.902 when using the standard paper-and-pencil psychometric tests (SPT) as the reference standard. Although hepatitis C has been found to induce cognitive deficits, this study showed no significant distinction in ICT results between cirrhotic patients with hepatitis C and those with other etiologies[6,25,26]. Moreover, there was no significant difference in ICT performance between patients with alcoholic cirrhosis and those with nonalcoholic cirrhosis[8]. These findings extend the clinical application of the ICT across different etiologies of liver cirrhosis. The PHES norms depend on the age bracket; however, ICT results, including lures and target accuracy, were found to be independent of age[8,11]. This is a crucial difference between the PHES and ICT, which may potentially simplify and generalize the ICT application for screening MHE. Additionally, compared with the learning effects of PHES, lure and target responses of ICT are closely associated between the initial and following testing sessions, which indicates that the ICT has good test-retest reliability[8,9,11]. Because of the high sensitivity and convenience of performance, the ICT is an effective diagnostic test for MHE in clinics, and it may help physicians to decide whether to send a patient for further evaluation or to administer treatment.
In an Indian population, the ICT showed a sensitivity of 92.6% and a specificity of 78.5% for screening MHE with a cut-off value of ≥ 14 lures when using the PHES as a reference standard[9]. The cut-off value of Indian patients in this study was higher than that reported earlier in the United States population[6,8]. Moreover, studies in India and Italy showed that the ICT had lower sensitivity, specificity, and AUROC for screening MHE compared to the results reported by Bajaj et al[6,8,13,14]. Therefore, Amodio et al[11] established a novel measurement method of ICT results in Italian patients, i.e. lures weighted by target accuracy, and it was shown to effectively identify MHE patients from the controls. When cirrhotic patients in India were given a questionnaire regarding which tests they would like to repeat during follow-up, only 12% of patients preferred the ICT over the PHES[27]. An explanation for this difference may be that most cirrhotic patients included in these studies were from an underdeveloped area and had lower educational levels. Furthermore, these patients were less familiar with computer games than United States controls, and unfamiliarity with a computer may lower the preference for ICT repetition and would be a critical influencing factor for the ICT results[9,10,27]. These results indicated that the ICT, like most psychometric tests, was influenced by demographic variables, and use of the ICT should be adjusted when it is performed in different populations. Most studies excluded cirrhotic patients receiving treatment with psychoactive medications such as benzodiazepines and antipsychotics. However, these psychoactive medications are used for treatment of 50% of patients with liver cirrhosis in real life, and this factor may disturb ICT performance and the sensitivity/specificity of unaffected patients observed[28].
The class of Child-Turcotte-Pugh (CTP) and the score of model for end-stage liver disease (MELD) are effective measurements for predicting survival in cirrhotic patients, and ICT results are closely related to both the CTP class and MELD score[9,29,30]. The ability of the ICT for predicting the development of OHE and the cumulative survival is found to be equivalent to the SPT and PHES[6,9]. Moreover, although the sample sizes for the transvenous intrahepatic portosystemic shunting (TIPS) and yogurt trial were limited, ICT results worsened after application of TIPS, and improved after yogurt treatment simultaneously with the SPT[8]. In contrast, in the study by Taneja et al[10] in India, there is no external validity of the ICT for diagnosing MHE due to the fact that there is no possible prediction of either survival or episodes of OHE during follow-up. The explanation to this finding could be attributed to the fact that there is no correlation of the ICT with the CTP class and MELD scores, which were adopted as the predictors of survival in this study. Additionally, several studies included patients with OHE history, and these studies combined the ICT results of cirrhotic patients with and without prior OHE[6,9-11]. Cirrhotic patients with OHE history have a high risk for recurrent OHE; therefore, further studies have focused on the ability of the ICT to OHE development prediction in a larger sample space with cirrhotic patients without prior OHE[31,32].
Because of impaired driving skills, cirrhotic patients with MHE have a higher reported occurrence of motor vehicle accidents and traffic violations[33]. Compared with the SPT, the ICT is significantly correlated with a higher rate of motor vehicle crashes in the last year and prospective follow-up for 1 year[34]. Navigation skill is an important aspect of driving difficulties induced by MHE, which is also associated with impairment of ICT results[35]. Therefore, the ICT may be superior to the SPT for screening MHE patients who are at risk of vehicle crashes during follow-up. Use of the ICT for screening MHE may reduce the risk of motor vehicle crashes.
