Published online Jul 6, 2020. doi: 10.12998/wjcc.v8.i13.2758
Peer-review started: November 13, 2019
First decision: March 15, 2020
Revised: May 14, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: July 6, 2020
Processing time: 236 Days and 14.9 Hours
Graft hepatocellular carcinoma (HCC) recurrence after liver transplant is more frequently encountered. Graft hepatectomy is technically challenging and is associated with high morbidity. Stereotactic body radiation therapy (SBRT) has been shown to be safe and effective for the treatment of primary HCC. However, its role in HCC recurrence in a liver graft remains unclear.
To evaluate the safety and efficacy of SBRT for the treatment of graft HCC recurrence after liver transplantation.
A retrospective study was conducted. From 2012 to 2018, 6 patients with intrahepatic HCC recurrence after liver transplant were treated with SBRT at Queen Mary Hospital, the University of Hong Kong. The primary outcome was time to overall disease progression and secondary outcomes were time to local progression and best local response, as assessed with the Modified response Evaluation Criteria for Solid Tumours criteria. Patients were monitored for treatment related toxicities and graft dysfunction.
A total of 9 treatment courses were given for 13 tumours. The median tumour size was 2.3 cm (range 0.7-3.6 cm). Two (22%) patients had inferior vena cava tumour thrombus. The best local treatment response was: 5 (55%) complete response, 1 (11%) partial response and 3 (33%) stable disease. After a median follow up duration of 15.5 mo, no local progression or mortality was yet observed. The median time to overall disease progression was 6.5 mo. There were 6 regional progression in the liver graft (67%) and 2 distant progression in the lung (22%). There was no grade 3 or above toxicity and there was no graft dysfunction after SBRT.
SBRT appears to be safe in this context. Regional progression is the mode of failure.
Core tip: From 2012 to 2018, 6 patients with intrahepatic hepatocellular carcinoma recurrence after liver transplant were treated with stereotactic body radiation therapy at Queen Mary Hospital, the University of Hong Kong. A total of 9 treatment courses were given for 13 tumours. The median tumour size was 2.3 cm (range 0.7-3.6 cm). Two (22%) patients had inferior vena cava tumour thrombus. Five patients had complete local response (55%). The median time to overall disease progression was 6.5 mo. There were 6 regional progression in the liver graft (67%). There was no grade 3 or above toxicity. Stereotactic body radiation therapy appears safe but regional progression is common.
- Citation: Au KP, Chiang CL, Chan ACY, Cheung TT, Lo CM, Chok KSH. Initial experience with stereotactic body radiotherapy for intrahepatic hepatocellular carcinoma recurrence after liver transplantation. World J Clin Cases 2020; 8(13): 2758-2768
- URL: https://www.wjgnet.com/2307-8960/full/v8/i13/2758.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i13.2758
Since the implementation of the model for end-stage liver disease allocation system, patients enlisted for hepatocellular carcinoma (HCC) have been given increased priority for cadaveric grafts[1]. Adoption of extended criteria also largely expanded the recipient pool[2-5]. With increasing numbers of liver transplants performed for HCC, recurrence is more frequently encountered[6]. One-third of post-transplant recurrence is confined to the liver graft[7]. In this context graft hepatectomy offers chance of cure, but is technically challenging due to hostile adhesions surrounding vital portal structures. Infective complications are not uncommon after such ultra-major operation in an immunocompromised host[8,9]. The demand for a safe and effective treatment modality is desperate.
Stereotactic body radiation therapy (SBRT), a precise delivery of conformal external beam irradiation, has been shown to be safe and effective for the treatment of primary HCC[10]. SBRT has become an appealing alternative to surgery in patients with inadequate liver function. However, the role of SBRT for HCC recurrence in a liver graft remains unclear. There is no literature to report the oncological benefits and the potential toxicity to the liver graft. Therefore, the current study is proposed to evaluate the safety and efficacy of SBRT for the treatment of intrahepatic HCC recurrence after liver transplantation.
A retrospective study was conducted at Queen Mary Hospital, the University of Hong Kong. Queen Mary Hospital was the tertiary referral centre and the only liver transplant centre in Hong Kong. All consecutive patients who received SBRT for recurrent HCC in the transplanted liver at this centre between 2012 and 2018 were included. A radiological diagnosis of HCC recurrence in the liver graft was made based on the typical enhancement pattern according to the dynamic imaging criteria[11]. The treatment decisions were discussed in a multidisciplinary tumour board among hepatobiliary and transplant surgeons, transplant hepatologists, radiation oncologists and medical oncologists.
Eligibility to SBRT were defined by: an Eastern Cooperative Oncology Group performance status ≤ 2[12]; uninvolved liver graft volume > 700 mL; adequate graft function i.e., international normalized ratio (INR) < 1.7, alanine aminotransferase and aspartate aminotransferase < 2.5 times upper limit of normal, with no ascites or hepatic encephalopathy and adequate renal function with creatinine < 1.5 times upper limit of normal, the number of tumour was limited to 5.