EncephalApp originated from the Stroop test, whose reputation is attributed to its assessment of the Stroop effect. The Stroop test assesses psychomotor speed and inhibitory control. There comes into conflict between word reading and color naming in the Stroop test. The individual has to suppress the brain’s word reading activity in order to conduct the color naming activity[36]. The Stroop test was previously performed using paper-and-pencil assessment methods, and it has been used to evaluate cognitive deficits due to brain damage, Alzheimer’s disease, and Parkinson's disease[37-39]. Based on the Stroop test, Bajaj et al[14] developed a computerized test, EncephalApp, for diagnosing MHE in 2013. The EncephalApp is operated by the iOS and Android system on the smartphone or computer, and the application and operational instructions of the EncephalApp can be easily downloaded from http://www.encephalapp.com.
The EncephalApp includes an easy “off” state and a difficult “on” state. In the “off” state, colored signs ### are presented on the screen, and subjects are required to identify the corresponding color (Figure 3A and 3B). In the “on” state, the app presents a discordant colored word; for example, the word “blue” will be presented in green color, and the correct answer is green (Figure 3C and 3D). The EncephalApp has practice runs before the test runs, and the order of tests are as follows: (1) Two practice “off” runs; (2) Five test “off” runs; (3) Two practice “on” runs; and (4) Five test “on” runs[14]. It takes about 15 min to complete all subtests of EncephalApp. Whether the EncephalApp is in the “on” or “off” state, it requires subjects to concentrate on the visual stimulus and choose the appropriate color by pressing on the screen, and the response time is calculated using “on-time” and “off-time”. Recent studies have found that “on-time” and “off-time” were highly associated with a wide spectrum of cognitive aspects, including visuomotor coordination, set-shifting, and response inhibition, indicating that the PHES and EncephalApp evaluate several similar cognitive domains in patients with MHE[13,14].
Clinical application of the EncephalApp for screening MHE was first reported by Bajaj et al[14] in 2013. This study showed that the EncephalApp had a sensitivity of 78% and specificity of 90% for diagnosing MHE when using the PHES as the reference standard. In a subsequent study also conducted by Bajaj et al[13], the EncephalApp identified cirrhotic patients with MHE with an AUROC value of 0.91, and the AUROC value was 0.88 for screening MHE in those without OHE. A multicenter study reported that the EncephalApp had a sensitivity of 80% based on the PHES and sensitivity of 70% based on the ICT for screening MHE when using adjusted United States population norms[12]. The PHES and EncephalApp, whether alone or in combination, were found to be equivalent for screening MHE, which indicates that single testing with the EncephalApp is sufficient for screening MHE in clinical practice[40]. The EncephalApp is a convenient, simple, and highly sensitive test for screening MHE, and application of the EncephalApp may increase the testing likelihood for MHE and subsequent therapy rates.
Previous research found that hepatitis C could induce cognitive impairment; however, the two above-mentioned studies found no differences in EncephalApp results in cirrhotic patients with hepatitis C compared to other etiologies of cirrhosis[14,41,42]. The reason for this finding may be that cognitive impairment due to hepatitis C is overshadowed by the impairment caused by liver cirrhosis, which expands the range of EncephalApp application and increases the rates of subsequent therapy for cirrhosis resulting from viral hepatitis. These studies have also established an instrumental comparison between the iPad version and iPod/iPhone version. Compared with the iPad version, the iPod/iPhone version was considered to be an easier approach; however, there was no significant distinction in EncephalApp results regardless of the device differences and familiarity with a smartphone, which might be attributed to the practice runs before each tests[13].
Although the effect of alcohol abuse on cognitive function was found to be confined to psychomotor speed, patients with alcoholic cirrhosis showed more serious cognitive impairment in the EncephalApp results despite similar severity of cirrhosis[14,43]. It was recognized that cirrhotic patients with red-green color blindness were excluded from studies because that color discrimination is indispensable for the EncephalApp test. As far as the present practices are concerned, the EncephalApp is not appropriate for screening MHE in cirrhotic patients with red-green color blindness and alcoholic cirrhotic patients without abstinence. As previously mentioned, patients with alcoholic cirrhosis should be tested for MHE using the ICT. In the studies we reviewed, patients receiving treatment with psychoactive medications and/or patients diagnosed with common comorbidities were excluded[12-14], which resulted in limiting generalizability and implementation of the EncephalApp for screening MHE. Therefore, it requires further study by increasing the number of clinically representative patients with alcoholic liver cirrhosis, psychotropic medications, and extrahepatic comorbidities.