Tumour location and volume were assessed with contrast computed tomography scan. The volume of uninvolved liver graft and other organs at risk were also assessed. Four-dimensional images were acquired using Philips Bellows DeviceTM (Philips Medical Systems, Cleveland, OH, United States) to synchronize respiratory motion. Gross tumor volume is defined as HCC focus that is visualized on contrast imaging. Individualized margin will be added to gross tumor volume to form the planning target volume to compensate for respiratory motions. We prescribe the dose according to Radiation Therapy Oncology Group 1112 protocol[13]. Stereotactic planning was performed to minimize collateral radiation to the surrounding organs-at risk including the normal liver graft, esophagus, heart, stomach, duodenum, small bowel, large bowel, kidneys, gallbladder, common bile duct, and spinal cord. The final dose is determined such that a maximum tumoricidal dose can be delivered to tumors while respecting the tolerance dose of organs-at-risk. Dose prescription was based on the volume of normal tissue irradiated and the volume of the target. A total dose of 20 to 50 Gy separated in 5 to 6 fractions were given over 5 to 14 d. Photon beam was delivered with respiratory gating to adjust for ventilatory movements. 6MeV photon beam was usually used while 10 MeV beam was used in selected patients for deeper penetration and better dose homogeneity.
Data was retrieved from a prospectively collected database. Patients were followed-up regularly by the radiation oncologists and transplant surgeons for monitoring graft function, adverse event and treatment response. Blood test was performed for complete blood count, renal and liver function, coagulation studies and alpha-fetoprotein at 2, 4, 8, 12, 26 and 52 wk after SBRT. Surveillance imaging was carried out every 3 to 6 mo with contrast computed tomography or primovist enhanced magnetic resonance imaging. Treatment response were evaluated according to the Modified response Evaluation Criteria for Solid Tumours criteria[14]. Treatment response of the index lesion was graded as complete response, partial response, stable disease or progressive disease. Toxicity was graded with the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0[15].
The primary outcome was time to progression, defined as the time between SBRT and the first imaging indicating disease progression. The patterns of disease progression included local, regional and distant. Local progression of the index lesion was defined according to Modified response Evaluation Criteria for Solid Tumours criteria. Regional progression was defined as intrahepatic disease progression completely distinct from the index lesion. Distinct progression referred to progression outside the liver. Continuous variables were presented as median and range. Survivals were studied with Kaplan-Meier method. Data was analysed with Statistical Package for the Social Sciences 16.0 (SPSS) for Windows (SPSS Inc., Chicago, IL, United States).