Age was found to be a significant influence factor of EncephalApp results[12-14]. Although current studies excluded cirrhotic patients with Alzheimer’s disease, Parkinson’s disease, and cerebrovascular disease, these age-related cognitive deficits may influence the reliability of the EncephalApp in the elderly population. Therefore, age-adjusted norms and cut-offs should be developed to reduce the related bias. Although education levels were found to be independent of EncephalApp results, cirrhotic patients included in studies conducted by Bajaj et al[13,14] had higher levels of education. On the contrary, epidemiological surveys in different countries showed that a fairly large number of cirrhotic patients had a lower educational background[3-5]. The relatively high educational levels of patients in the current studies may affect the potential influence of education on these results and limit the generalizability of the EncephalApp. Additionally, the EncephalApp has been translated into several languages, including English, German, and Chinese; thus, linguistic differences and ethnic origin may have certain effects that should be considered[44]. Therefore, further validation of the use of the EncephalApp for screening MHE should control for possible influence factors, including educational background, age, and cultural differences.
Current studies have demonstrated that the EncephalApp has stable test-retest reliability and external validity for screening MHE, indicating that the EncephalApp is potentially appropriate for repeated tests during follow-up[12-14]. A multivariable analysis showed that EncephalApp abnormalities and the MELD score were significant predictors of the initial episode of OHE in cirrhotic patients in a 10-mo follow-up, independent of age and alcoholic etiology[12]. The PHES or EncephalApp alone, or a combination of the PHES and EncephalApp are all acceptable strategies to predict initial OHE development[40]. Therefore, whether based on reference standards or based on population norms, EncephalApp results independently predict the development of an OHE episode.
TIPS and hyponatremia are considered to be the common etiology of MHE, and studies have shown that EncephalApp results worsened after TIPS placement and improved after hyponatremia correction[13,14]. These results showed that the EncephalApp can evaluate cognitive impairment in decompensated cirrhosis and extend the realm of its performance across the range of cognitive deficits in liver cirrhosis. The EncephalApp specifically was associated with basic domains involved in psychomotor speed, visuomotor coordination, and reaction time. Similar to the ICT, EncephalApp results were associated with illegal turns and crashes in driving simulation tests, which highlights its face validity[13].
The CFF test is a neurophysiological assessment method that evaluates the ability of the cerebral cortex to identify flickering lights. It was originally designed to examine optic nerve lesions in ophthalmology clinics[45]. In the CFF assessment that utilizes the portable Hepatonorm Analyzer (R&R Medical Business Freiburg GmbH, Freiburg, Germany), light pulses decreasing in frequency from 60 to 25 Hz are presented to a subject, and the subject has to press a button as soon as the impression of fused light shifts to that of flickering light. After a training session, flicker frequencies are measured eight to ten times, and the mean value of these runs is calculated to determine the result of the CFF test. The test takes about ten minutes to complete. CFF is well tolerated and easily understood by cirrhotic patients, and it does not require the supervision of specialized personnel. However, CFF assessment requires good eyesight, the absence of red-green blindness, and specialized equipment. Moreover, CFF results are mainly influenced by the luminance of the stimuli, the visual angle, and the distance between eyes and the light source during assessment[16].
CFF was recommended for screening MHE in 2002, and it has been performed in Germany, Spain, Turkey, Egypt, and India[15,17,46-49] (Table 1). Using a cut-off of 39 Hz, CFF showed a sensitivity of 55% and a specificity of 100% for screening MHE in German cirrhotic patients[46]. Similarly, in Indian cirrhotic patients, a CFF threshold of 38 Hz identified MHE patients with a sensitivity of 72.4% and a specificity of 77.2% using the PHES as the reference standard[15]. Moreover, in comparison with PHES, CFF also had a sensitivity of 91.1%, specificity of 92.7%, and area under the curve of 0.937 for screening MHE in Egypt[17]. A meta-analysis of nine studies indicated that CFF has a high summary specificity (79%) and moderate sensitivity (61%) for screening MHE[50]. These results showed that CFF is a promising method for screening MHE, and its results are independent of educational background, verbal fluency, language, and numeracy[15,17,49].
Although there was a significant correlation between CFF and PHES test results, CFF had a lower sensitivity of 39% for detecting MHE in Turkish cirrhotic patients with a cut-off value of < 39 Hz[48]. Similarly, Kircheis et al[51] reported that the diagnostic agreement value between CFF and conventional PHES in MHE patients was only 54%. The explanation for this discrepancy of CFF sensitivity is that CFF has advantages in the evaluation of attention abnormalities and is not specialized in assessing motor function, suggesting that CFF could only detect certain features of MHE. Furthermore, several studies have found that CFF values decreased by 0.6–0.7 Hz during every decade of life, and that patients with alcoholic cirrhosis had significantly lower CFF than those with liver cirrhosis due to other etiologies[23,46,47]. Therefore, a fixed cut-off may induce biased CFF results in elderly patients and in patients with alcoholic cirrhosis, and age-matched cut-off values should accordingly be established and applied. All things considered, CFF is a simple and easy-to-perform test for screening MHE in cirrhotic patients, which nevertheless should be used as an adjunct to, and not a replacement of, conventional PHES.
CFF was found to have no learning effects, which indicates that it is appropriate for follow-up studies[27]. The study by Sharma et al[49] showed that CFF is a reliable and accurate test not only for MHE screening but also for the assessment of recovery of MHE patients treated with lactulose for 1 mo. In Spain, a prospective study of 117 consecutive cirrhotic patients showed that CFF predicted the first appearance of OHE during the 5-year follow-up period[52]. Moreover, CFF < 39 Hz, not the PHES, was associated with lower survival, irrespective of MELD scores[52]. Similarly, there was a correlation between CFF results and CTP scores but not with MELD scores, and Cox regression showed that CFF together with CTP scores was independently associated with a risk for the development of OHE[15]. TIPS is a common etiology of MHE, and it can increase the incidence of OHE. Compared to the PHES, CFF has a good negative predictive value (91%) for the risk of recurrent OHE after TIPS, which suggests that CFF may help select cirrhotic patients for TIPS and decrease the incidence of OHE after TIPS[53]. These results indicated that CFF can predict the survival of and the occurrence of OHE in cirrhotic patients with MHE, which is crucial for early prevention and subsequent treatment of MHE.
The Stroop test has been validated for screening MHE in adults. In the study by Suresh et al[54], the Tamil language version of the computerized Stroop test, called StroopEffect-2, had a sensitivity of 89.6% and specificity of 100% for diagnosing MHE in Indian children with extrahepatic portal vein obstruction with a cut-off of > 180.4 s and an AUROC of 0.97. This study indicated that the computerized Stroop test is suitable for MHE screening in children. Moreover, Wuensch et al[55], who was inspired by the paper-pencil number connection test version A, developed a novel electronic number connection test. The electronic number connection test exhibited high sensitivity (> 82%) and high specificity (> 85%) in MHE detection with excellent test–retest reliability.
The cognitive drug research (CDR) assessment battery is a computerized cognitive testing tool developed for the assessment of human cognitive function impairment. The stimuli of the task are displayed on a laptop, and both accuracy and reaction time of participants’ responses are recorded with the “yes” and “no” buttons on a two-button response box[56]. Mardini et al[57] reported that the CDR assessment battery has a sensitivity of 86.4% and a specificity of 81.0% for screening MHE in cirrhotic patients compared with the PHES as the reference standard. Furthermore, the CDR system has shown appreciable test-retest reliability, which may help evaluate cognitive improvement when cirrhotic patients with MHE are retested after treatment[56,58]. However, the CDR assessment battery requires a practice session before administration, which induces the learning effect that may influence the reliability of the battery for screening MHE.