Twenty-three patients were diagnosed with intrahepatic HCC recurrence after liver transplantation. Six of them received SBRT (Table 1). Four (67%) of them were transplanted with deceased whole graft while 2 (33%) received a right lobe graft from a living donor. In 4 patients, the HCC before transplant were within the University of California San Francisco criteria[16]. A total of 9 courses of radiotherapy were given for 13 tumours. Two patients were irradiated more than once for metachronous recurrence (No. 1 and 2). The median age at the time of SBRT was 59 (range 31-67) years (Table 2). All patients had an Eastern Cooperative Oncology Group performance status ≤ 1. The median time to recurrence of the index tumour was 66.5 (range 1.2-86.7) mo. All patients had normal liver function with no evidence of graft cirrhosis. One patient was warfarinized for tumour thrombus in the inferior vena cava (IVC) and his INR was 1.5. The INR of the remaining patients were ≤ 1.2. The level of serum bilirubin and albumin were all within normal ranges.
| No. | Sex | Age | Index recurrence | Distant recurrence | Systemic therapy | Regional therapy | SBRT (dose/fraction) | Best local response | Disease progression | Time to progression (mo) | Follow up time | Toxicity/Grade | |||||
| Time after transplant (mo) | First | No. | Size (cm) | Location | From first recurrence (mo) | From SBRT (mo) | |||||||||||
| Recurrence | |||||||||||||||||
| 1 | M | 36 | 58.2 | No | 1 | 2.4 | S7 | No | SRL | No | 50 Gy/5 | CR | Regional/distant | 6.5 | 132 | 84.2 | Dyspepsia/1 |
| 37 | 66.5 | No | 1 | 1.9 | S6 | Lung | SRL/SECOX | No | 50 Gy/5 | CR | Distant | 10.9 | |||||
| 2 | M | 66 | 69.4 | Yes | 2 | 3.6 | S8 | No | EVL | No | 45 Gy/5 | PR | Regional | 9.5 | 31.3 | 28 | |
| 3.1 | S7/IVC thrombus | ||||||||||||||||
| 66 | 81.8 | No | 2 | 2 | S8 | No | EVL | No | 40 Gy/5 | SD | Regional | 3.3 | |||||
| 0.7 | S7 | ||||||||||||||||
| 67 | 86.7 | No | 1 | 1.2 | S6 | No | EVL | No | 40 Gy/5 | SD | Regional | 3.2 | |||||
| 3 | M | 60 | 12.5 | Yes | 2 | 3.3 | S7/IVC thrombus | No | EVL/Sorafenib | No | 37.5 Gy/5 | CR | Regional | 10.3 | 19 | 15.5 | Dyspepsia/1 |
| 2.3 | S6 | ||||||||||||||||
| 4 | M | 63 | 24.5 | Yes | 1 | 2.8 | S3 | No | EVL | No | 37.5 Gy/5 | SD | Regional | 3.3 | 9 | 6.4 | Gastric ulcer/2 |
| 5 | M | 57 | 1.2 | Yes | 1 | 1 | Portal LN | No | EVL/Lenvatinib | No | 45 Gy/5 | CR | No | - | 6.9 | 6.3 | |
| 6 | M | 58 | 75.8 | No | 1 | 2.5 | S5 | No | EVL | TACE | 50 Gy/5 | CR | No | - | 30.8 | 1.8 | Diarrhoea/1 |
| Range (%) | |
| Age | 59 (31-66) |
| Gender (male), n (%) | 6 (100) |