The central nervous system vital signs (CNSVS) is a computerized neurocognitive test battery that was developed into a routine clinical screening instrument. It includes seven tests: verbal and visual memory, finger tapping, symbol digit coding, the Stroop test, a test of shifting attention, and the continuous performance test[59]. The CNSVS is able to evaluate verbal memory, processing speed, executive function, reaction time, cognitive flexibility, and complex attention. Prakash et al[60] prospectively enrolled 100 cirrhotic patients and 110 controls, and a high correlation between the CNSVS and PHES was observed. In this study, the CNSVS identified MHE with 85% sensitivity, 64% specificity, and AUROC value of 0.74[60]. It seems promising, but it still requires validation with larger populations in multicenter studies.
The immediate postconcussion assessment and cognitive test (ImPACT) is a computer-based program for assessing neurocognitive function and concussion symptoms. The ImPACT executes measurement of attention span, working memory, processing speed, and reaction time and yields four composite scores: verbal memory, visual memory, visual motor speed, and reaction time[61]. Tsushima et al[62] found the correlation between the ImPACT scores and results of paper-and-pencil testing and health-related quality of life. In comparison with paper-and-pencil testing, the ImPACT showed 62.5% sensitivity and 94.7% specificity for screening MHE[62]. The ImPACT offers web-based neuropsychological evaluation of MHE in a brief, user-friendly, and effective approach. However, it has not been validated in multicenter studies and is innovated for subjects of college enrollment; thus, the usage of which for most patients with liver cirrhosis is not suitable.
MHE lacks a universal and standard method for diagnosis. Novel computerized psychometric tests are rapid, effective, and convenient methods for screening MHE in clinical practice and for identifying high-risk cirrhotic patients for further validation by the PHES test. Computerized psychometric tests may help facilitate early diagnosis and treatment of MHE, which may improve patients’ quality of life, reduce the risk of progression to OHE, and consequently reduce the risk of motor vehicular accidents. However, diagnostic accuracy of computerized psychometric tests is influenced by educational background, age, and cultural differences. Therefore, calibration and validation of these tests are required to reach more convincing conclusions before their widespread clinical application in cirrhotic patients at risk for MHE.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yücel O S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Xing YX
| 1. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1487] [Article Influence: 123.9] [Reference Citation Analysis (1)] |
| 2. | Basu PP, Shah NJ. Clinical and Neurologic Manifestation of Minimal Hepatic Encephalopathy and Overt Hepatic Encephalopathy. Clin Liver Dis. 2015;19:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Wang JY, Zhang NP, Chi BR, Mi YQ, Meng LN, Liu YD, Wang JB, Jiang HX, Yang JH, Xu Y, Li X, Xu JM, Zhang G, Zhou XM, Zhuge YZ, Tian DA, Ye J, Liu YL. Prevalence of minimal hepatic encephalopathy and quality of life evaluations in hospitalized cirrhotic patients in China. World J Gastroenterol. 2013;19:4984-4991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 4. | Seo YS, Yim SY, Jung JY, Kim CH, Kim JD, Keum B, An H, Yim HJ, Lee HS, Kim CD, Ryu HS, Um SH. Psychometric hepatic encephalopathy score for the detection of minimal hepatic encephalopathy in Korean patients with liver cirrhosis. J Gastroenterol Hepatol. 2012;27:1695-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Mina A, Moran S, Ortiz-Olvera N, Mera R, Uribe M. Prevalence of minimal hepatic encephalopathy and quality of life in patients with decompensated cirrhosis. Hepatol Res. 2014;44:E92-E99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS, Saeian K, Verber MD, Hischke D, Hoffmann RG, Franco J, Varma RR, Rao SM. Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. Am J Gastroenterol. 2007;102:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Bajaj JS, Etemadian A, Hafeezullah M, Saeian K. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology. 2007;45:833-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, Hischke D, Hammeke TA, Pinkerton SD, Saeian K. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591-1600.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Gupta D, Ingle M, Shah K, Phadke A, Sawant P. Prospective comparative study of inhibitory control test and psychometric hepatic encephalopathy score for diagnosis and prognosis of minimal hepatic encephalopathy in cirrhotic patients in the Indian subcontinent. J Dig Dis. 2015;16:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Taneja S, Dhiman RK, Khatri A, Goyal S, Thumbru KK, Agarwal R, Duseja A, Chawla Y. Inhibitory control test for the detection of minimal hepatic encephalopathy in patients with cirrhosis of liver. J Clin Exp Hepatol. 2012;2:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Amodio P, Ridola L, Schiff S, Montagnese S, Pasquale C, Nardelli S, Pentassuglio I, Trezza M, Marzano C, Flaiban C, Angeli P, Cona G, Bisiacchi P, Gatta A, Riggio O. Improving the inhibitory control task to detect minimal hepatic encephalopathy. Gastroenterology. 2010;139:510-518, 518.e1-518.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Allampati S, Duarte-Rojo A, Thacker LR, Patidar KR, White MB, Klair JS, John B, Heuman DM, Wade JB, Flud C, O'Shea R, Gavis EA, Unser AB, Bajaj JS. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. Am J Gastroenterol. 2016;111:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 13. | Bajaj JS, Heuman DM, Sterling RK, Sanyal AJ, Siddiqui M, Matherly S, Luketic V, Stravitz RT, Fuchs M, Thacker LR, Gilles H, White MB, Unser A, Hovermale J, Gavis E, Noble NA, Wade JB. Validation of EncephalApp, Smartphone-Based Stroop Test, for the Diagnosis of Covert Hepatic Encephalopathy. Clin Gastroenterol Hepatol. 2015;13:1828-1835.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Bajaj JS, Thacker LR, Heuman DM, Fuchs M, Sterling RK, Sanyal AJ, Puri P, Siddiqui MS, Stravitz RT, Bouneva I, Luketic V, Noble N, White MB, Monteith P, Unser A, Wade JB. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology. 2013;58:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Romero-Gómez M, Córdoba J, Jover R, del Olmo JA, Ramírez M, Rey R, de Madaria E, Montoliu C, Nuñez D, Flavia M, Compañy L, Rodrigo JM, Felipo V. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Lauridsen MM, Jepsen P, Vilstrup H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: a comparative study of 154 patients with liver disease. Metab Brain Dis. 2011;26:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Metwally MA, Biomy HA, Omar MZ, Sakr AI. Critical flickering frequency test: a diagnostic tool for minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2019;31:1030-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ford JM, Gray M, Whitfield SL, Turken AU, Glover G, Faustman WO, Mathalon DH. Acquiring and inhibiting prepotent responses in schizophrenia: event-related brain potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2004;61:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Konrad K, Gauggel S, Manz A, Schöll M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD). Brain Inj. 2000;14:859-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Buckley J, Cohen JD, Kramer AF, McAuley E, Mullen SP. Cognitive control in the self-regulation of physical activity and sedentary behavior. Front Hum Neurosci. 2014;8:747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Verbruggen F, Best M, Bowditch WA, Stevens T, McLaren IP. The inhibitory control reflex. Neuropsychologia. 2014;65:263-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Goldbecker A, Weissenborn K, Hamidi Shahrezaei G, Afshar K, Rümke S, Barg-Hock H, Strassburg CP, Hecker H, Tryc AB. Comparison of the most favoured methods for the diagnosis of hepatic encephalopathy in liver transplantation candidates. Gut. 2013;62:1497-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Pawar VB, Surude RG, Sonthalia N, Zanwar V, Jain S, Contractor Q, Rathi PM. Minimal Hepatic Encephalopathy in Indians: Psychometric Hepatic Encephalopathy Score and Inhibitory Control Test for Diagnosis and Rifaximin or Lactulose for Its Reversal. J Clin Transl Hepatol. 2019;7:304-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Córdoba J, Flavià M, Jacas C, Sauleda S, Esteban JI, Vargas V, Esteban R, Guardia J. Quality of life and cognitive function in hepatitis C at different stages of liver disease. J Hepatol. 2003;39:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Sharma P, Kumar A, Singh S, Tyagi P, Kumar A. Inhibitory control test, critical flicker frequency, and psychometric tests in the diagnosis of minimal hepatic encephalopathy in cirrhosis. Saudi J Gastroenterol. 