| Type of graft | |
| Whole | 4 (67) |
| Right lobe | 2 (33) |
| Within UCSF, n (%) | 4 (67) |
| Time to first recurrence (mo) | 18.5 (1.2-69.4) |
| Time to index recurrence (mo) | 66.5 (1.2-86.7) |
| ECOG | 1 (0-1) |
| Bilirubin (umol/L) | 9 (4-23) |
| Albumin (g/L) | 43 (39-47) |
| ALT (U/L) | 28 (19-44) |
| AST (U/L) | 33 (18-58) |
| INR | 1.0 (1.0-1.5)1 |
| Platelet (× 109/L) | 124 (43-255) |
| Creatinine (umol/L) | 107 (78-121) |
| Index lesion as first recurrence, n (%) | 4 (44) |
| Number of index lesions | 1 (1-2) |
| Tumour size (cm) | 2.3 (0.7-3.6) |
| IVC invasion, n (%) | 2 (22) |
| Distant recurrence, n (%) | 1 (11) |
| AFP (ng/mL) | 4 (2-1354) |
| Prescribed dose (Gy) | 45 (37.5-50) |
| Number of fractions | 5 (5-6) |
| Dose per fraction (Gy) | 8 (7.5-10) |
| Treatment duration (d) | 5 (5-14) |
| Concomitant sysetmic treatment, n (%) | 6 (100) |
Four (44%) of the index lesions were the first-time recurrence after liver transplantation. Although the selection criteria were less than or equal to 5 tumours, most patients in our series had a solitary recurrence (range 1-2). The clinician could have selected more favourable tumours as we had limited experience for liver graft irradiation. The median tumour size was 2.3 cm (range 0.7-3.6 cm). One (11%) patient has synchronous pulmonary metastasis managed with concurrent SBRT to the lung. Two (22%) patients had IVC tumour thrombus. There was no portal venous invasion in this series. The serum level of alpha-fetoprotein ranged from 2 to 1354 ng/mL. The median treatment dose to tumour was 45 (range 37.5-50) Gy. Irradiation was given over 5-6 fractions of a median 8 (range 7.5-10) Gy. All patients had concomitant systemic treatment during the study period (Table 1). All of them received mammalian target of rapamycin inhibitor (sirolimus or everolimus) as immunosuppression. Two patients were treated with sorafenib: one as single agent (No. 3) and the other (No. 1) combined with capecitabine and oxaliplatin (SECOX). One patient (No. 5) received lenvatinib upon completion of SBRT. One patient (No. 6) received trans-arterial chemoembolization (TACE) while awaiting SBRT.
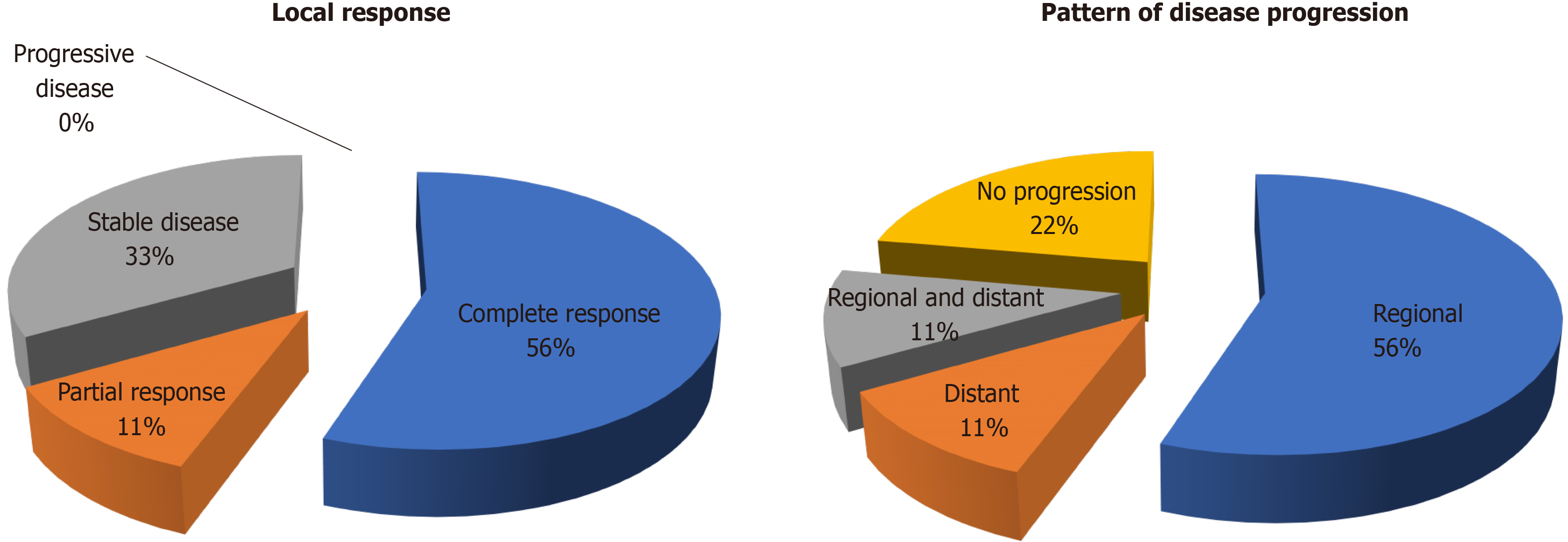
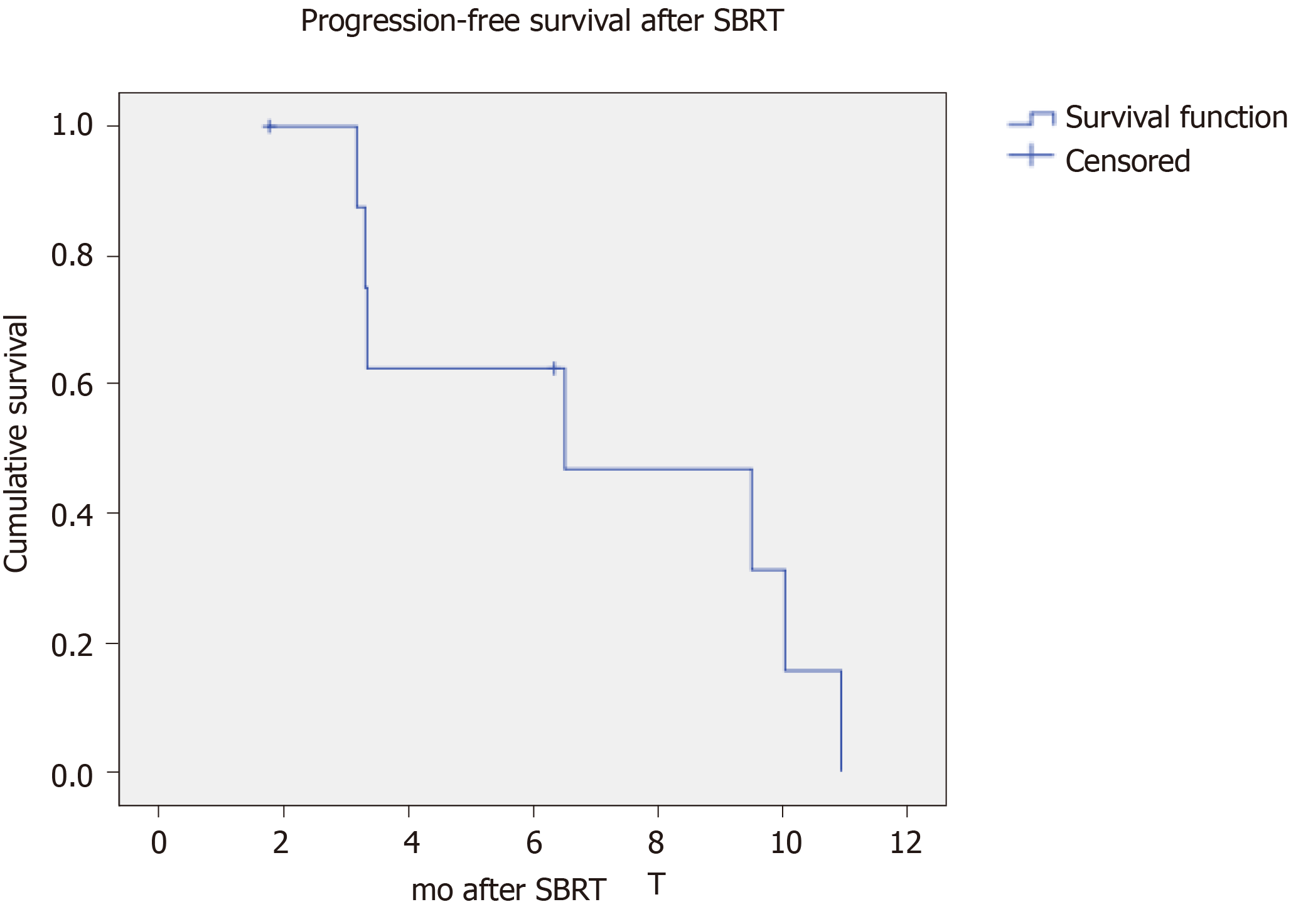
The best local treatment response after SBRT was: 5 (55%) complete response, 1 (11%) partial response and 3 (33%) stable disease (Figure 1A). The median follow-up duration after SBRT were 15.5 mo from SBRT and 24.9 mo from the first recurrence. No local progression or mortality was yet observed. There were 7 disease progressions (78%, including 5 regionals i.e., in the liver graft (56%), 1 distant in the lung (11%) and 1 concurrent in liver and lung (11%) (Figure 1B). The median time to overall disease progression was 6.5 mo (Figure 2). All patients were surviving at the time of writing.
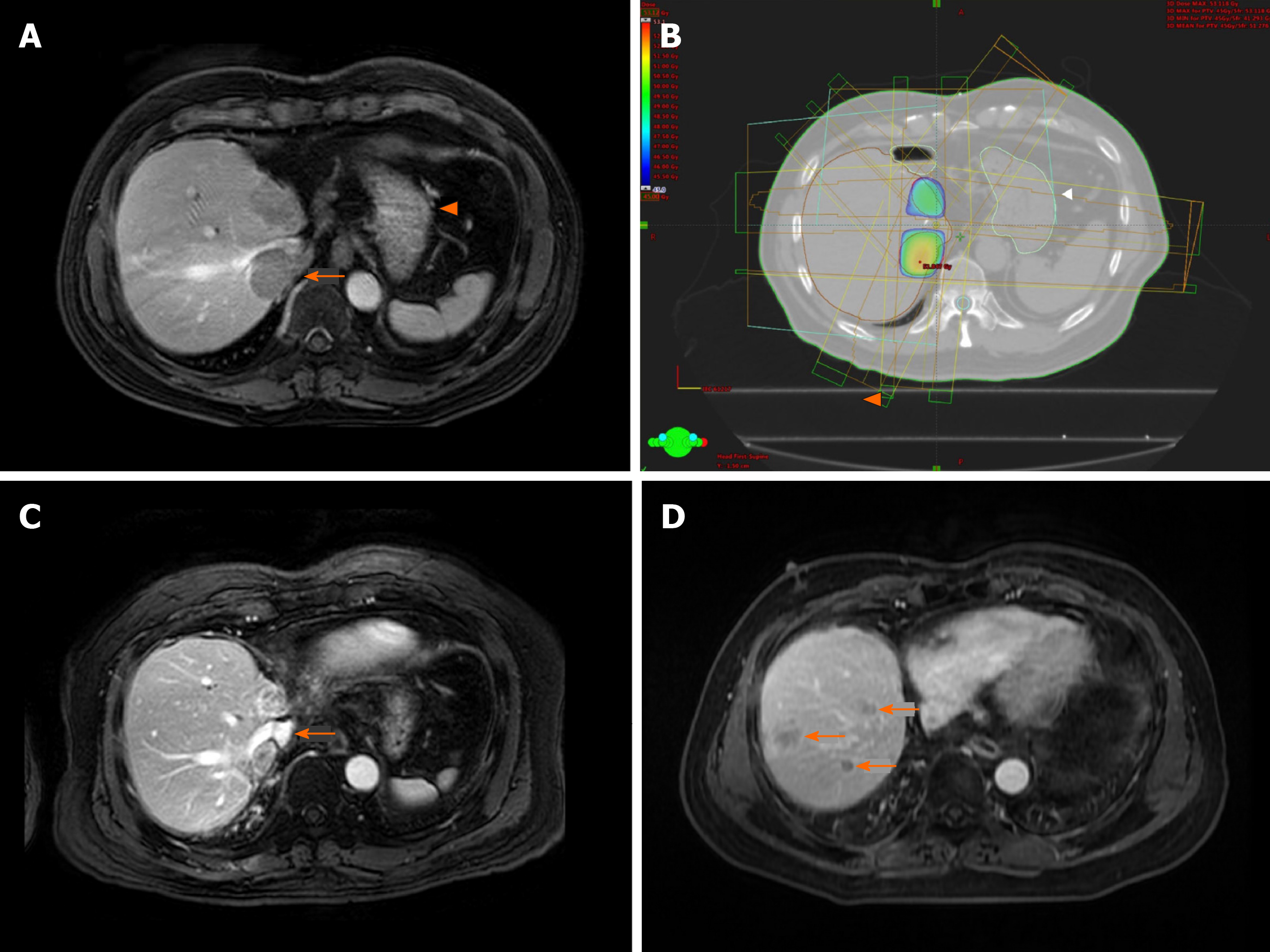
The representative images of patient No. 2 were shown in Figure 3. He received a total of 3 courses of SBRT for repeated intrahepatic recurrence after right lobe liver transplant. There were 2 tumours upon initial recurrence, one at S8 and one at S7 extending into the IVC (Figure 3A). Stereotactic irradiation was performed to both tumours while minimizing collateral radiation to the normal liver graft and stomach (Figure 3B). The irradiated tumours showed partial response with re-cannulation of the IVC (Figure 3C). Subsequent regional recurrences were treated with further courses of SBRT. Eventually multifocal intrahepatic recurrence developed (Figure 3D), and the treatment was converted to TACE.
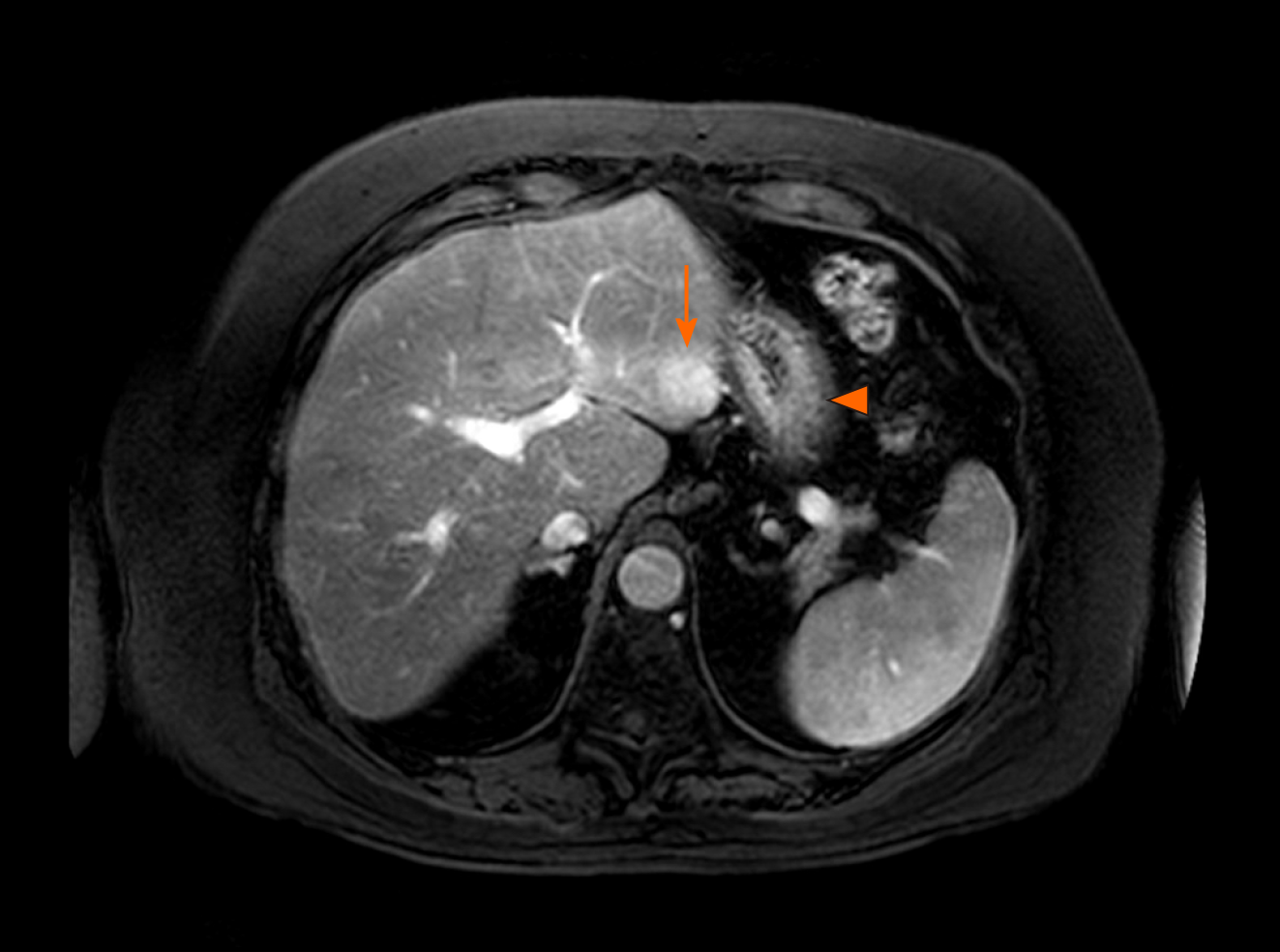
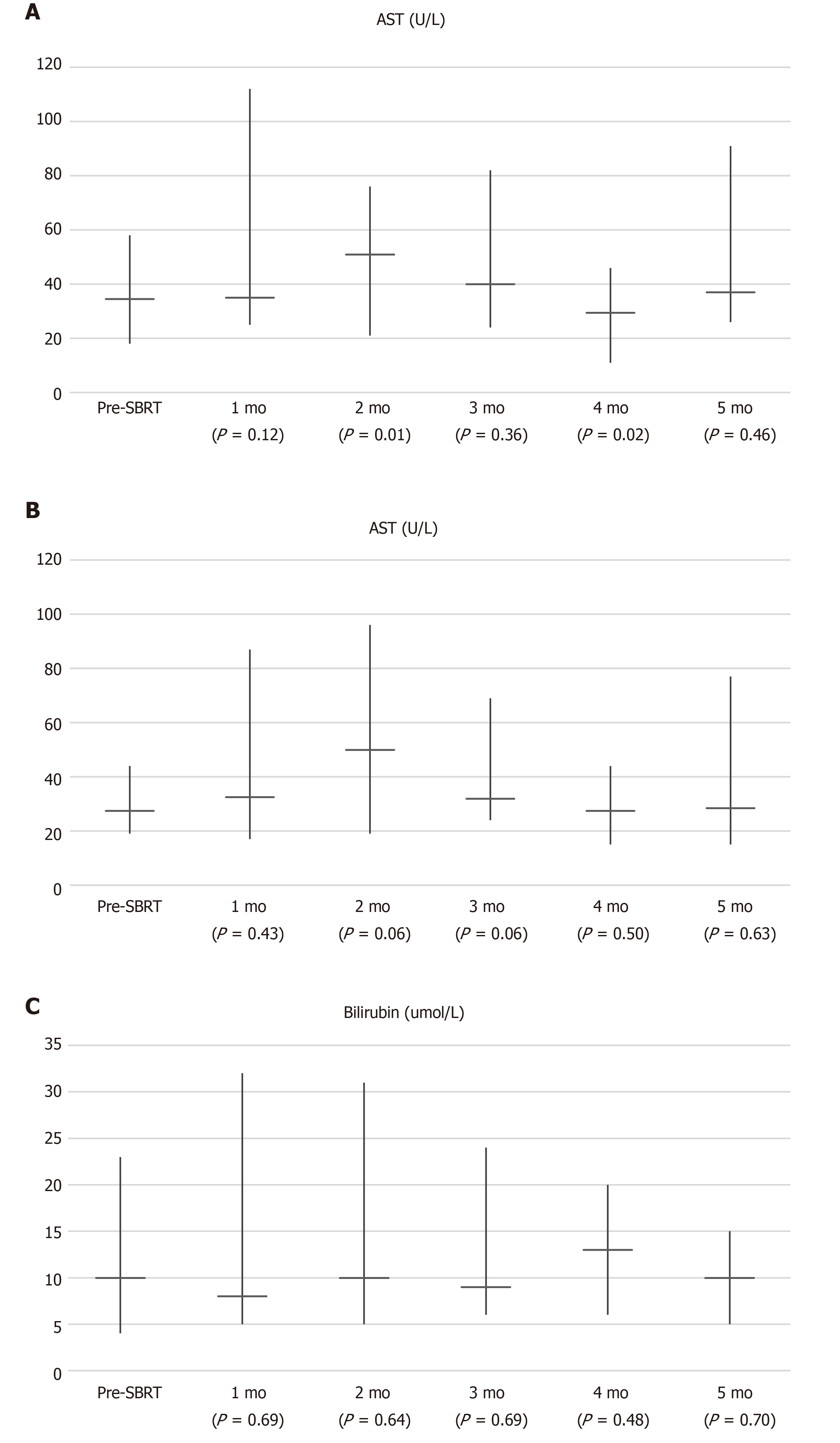
Four adverse events (graded 1-2) were observed and they were related to the gastrointestinal system (Table 1). One patient suffered from gastric ulcer after irradiation of segment III recurrence in close proximity to stomach (Figure 4). He required oral proton pump inhibitor therapy. Two other had dyspepsia while one suffered from diarrhoea which was self-limiting. There was no grade 3 or above toxicity. No graft dysfunction occurred after SBRT. Figure 5 showed the liver function parameters after SBRT. SBRT appeared to be associated with transient and self-limiting elevation of aspartate transaminase (P = 0.01) and alanine transaminase (P = 0.06) at 2 mo after the treatment session. There was no significant change in levels of bilirubin after SBRT.
The current series demonstrated that SBRT for post-transplant intrahepatic HCC recurrence conferred effective local control without adversely affecting graft function. Complete response was achieved in majority of the patients and the median time to local progression has yet been achieved after 15.5 mo, in the presence of two locally advanced tumour i.e., with IVC tumour thrombi. Regional progression was the mode of failure, which occurred commonly and shortly after SBRT. Disease progression occurred over a median of half year, with two-third recurring in the other parts of the liver. These results affirm the safety and local efficacy of SBRT but call for the necessity of additional regional control.
For primary HCC, regional and distant progression occurred in 19% and 11% of patients respectively after SBRT[10]. The treatment outcomes for post-transplant recurrence compared unfavourably especially in terms of regional progression (67%). The disease nature itself is held responsible. Post-transplant HCC recurrence is, by definition, metastatic disease from the native liver. Even isolated intrahepatic recurrence represents a local phenomenon of an ongoing systemic event, and should be managed with a combination of systemic and loco-regional treatment[17].
Apart from SBRT, all patients in our series received systemic anti-tumour therapy with at least a mammalian target of rapamycin inhibitor (Table 1). However, this study revealed a significant shortcoming of SBRT and systemic therapy combination in this context. Regional control is inadequate. Given the effectiveness of radiotherapy in local control, it may appear sensible to expand the irradiation field to cover the remaining liver graft. However, historical experience in whole liver irradiation has shown that the liver has a relatively limited tolerance[18]. Several attempts by the Radiation Therapy Oncology Group have failed to establish a safe and effective dose for whole liver irradiation[19,20]. When combined with conformal irradiation to the tumour, the risk of radiation induced liver disease is expected to be further amplified. Perhaps hepatic disease can be better handled with regional chemotherapy. Intra-arterial delivery of platinum drug carried in an emulsion with lipiodol via TACE is a well-established treatment modality for primary HCC[21]. The chemotherapeutic effect is further enhanced by gelfoam embolization, which induces tumour necrosis. The efficacy of TACE for post-transplant recurrence has been addressed a prospective case-control study consisting 28 patients[22]. The best outcome was partial response, which was elicited in 57% of the patients. The local control compared inferiorly to SBRT in our series (55% complete response and 11% partial response). Nevertheless, improved overall survival was reported compared to no chemoembolization (1-year survival 86% vs 50%, P = 0.01), acknowledging the oncological benefits of regional therapy.
Based on these results, we look forward to a combination treatment of SBRT with TACE. Their respective roles in local and regional control potentially complement each other. Of course, concomitant systemic therapy is of utmost importance and should not be omitted. In fact, the last patient in our series (No. 6) received this combination. He was treated with everolimus and TACE, followed by SBRT at 6-wk interval. The oncological outcomes cannot be ascertained at this juncture due to limited follow up. This would be answered by future studies with larger sample size and longer follow up time. SBRT/TACE combination has been shown to be safe in patients with cirrhosis and primary HCC[23]. In a transplanted liver, the additional concerns are graft toxicities and biliary complications. So far, no graft failure or biliary complications have been reported[22]. Nevertheless, pre-existing biliary complications are found in 20% of transplant recipients[24]. Whether SBRT/TACE combination could benefit patients with graft HCC recurrence remains to be answered by future studies.
In this study, patients were selected for SBRT based on limited disease burden i.e., oligo-recurrence, technical feasibility and adequate graft function. Oligo-recurrence describes recurrent disease limited in number and location, so that loco-regional treatment confer survival benefits[25]. All patients were surviving after a median follow up duration was 24.9 mo. One patient (No. 1) survived for more than 10 years after developing recurrence. This was encouraging, considering the disease was metastatic in nature. This was a result of repeated courses of loco-regional treatment combined with multiple lines of systemic therapy, in a highly selected patient cohort. Though long-term survival deems possible, the million-dollar question remains. Not until more patients have been managed can we predict who will benefit from a more aggressive approach.
The current study is limited by its retrospective and descriptive nature. Sample size and the follow up duration were limited. Nevertheless, this is the first series in the literature to report the efficacy and safety regarding SBRT for graft HCC recurrence after liver transplantation. We have revealed a significant shortcoming of SBRT in this cohort to guide future studies.
Graft hepatocellular carcinoma (HCC) recurrence after liver transplant is not uncommon. Graft hepatectomy is technically challenging and is associated with high morbidity. Stereotactic body radiation therapy (SBRT) could be a safe alternative treatment modality to graft HCC recurrence.
The role of SBRT for HCC recurrence in a liver graft remains unclear.
The current study aims to evaluate the safety and efficacy of SBRT for graft HCC recurrence after liver transplantation.
A retrospective study of 6 patients and 9 treatment courses for 13 recurrent tumours in the liver graft.
Five patient had complete local response (55%). The median time to overall disease progression was 6.5 mo. There were 6 regional progression in the liver graft (67%). There was no grade 3 or above toxicity.
SBRT appears safe but regional progression is common.
The role of SBRT combined with additional regional treatment could be explored.
| 1. | Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127:S261-S267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 198] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1714] [Article Influence: 68.6] [Reference Citation Analysis (1)] |
| 3. | Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Silva M, Moya A, Berenguer M, Sanjuan F, López-Andujar R, Pareja E, Torres-Quevedo R, Aguilera V, Montalva E, De Juan M, Mattos A, Prieto M, Mir J. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl. 2008;14:1449-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 380] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 6. | Yao FY, Bass NM, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: lessons from the first year under the Model of End-Stage Liver Disease (MELD) organ allocation policy. Liver Transpl. 2004;10:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | de'Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J Gastroenterol. 2015;21:11185-11198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Sommacale D, Dondero F, Sauvanet A, Francoz C, Durand F, Farges O, Kianmanesh R, Belghiti J. Liver resection in transplanted patients: a single-center Western experience. Transplant Proc. 2013;45:2726-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Chok KSh. Management of recurrent hepatocellular carcinoma after liver transplant. World J Hepatol. 2015;7:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447-e453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 11. | Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, Massironi S, Della Corte C, Ronchi G, Rumi MG, Biondetti P, Colombo M. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7038] [Cited by in RCA: 8241] [Article Influence: 191.7] [Reference Citation Analysis (0)] |
| 13. | Dawson LA, Hospital PM, Zhu A, Knox J, Krishnan S, Craig T, Guha C, Kachnic L, Gillin MT, Hong TS, Winter K. Radiation Therapy Oncology Group Rtog 1112 Randomized Phase Iii Study Of Sorafenib Versus Stereotactic Body Radiation Therapy Followed By Sorafenib In Hepatocellular Carcinoma. Available from: https://www.rtog.org/Portals/0/RTOG%20Broadcasts/Attachments/1112_master_w_update_5.7.13.pdf. |
| 14. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3427] [Article Influence: 214.2] [Reference Citation Analysis (43)] |
| 15. | National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. |
| 16. | Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 264] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 17. | Au KP, Chok KSH. Multidisciplinary approach for post-liver transplant recurrence of hepatocellular carcinoma: A proposed management algorithm. World J Gastroenterol. 2018;24:5081-5094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, Ten Haken RK. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 540] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 19. | Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3227] [Cited by in RCA: 3113] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 20. | Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 118] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (32)] |
| 22. | Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, Zeng XC. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Wong TC, Chiang CL, Lee AS, Lee VH, Yeung CS, Ho CH, Cheung TT, Ng KK, Chok SH, Chan AC, Dai WC, Wong FC, Luk MY, Leung TW, Lo CM. Better survival after stereotactic body radiation therapy following transarterial chemoembolization in nonresectable hepatocellular carcinoma: A propensity score matched analysis. Surg Oncol. 2019;28:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Chok KS, Chan SC, Cheung TT, Sharr WW, Chan AC, Lo CM, Fan ST. Bile duct anastomotic stricture after adult-to-adult right lobe living donor liver transplantation. Liver Transpl. 2011;17:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1491] [Cited by in RCA: 1964] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Sugawara Y, Takaki A, Tomizawa M S-Editor: Dou Y L-Editor: A E-Editor: Xing YX