2013;19:40-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Bajaj JS, Thacker LR, Heuman DM, Sterling RK, Stravitz RT, Sanyal AJ, Luketic V, Fuchs M, Gilles HC, Wade JB. Cognitive performance as a predictor of hepatic encephalopathy in pretransplant patients with cirrhosis receiving psychoactive medications: a prospective study. Liver Transpl. 2012;18:1179-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Bie CQ, Yang DH, Tang SH, Huang W. The value of model for end-stage liver disease and Child-Turcotte-Pugh scores over time in evaluating the prognosis of patients with decompensated cirrhosis: experience in the Chinese mainland. Hepatol Res. 2009;39:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Chawla YK, Kashinath RC, Duseja A, Dhiman RK. Predicting Mortality Across a Broad Spectrum of Liver Disease-An Assessment of Model for End-Stage Liver Disease (MELD), Child-Turcotte-Pugh (CTP), and Creatinine-Modified CTP Scores. J Clin Exp Hepatol. 2011;1:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A Quality Improvement Initiative Reduces 30-Day Rate of Readmission for Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 32. | Tapper EB, Halbert B, Mellinger J. Rates of and Reasons for Hospital Readmissions in Patients With Cirrhosis: A Multistate Population-based Cohort Study. Clin Gastroenterol Hepatol. 2016;14:1181-1188.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 33. | Bajaj JS, Hafeezullah M, Hoffmann RG, Saeian K. Minimal hepatic encephalopathy: a vehicle for accidents and traffic violations. Am J Gastroenterol. 2007;102:1903-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Bajaj JS, Saeian K, Schubert CM, Hafeezullah M, Franco J, Varma RR, Gibson DP, Hoffmann RG, Stravitz RT, Heuman DM, Sterling RK, Shiffman M, Topaz A, Boyett S, Bell D, Sanyal AJ. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology. 2009;50:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 35. | Bajaj JS, Hafeezullah M, Hoffmann RG, Varma RR, Franco J, Binion DG, Hammeke TA, Saeian K. Navigation skill impairment: Another dimension of the driving difficulties in minimal hepatic encephalopathy. Hepatology. 2008;47:596-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Scarpina F, Tagini S. The Stroop Color and Word Test. Front Psychol. 2017;8:557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 802] [Article Influence: 89.1] [Reference Citation Analysis (1)] |
| 37. | Guise BJ, Thompson MD, Greve KW, Bianchini KJ, West L. Assessment of performance validity in the Stroop Color and Word Test in mild traumatic brain injury patients: a criterion-groups validation design. J Neuropsychol. 2014;8:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Park KW, Kim HS, Cheon SM, Cha JK, Kim SH, Kim JW. Dementia with Lewy Bodies versus Alzheimer's Disease and Parkinson's Disease Dementia: A Comparison of Cognitive Profiles. J Clin Neurol. 2011;7:19-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 39. | Sisco SM, Slonena E, Okun MS, Bowers D, Price CC. Parkinson's disease and the Stroop color word test: processing speed and interference algorithms. Clin Neuropsychol. 2016;30:1104-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Duarte-Rojo A, Allampati S, Thacker LR, Flud CR, Patidar KR, White MB, Klair JS, Heuman DM, Wade JB, Gavis EA, Bajaj JS. Diagnosis of covert hepatic encephalopathy: a multi-center study testing the utility of single versus combined testing. Metab Brain Dis. 2019;34:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Kuhn T, Sayegh P, Jones JD, Smith J, Sarma MK, Ragin A, Singer EJ, Albert Thomas M, Thames AD, Castellon SA, Hinkin CH. Improvements in brain and behavior following eradication of hepatitis C. J Neurovirol. 2017;23:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Hashemi F, Fathi Ashtiani A, Mirminachi B, Sharafkhah M, Ekhlasi G, Jafari E, Poustchi H. Impact of Hepatitis C Virus Infection on Cognitive Function in Patients With Covert Hepatic Encephalopathy. Hepat Mon. 2015;15:e30507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Gunn C, Mackus M, Griffin C, Munafò MR, Adams S. A systematic review of the next-day effects of heavy alcohol consumption on cognitive performance. Addiction. 2018;113:2182-2193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 44. | Amodio P, Cordoba J. Smart applications for assessing minimal hepatic encephalopathy: novelty from the app revolution. Hepatology. 2013;58:844-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Nakajima H, Motomura M, Tanaka K, Fujikawa A, Nakata R, Maeda Y, Shima T, Mukaino A, Yoshimura S, Miyazaki T, Shiraishi H, Kawakami A, Tsujino A. Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ Open. 2015;5:e007766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Häussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology. 2002;35:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 263] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Dhiman RK, Kurmi R, Thumburu KK, Venkataramarao SH, Agarwal R, Duseja A, Chawla Y. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci. 2010;55:2381-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 48. | Özel Coşkun BD, Özen M. Critical flicker frequency test for diagnosing minimal hepatic encephalopathy in patients with cirrhosis. Turk J Gastroenterol. 2017;28:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Sharma P, Sharma BC, Sarin SK. Critical flicker frequency for diagnosis and assessment of recovery from minimal hepatic encephalopathy in patients with cirrhosis. Hepatobiliary Pancreat Dis Int. 2010;9:27-32. [PubMed] |
| 50. | Torlot FJ, McPhail MJ, Taylor-Robinson SD. Meta-analysis: The diagnostic accuracy of critical flicker frequency in minimal hepatic encephalopathy. Aliment Pharmacol Ther. 2013;37:527-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Kircheis G, Hilger N, Häussinger D. Value of critical flicker frequency and psychometric hepatic encephalopathy score in diagnosis of low-grade hepatic encephalopathy. Gastroenterology. 2014;146:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Ampuero J, Simón M, Montoliú C, Jover R, Serra MÁ, Córdoba J, Romero-Gómez M. Minimal hepatic encephalopathy and critical flicker frequency are associated with survival of patients with cirrhosis. Gastroenterology. 2015;149:1483-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 53. | Berlioux P, Robic MA, Poirson H, Métivier S, Otal P, Barret C, Lopez F, Péron JM, Vinel JP, Bureau C. Pre-transjugular intrahepatic portosystemic shunts (TIPS) prediction of post-TIPS overt hepatic encephalopathy: the critical flicker frequency is more accurate than psychometric tests. Hepatology. 2014;59:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Suresh MV, Jagadisan B, Kandasamy P, Senthilkumar GP. Stroop Test Validation to Screen for Minimal Hepatic Encephalopathy in Pediatric Extrahepatic Portal Venous Obstruction. J Pediatr Gastroenterol Nutr. 2018;66:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 55. | Wuensch T, Ruether DF, Zöllner C, Mueller T, Jung T, Kaffarnik M, Kassner U, Schott E, Kiefer S, Pratschke J, Stockmann M, Jara M. Performance characterization of a novel electronic number connection test to detect minimal hepatic encephalopathy in cirrhotic patients. Eur J Gastroenterol Hepatol. 2017;29:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Collerton J, Collerton D, Arai Y, Barrass K, Eccles M, Jagger C, McKeith I, Saxby BK, Kirkwood T; Newcastle 85+ Study Core Team. A comparison of computerized and pencil-and-paper tasks in assessing cognitive function in community-dwelling older people in the Newcastle 85+ Pilot Study. J Am Geriatr Soc. 2007;55:1630-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Mardini H, Saxby BK, Record CO. Computerized psychometric testing in minimal encephalopathy and modulation by nitrogen challenge and liver transplant. Gastroenterology. 2008;135:1582-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Wesnes KA, McKeith IG, Ferrara R, Emre M, Del Ser T, Spano PF, Cicin-Sain A, Anand R, Spiegel R. Effects of rivastigmine on cognitive function in dementia with lewy bodies: a randomised placebo-controlled international study using the cognitive drug research computerised assessment system. Dement Geriatr Cogn Disord. 2002;13:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21:623-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 607] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 60. | Prakash R, Chikkanna R, Anna K, Karanth P, Allampati SK, Theethira TG, Dosanjh G, Perzynski AT, Mullen KD. Sa1039 USA validated computerized psychometric testing system for the diagnosis of minimal hepatic encephalopathy - at last. Gastroenterology. 2012;142:S-952. [DOI] [Full Text] |
| 61. | Allen BJ, Gfeller JD. The Immediate Post-Concussion Assessment and Cognitive Testing battery and traditional neuropsychological measures: a construct and concurrent validity study. Brain Inj. 2011;25:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Tsushima M, Tsushima W, Tsushima V, Lim N, Madrigal E, Jackson C, Mendler MH. Use of ImPACT to diagnose minimal hepatic encephalopathy: an accurate, practical, user-friendly internet-based neuropsychological test battery. Dig Dis Sci. 2013;58:2673-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |