Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2542
Peer-review started: February 24, 2020
First decision: March 27, 2020
Revised: April 1, 2020
Accepted: June 2, 2020
Article in press: June 2, 2020
Published online: June 26, 2020
Processing time: 120 Days and 21.3 Hours
The anthracycline chemotherapeutic drugs are cardiotoxic. Studies have found some indicators related to cardiotoxicity. However, there is currently no accurate indicator that can predict cardiac toxicity early.
To explore the diagnostic value of real-time three-dimensional echocardiography (RT3DE) in predicting cardiac toxicity in breast cancer patients undergoing chemotherapy.
Female breast cancer patients who underwent radical mastectomy and postoperative chemotherapy at the Affiliated Hanzhou First People’s Hospital, Zhejiang University School of Medicine were recruited. All patients were routinely administered with chemotherapy for four cycles (T1-T4) after surgery. Two-dimensional (2D) echocardiography, RT3DE, and serological examinations were performed after each cycle of chemotherapy. Patients were divided into a toxic group and a non-toxic group based on whether patients had Δ left ventricular ejection fraction > 10% after one year of chemotherapy. Repeated measurement analysis of variance was used to compare the changes in 2D echocardiographic indicators, serological indicators, and RT3DE indicators before and after chemotherapy. Multivariate logistic regression was used to identify independent predictive indicators for cardiac toxicity in postoperative chemotherapy patients. Receiver operating characteristics (ROC) curve analysis was performed to analyze the diagnostic value of potential indicators in the diagnosis of cardiotoxicity.
A total of 107 female breast cancer patients were included in the study. T4 maximum peak velocity in early diastole (E peak)/mitral annulus lateral tissue Doppler (e' peak) (E/e'), serological indicators [T4 cardiac troponin I (cTnI) and T4 pro-brain natriuretic peptide (Pro-BNP)], T3 minimum left atrial volume (LAV), T4 LAVmin, T3 LAV before the start of the P wave (LAVprep), and T4 LAVprep in the toxicity group were significantly higher than those in the non-toxic group. Multivariate logistic regression found that T4 cTnI, T4 Pro-BNP, T3 LAVmin, T4 LAVmin, T3 LAVprep, and T4 LAVprep had potential predictive value for cardiac toxicity (P < 0.05). ROC results showed that T4 LAVmin had the highest accuracy for diagnosing cardiac toxicity [area under the curve (AUC) = 0.947; sensitivity = 78.57%; specificity = 94.62%], followed by T4 LAVprep (AUC = 0.899; sensitivity = 100%; specificity = 66.67%). The accuracies of LAVprep and LAVprep in predicting cardiac toxicity were higher than those of T3 LAVmin and T3 LAVprep.
RT3DE of left atrial volume can be used to predict the cardiotoxicity caused by chemotherapy, and it is expected to guide the clinical adjustment of dose and schedule in time.
Core tip: Although chemotherapeutics can improve the curative effect of breast cancer, there is a certain degree of cardiotoxicity. The patient's cardiac function needs to be closely monitored during chemotherapy. Studies have found that left ventricular diastolic dysfunction can indicate early cardiac impairment. Therefore, the analysis of left ventricular diastolic function to evaluate the cardiac toxicity is the research focus. However, the current indicators can only prove that they are related to cardiotoxicity, but they cannot early predict the occurrence of cardiotoxicity. In this study, RT3DE was used to measure the left atrial volume, and it was found that the left atrial volume index was the most accurate in predicting the cardiotoxicity caused by chemotherapy in the early stage.
- Citation: Zhou F, Niu L, Zhao M, Ni WX, Liu J. Real-time three-dimensional echocardiography predicts cardiotoxicity induced by postoperative chemotherapy in breast cancer patients. World J Clin Cases 2020; 8(12): 2542-2553
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2542
Anthracyclines-based combined chemotherapy can improve the surgical outcome of patients with breast cancer and prolong their lifetime. The anthracycline chemotherapeutic drugs are cardiotoxic, and excessive use will lead to left ventricular diastolic dysfunction[1,2]. The impaired diastolic function can cause systolic dysfunction, and in severe cases, it will cause progressive and irreversible heart damage, which significantly affects the prognosis of breast cancer patients. Therefore, it is of great significance to evaluate the cardiac function in time to reduce the adverse effects of chemotherapeutic drugs on the heart. The expert consensus of European Association of Cardiovascular Imaging has pointed out that a reduction of left ventricular ejection fraction (LVEF) of more than 10% can be used as a cutoff value for the diagnosis of cardiac toxicity related to chemotherapy[3]. However, LVEF is not sensitive as an indicator for the early diagnosis of cardiac toxicity, which is not helpful for the early detection of the negative impact of chemotherapy drugs on patients' hearts. Left ventricular diastolic dysfunction usually occurs before systolic dysfunction. It is popular recently for detecting changes in left ventricular diastolic function to evaluate the cardiotoxicity caused by chemotherapy drugs. There are some methods to assess the left ventricular diastolic function, including echocardiographic indicators, two-dimensional (2D) ultrasound speckle tracking imaging, serum pro-B type natriuretic peptide (pro-BNP), and cardiac troponin. However, all these indicators have their shortcomings in the diastolic function assessment[4-7]. New detection indicators are needed for a more accurate assessment of left ventricular diastolic function.
It is known that the left atrial volume (LAV) reveals the extent and duration of left ventricular diastolic perfusion pressure elevation. The American Echocardiographic Association guidelines suggest that LAV can be used for predicting impaired cardiac function[8]. To date, real-time three-dimensional echocardiography (RT3DE) is feasible for measuring the irregular volume of the left atrium. RT3DE can independently observe the movements and volume changes of the left atrium[9-11]. A study by Hatipoglu et al[12] has shown that minimum LAV (LAVmin) and maximum LAV (LAVmax) are correlated with left ventricular diastolic blood pressure measured using cardiac catheters. Yamano et al[13] found that different degrees of left ventricular diastolic dysfunction were related to the LAV index. However, these studies only focus on the relationship between LAV and left ventricular diastolic function. There are few studies analyzing the value of LAV in early predicting cardiac toxicity during chemotherapy.
The aim of this study was to preliminarily analyze the value of RT3DE-related indicators for early prediction of cardiac toxicity caused by chemotherapy among breast cancer patients, with a view to providing information for the adjustment of clinical medication schedule.
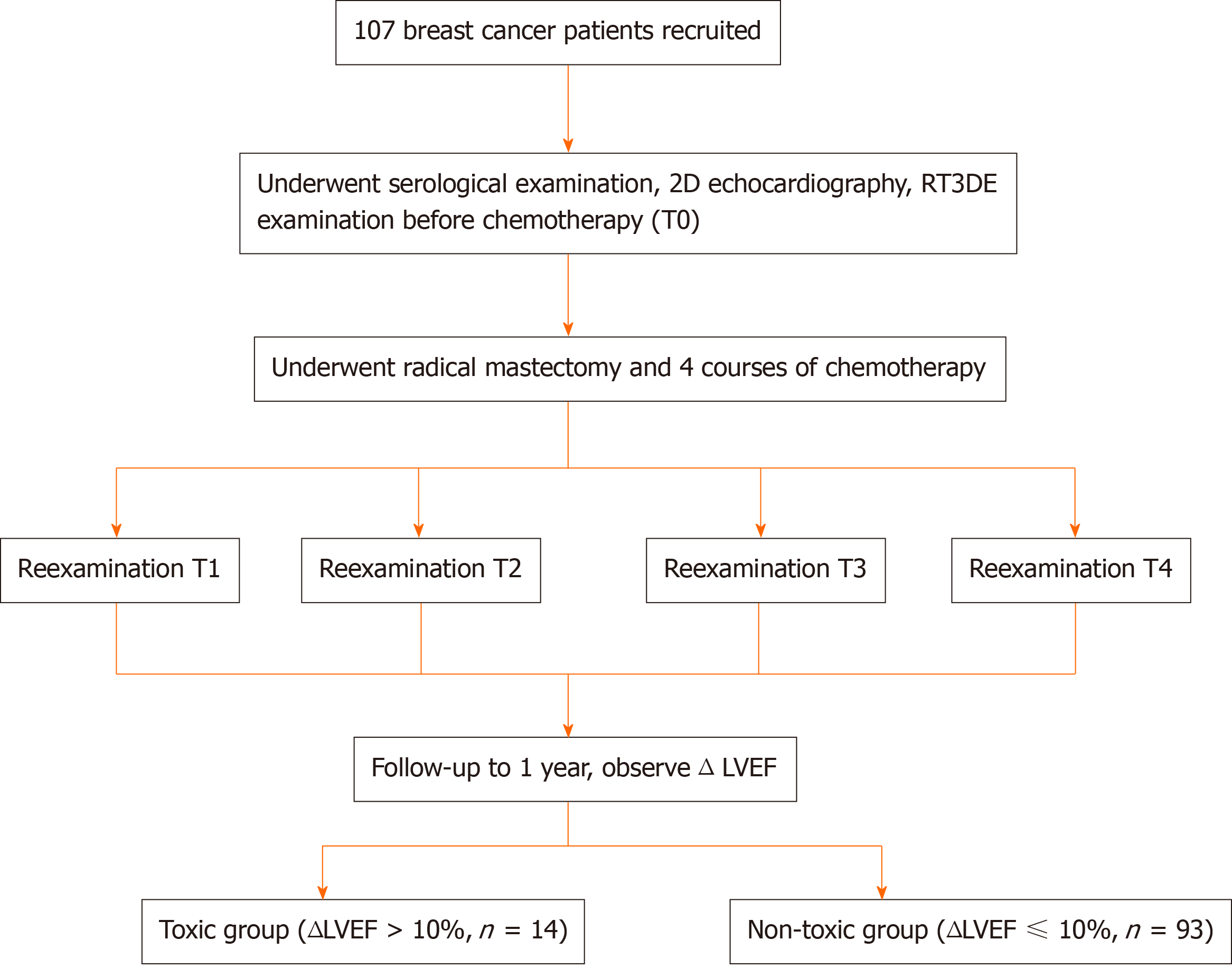
From March 2016 to March 2018, 107 female breast cancer patients aged 28 to 65 years who underwent surgical treatment and postoperative chemotherapy at Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine were recruited. The inclusion criteria were: (1) Adult female breast cancer patients who were confirmed with invasive ductal carcinoma by pathology after surgery; (2) No previous history of other tumors; (3) Predicted survival time > 24 mo; and (4) No complications that significantly affected the ultrasound detection, such as thoracic deformity, obesity, spinal deformity, and respiratory diseases. The exclusion criteria were: (1) Patients with a history of diseases that could affect cardiac function, including congenital heart disease, hypertension, coronary heart disease, and myocarditis; (2) ECG, echocardiography, and other tests before chemotherapy revealed significant abnormal cardiac function; and (3) Patients received chemotherapy or radiation therapy previously. This study was approved by the ethics committee of Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, and all patients provided informed consent.
Serological examination: Fasting venous blood was collected through the median cubital vein, and the supernatant was centrifuged for 5 min at 4 °C and 10000 rpm. The Vitros 5600 analyzer (Ortho Clinical Diagnostics, Johnson & Johnson, Buckinghamshire, United Kingdom) was used to detect cardiac troponin I (cTnI) and pro-brain natriuretic peptide (Pro-BNP).
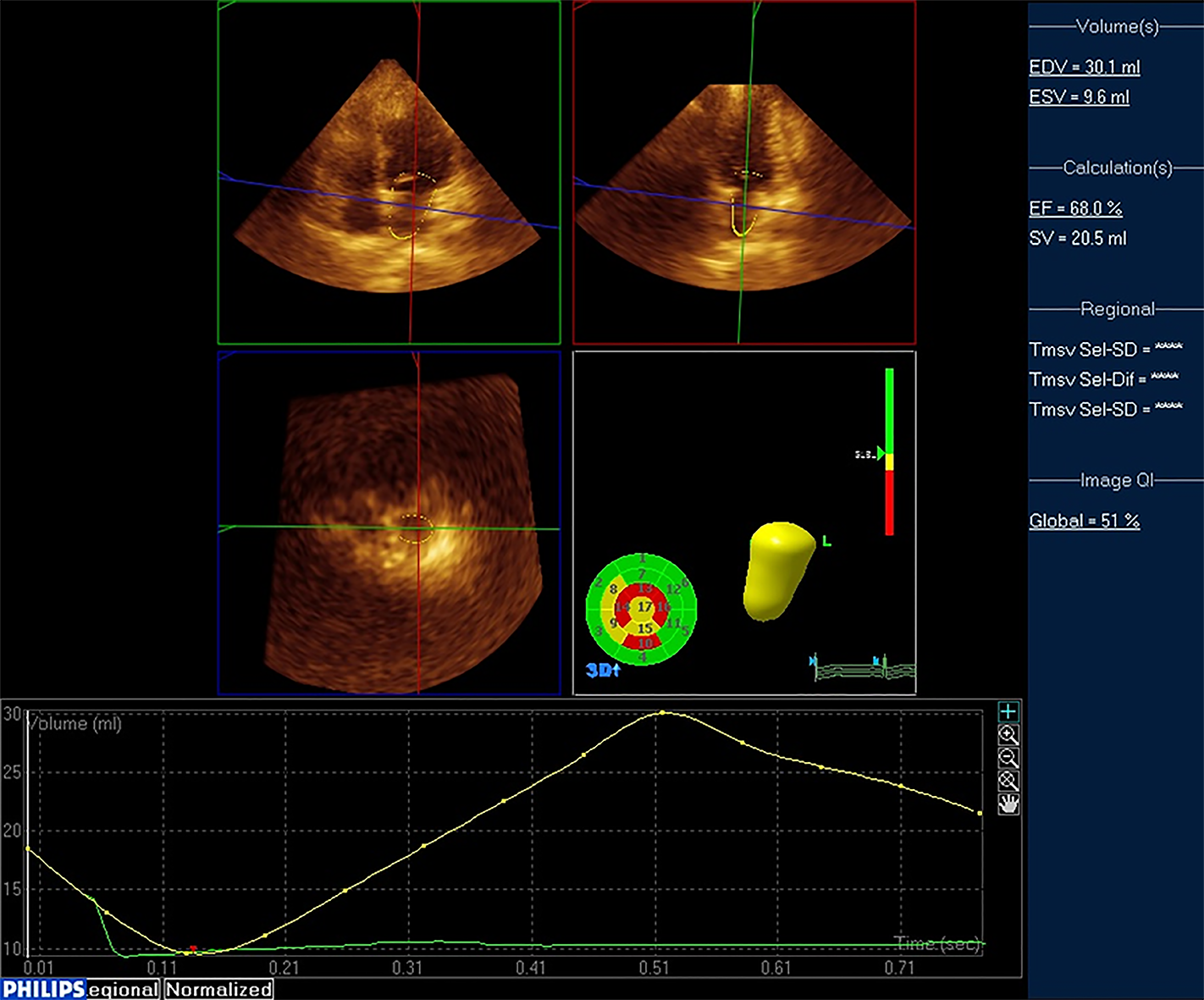
RT3DE examination: A Philips IU Elite ultrasound diagnostic instrument (Philips Healthcare, Seattle, WA, United States) equipped with S5-1 probe (frequency 2.0-3.5 MHz) and X5-1 probe (frequency 2.0-3.5 MHz) was used to perform echo-cardiography examination on patients to obtain 2D and 3D ultrasound images and indicators. During the examination, the patient was placed in the left lateral position, and the ECG was recorded. Measurement of left ventricular diastolic diameter (LVDd), left atrial diameter (LAd), E/A peak of mitral valve blood flow spectrum (A peak: maximum peak velocity in late diastole, E peak: maximum peak velocity in early diastole), LVEF, E/ e' (e' peak: Mitral annulus lateral tissue Doppler), and left ventricular fraction shortening (LVFS) were obtained. Subsequently, RT3DE was switched and a standard apex four-chamber view was obtained. A clear 3D image of the left atrium was collected in RT3DE mode (Figure 1). The QLab software 3DQa module was used for online analysis. With ECG as the reference standard, the LAV at the R wave position was defined as LAVmin. The LAV when the mitral valve was about to open was defined as LAVmax. The LAV before the start of the P wave was defined as LAVprep. LAEF (%) was calculated as (LAVmax-LAVmin) /LAVmax.
Patient treatment protocol: All patients underwent radical mastectomy and started to receive a combination of adriamycin-based chemotherapy on the 6th day after surgery. Doxorubicin was administered at a dose of 50 mg/m2. Patients need to complete four chemotherapy cycles, with each chemotherapy interval of 21 d. 2D echocardiography, RT3DE, and serological examinations were performed on the first day after each chemotherapy course. According to the examination time point, there were five subgroups: T0 (before chemotherapy), T1, T2, T3, and T4.
All patients were followed after four times of chemotherapy. The follow-up time was set to 1 year from the start of chemotherapy. At the end of follow-up, the LVEFs of all the patients were examined using echocardiography. With ΔLVEF > 10% (ΔLVEF = LVEFbefore chemotherapy - LVEF1st year after chemotherapy) as the standard, the patients were included in a toxic group (ΔLVEF > 10%), and the remaining patients were included in a non-toxic group (ΔLVEF ≤ 10%). The effects of 2D, RT3DE, and serological indicators on the prediction of cardiac toxicity in breast cancer patients receiving chemotherapy were analyzed (Figure 2).
SPSS (version 22.0; SPSS Inc., Chicago, IL, United States) and Medcalc (Version 22.0.1; MedCalc Software, Ostend, Belgium) were used for statistical analyses. The numerical data are expressed as the mean ± SD. Repeated-measures analysis of variance was used to study the factors influencing the patient's cardiotoxicity. Multivariate logistic regression was used to find independent risk factors for cardiotoxicity. Receiver operating characteristic (ROC) curve analysis was performed to analyze the diagnostic value of potential indicators in the diagnosis of cardiotoxicity caused by chemotherapy. Statistical significance was defined at 2-tailed P < 0.05 for all tests.
A total of 107 breast cancer patients were included in this study. The average tumor size was 2.31 ± 0.92 cm. According to WHO classification, there were eight patients with grade 1 disease, 61 with grade 2, and 38 with grade 3. All patients were diagnosed with invasive ductal carcinoma of the breast, of whom 33 had lymph node metastasis. At the end of the follow-up, 14 patients were found to have a ΔLVEF > 10% and included in a toxic group, and the remaining 93 patients were included in a non-toxic group. The incidence of cardiac toxicity after chemotherapy for breast cancer was 13.1%.
In this study, we compared the changes in routine detection indicators (echocardiographic indicators, troponin, and pro-BNP) of breast cancer patients before and after chemotherapy in the toxic and non-toxic groups. According to the repeated measurement analysis of variance results, it was found that E/e', cTnI, and Pro-BNP showed an increasing trend under different chemotherapy cycles (Pwithin group< 0.05). There was no significant difference in the routine detection indicators between the toxic group and the non-toxic group (Pbetween group > 0.05). However, it is worth noting that the E/e', cTnI, and Pro-BNP in the toxic group were significantly higher than those in the non-toxic group at T4 of the chemotherapy cycle (P < 0.05). The interactions in the changes of E/e', cTnI, and Pro-BNP between the two groups under different chemotherapy cycles were valid (Pinteraction < 0.05). The other routine detection indicators, including LAd, LVDd, mitral valve E/A, LVEF, ΔLVEF, and LVFS had no statistical significance in within-group comparison, between-group comparison, and interaction analysis (P > 0.05; Table 1).
| Routine de-tection in-dicator | Group | T0 | T1 | T2 | T3 | T4 | Fwithin group | Pwithin group value | Fbetween group | Pbetween group value | Finteraction | Pinteraction value |
| LAd (mm) | Toxic | 3.31 ± 0.46 | 3.34 ± 0.45 | 3.40 ± 0.57 | 3.37 ± 0.62 | 3.42 ± 0.89 | 0.921 | 0.401 | 1.111 | 0.332 | 1.442 | 0.241 |
| Non-toxic | 3.21 ± 0.88 | 3.31 ± 0.69 | 3.20 ± 0.82 | 3.27 ± 0.92 | 3.23 ± 0.87 | |||||||
| LVDd (mm) | Toxic | 4.39 ± 0.45 | 4.47 ± 0.62 | 4.45 ± 0.65 | 4.55 ± 0.58 | 4.61 ± 0.54 | 0.971 | 0.382 | 1.540 | 0.220 | 0.201 | 0.823 |
| Non-toxic | 4.22 ± 0.63 | 4.21 ± 0.73 | 4.28 ± 0.89 | 4.30 ± 0.84 | 4.25 ± 0.93 | |||||||
| Mitral valve E/A | Toxic | 1.34 ± 0.38 | 1.33 ± 0.37 | 1.31 ± 0.31 | 1.29 ± 0.41 | 1.27 ± 0.34 | 0.533 | 0.593 | 1.251 | 0.291 | 1.252 | 0.473 |
| Non-toxic | 1.51 ± 0.51 | 1.44 ± 0.46 | 1.47 ± 0.52 | 1.43 ± 0.46 | 1.41 ± 0.51 | |||||||
| LVEF (%) | Toxic | 64.92 ± 5.38 | 63.29 ± 6.39 | 63.27 ± 6.83 | 62.36 ± 6.90 | 62.39 ± 5.59 | 0.754 | 0.628 | 0.473 | 0.183 | 0.982 | 0.377 |
| Non-toxic | 65.24 ± 6.49 | 65.37 ± 7.49 | 64.63 ± 6.72 | 63.93 ± 5.89 | 63.28 ± 7.39 | |||||||
| ΔLVEF (%) | Toxic | - | 0.93 ± 0.31 | 1.38 ± 0.38 | 1.42 ± 0.33 | 2.39 ± 0.48 | 0.552 | 0.579 | 1.583 | 0.211 | 0.942 | 0.392 |
| Non-toxic | - | 0.68 ± 0.35 | 1.09 ± 0.41 | 1.28 ± 0.52 | 2.01 ± 0.45 | |||||||
| LVFS (%) | Toxic | 37.2 ± 3.4 | 37.4 ± 3.3 | 36.8 ± 2.9 | 36.7 ± 3.6 | 36.9 ± 3.1 | 0.764 | 0.472 | 1.622 | 0.203 | 0.740 | 0.481 |
| Non-toxic | 36.8 ± 3.6 | 37.1 ± 3.5 | 37.4 ± 4.2 | 36.4 ± 3.8 | 36.5 ± 2.9 | |||||||
| E/e' | Toxic | 6.36 ± 1.04 | 6.89 ± 1.38 | 7.02 ± 1.62 | 7.19 ± 1.48 | 7.82 ± 1.49a | 3.912 | 0.023 | 3.172 | 0.072 | 3.961 | 0.022 |
| Non-toxic | 6.17 ± 1.67 | 6.53 ± 1.58 | 6.88 ± 1.49 | 7.08 ± 1.63 | 7.32 ± 1.73 | |||||||
| cTnI (μg/L) | Toxic | 0.0231 ± 0.0106 | 0.0247 ± 0.0128 | 0.0256 ± 0.0193 | 0.0289 ± 0.0147 | 0.0422 ± 0.0166a | 5.201 | 0.007 | 2.974 | 0.084 | 4.123 | 0.019 |
| Non-toxic | 0.0204 ± 0.0148 | 0.0226 ± 0.0175 | 0.0214 ± 0.0149 | 0.0218 ± 0.0128 | 0.0228 ± 0.0145 | |||||||
| Pro-BNP (ng/L) | Toxic | 94.44 ± 19.39 | 95.92 ± 19.73 | 100.93 ± 21.49 | 112.38 ± 20.38 | 128.68 ± 22.29a | 5.573 | 0.005 | 3.063 | 0.082 | 4.452 | 0.014 |
| Non-toxic | 91.29 ± 28.94 | 92.39 ± 31.29 | 94.92 ± 29.30 | 95.93 ± 28.39 | 97.11 ± 30.61 |
In this study, RT3DE was used to compare the changes in 3D indicators of the left atrium before and after chemotherapy in the toxic and non-toxic groups. The repeated analysis of variance analysis showed that LAVmin and LAVprep showed an increasing trend under different chemotherapy cycles (Pwithin group< 0.05). LAVmin and LAVprep in the toxic group were significantly larger than those in the non-toxic group (Pbetween group< 0.05). T3 LAVmin, T4 LAVmin, T3 LAVprep, and T4 LAVprep in the toxic group were larger than those in the non-toxic group, and the differences were statistically significant (P < 0.05). The interactions in the changes of LAVmin and LAVprep between the two groups under different chemotherapy cycles were valid (bothPinteraction < 0.05). LAVmax and LAEF had no statistical significance in within-group comparison, between-group comparison, and interaction analysis (P > 0.05, Table 2).
| Three-dimen-sional in-dicator | Group | T0 | T1 | T2 | T3 | T4 | Fwithin group | Pwithin group value | Fbetween group | Pbetween group value | Finteraction | Pinteraction value |
| LAVmin (mL) | Toxic | 14.93 ± 2.07 | 16.93 ± 2.51 | 19.62 ± 2.07 | 21.94 ± 1.91a | 23.11 ± 2.04a | 5.201 | 0.007 | 4.533 | 0.013 | 10.072 | 0.000 |
| Non-toxic | 14.73 ± 3.92 | 15.63 ± 3.29 | 17.39 ± 2.84 | 18.84 ± 2.72 | 17.96 ± 2.28 | |||||||
| LAVmax (mL) | Toxic | 34.82 ± 4.96 | 35.11 ± 5.23 | 35.28 ± 5.28 | 36.82 ± 6.02 | 37.72 ± 7.62 | 2.941 | 0.057 | 2.563 | 0.082 | 2.792 | 0.066 |
| Non-toxic | 32.18 ± 5.29 | 33.72 ± 6.28 | 34.13 ± 5.65 | 34.89 ± 5.39 | 35.27 ± 6.39 | |||||||
| LAVprep (mL) | Toxic | 23.92 ± 5.29 | 24.29 ± 4.68 | 25.83 ± 5.17 | 27.99 ± 5.53a | 29.79 ± 4.40a | 4.713 | 0.011 | 5.063 | 0.008 | 8.391 | 0.000 |
| Non-toxic | 22.88 ± 4.02 | 23.18 ± 3.79 | 23.69 ± 4.03 | 23.11 ± 4.18 | 22.89 ± 3.03 | |||||||
| LAEF (%) | Toxic | 58 ± 8 | 58 ± 7 | 57 ± 8 | 56 ± 8 | 56 ± 7 | 1.240 | 0.294 | 1.731 | 0.182 | 1.582 | 0.211 |
| Non-toxic | 60 ± 9 | 59 ± 8 | 59 ± 9 | 58 ± 8 | 57 ± 8 |
In this study, we performed multivariate logistic regression analysis on indicators with differences between the two groups to assess the predictive effect of these indicators on cardiac toxicity after breast cancer chemotherapy. The results showed that T4 E/e' had no significant effect on the occurrence of cardiac toxicity (P > 0.05). T4 cTnI, T4 Pro-BNP, T3 LAVmin, T4 LAVmin, T3 LAVprep, and T4 LAVprep had significant effects on cardiac toxicity (P < 0.05; Table 3).
| Indicator | B | S.E. | Wald | P value | OR | 95%CI | |
| Lower limit | Upper limit | ||||||
| T4 E/e' | 0.250 | 0.204 | 2.183 | 0.125 | 1.284 | 0.861 | 1.915 |
| T4 cTnI | 0.733 | 0.317 | 5.024 | 0.025 | 2.081 | 1.118 | 3.874 |
| T4 Pro-BNP | 0.171 | 0.021 | 6.072 | 0.016 | 1.187 | 1.139 | 1.237 |
| T3 LAVmin | 0.387 | 0.021 | 9.024 | 0.003 | 1.472 | 1.413 | 1.534 |
| T4 LAVmin | 0.467 | 0.182 | 9.592 | 0.001 | 1.595 | 1.116 | 2.279 |
| T3 LAVprep | 0.290 | 0.017 | 8.815 | 0.005 | 1.336 | 1.292 | 1.381 |
| T4 LAVprep | 0.344 | 0.013 | 9.723 | 0.000 | 1.411 | 1.376 | 1.447 |
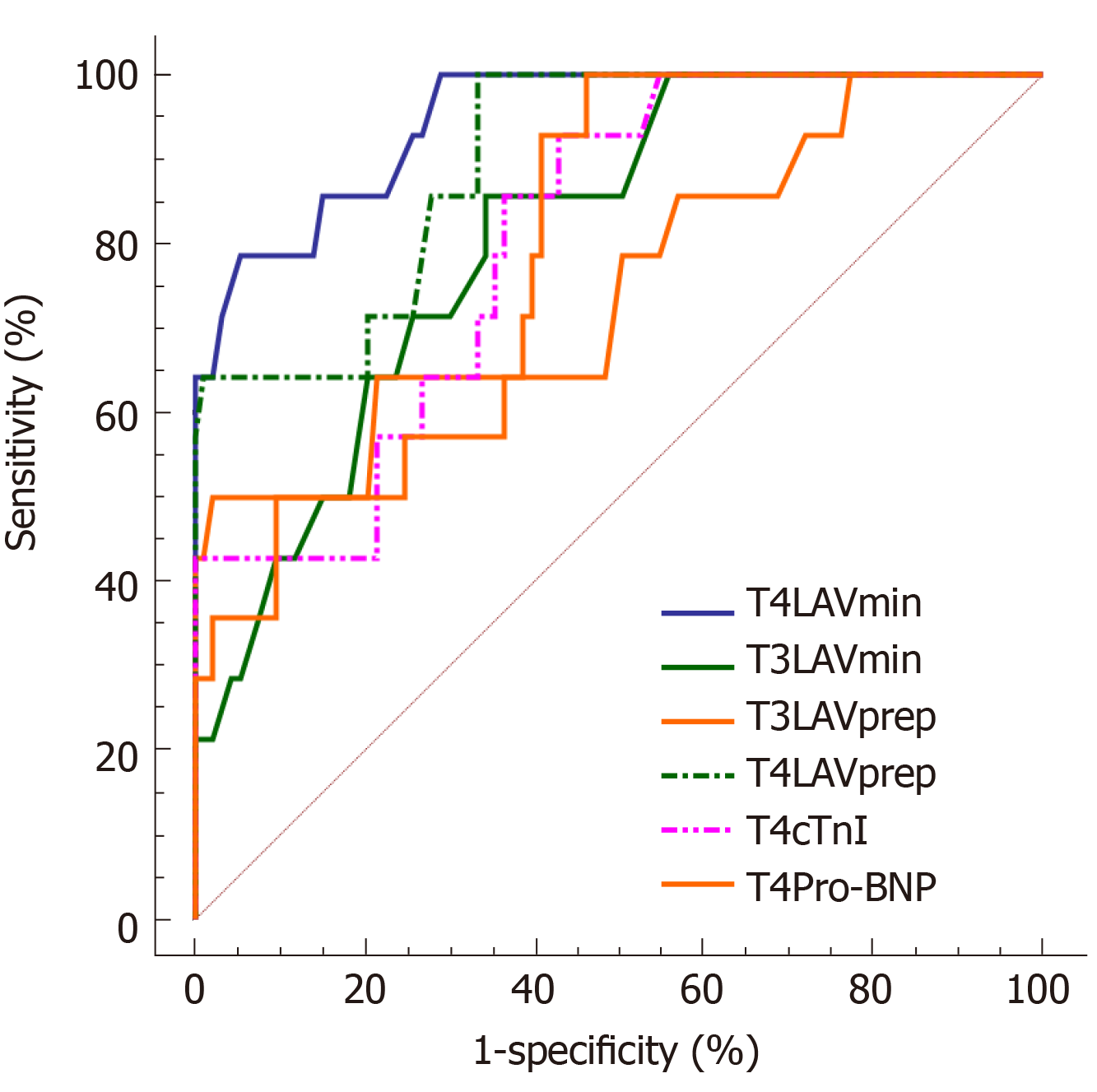
ROC curve analysis was performed to further analyze the predictive accuracy of T4 cTnI, T4 Pro-BNP, T3 LAVmin, T4 LAVmin, T3 LAVprep, and T4 LAVprep for cardiac toxicity after chemotherapy. The results showed that T4 LAVmin had the highest area under the curve (AUC) for predicting cardiotoxicity (AUC = 0.947), suggesting that LAVmin at the 4th chemotherapy cycle can accurately predict cardiac toxicity in patients. The best diagnostic value was 21.2 mL, and the sensitivity and specificity were 78.57% and 94.62%, respectively. T4 LAVprep (AUC = 0.899) followed, but the AUC of T4 LAVprep was lower than 0.9. T3 LAVmin, T3 LAVprep, T4 cTnI, and T4 Pro-BNP had lower accuracy in predicting cardiac toxicity (AUC between 0.7-0.8) and could not be used to predict cardiac toxicity (Table 4 and Figure 3).
| Indicator | AUC | 95%CI | Cut off point | Sensitivity (%) | Specificity (%) |
| T3LAVmin | 0.808 | 0.721-0.878 | 20.1 | 85.71 | 65.59 |
| T4LAVmin | 0.947 | 0.886-0.981 | 21.2 | 78.57 | 94.62 |
| T3LAVprep | 0.753 | 0.660- 0.831 | 30.1 | 50.00 | 97.85 |
| T4LAVprep | 0.899 | 0.826-0.949 | 24.4 | 100 | 66.67 |
| T4cTnI | 0.806 | 0.718-0.876 | 0.024 | 92.86 | 56.99 |
| T4Pro-BNP | 0.793 | 0.704-0.866 | 100.6 | 100 | 53.76 |
Cardiotoxicity caused by anthracycline chemotherapy drugs can obviously affect the prognosis of breast cancer patients. Early and accurate prediction of cardiac toxicity can effectively prevent the occurrence of cardiac toxicity. Left ventricular diastolic dysfunction usually occurs before systolic dysfunction, so the examination of left ventricular diastolic function can be utilized for early evaluation of cardiotoxicity caused by chemotherapy drugs. Heart biopsy is the gold standard for diagnosing heart injury, but its popularity is low as an invasive test[14]. The tissue Doppler index E/e' can be used to evaluate changes in left ventricular diastolic function, but it has diagnostic value only when it is greater than 15[15]. Although serological indicators are highly sensitive, studies by Christenson et al[16] and Cao et al[17] suggested that it was difficult to accurately reveal the changes of diastolic function merely relying on a single serological indicator, and the specificity was not high. Two-dimensional ultrasound speckle tracking imaging is one of the most sensitive methods for detecting left ventricular diastolic function, however, Leitman et al[18] found that it was angularly dependent and had poor repeatability in practical applications. Three-dimensional ultrasound speckle tracking imaging is free of angle dependence, and can realize the strain analysis of the left ventricle segments. But it has the disadvantages of complicated operation and being difficult for promotion[19]. Therefore, new methods need to be explored to help early predictions of impaired cardiac function. The left atrium volume indicates the extent and duration of the elevation of left ventricular diastolic perfusion pressure and can predict cardiac toxicity early. Recently, studies have used RT3DE technology to measure left atrial diastolic function to assess cardiac injury and arrhythmia[20,21]. This technology can quickly obtain 3D information of the left atrium to assess early cardiac damage. However, its role in early prediction of chemotherapy-related cardiac toxicity is rare. Therefore, in this study, the left atrium data were examined by 2D echocardiography and RT3DE, and the 2D and 3D indicators of the left atrium during chemotherapy were used to predict the occurrence of chemotherapy-related cardiac toxicity in the early stage.
Since the cardiotoxicity generated by anthracyclines during chemotherapy is mostly in the subclinical stage, it is often difficult to detect the slight differences caused by heart damage due to the strong compensation mechanism of the heart[22]. The results in this study showed that 2D echocardiographic indicators such as LAd, LVDd, mitral valve E/A, LVEF, ΔLVEF, and LVFS did not show significant differences before and after chemotherapy, indicating that traditional 2D indicators are not feasible for early prediction of cardiac toxicity, which is consistent with the research of Lotrionte et al[23]. It is worth noting that the E/e' and serological indicators (cTnI and Pro-BNP) were different between the two groups, indicating that these indicators are more sensitive than traditional 2D ultrasound indicators and have potential for early diagnosis of cardiac toxicity. After screening by multivariate logistic regression analysis, only T4 cTnI and T4 Pro-BNP might independently predict cardiac toxicity in patients after chemotherapy. The reason may be that cTnI and Pro-BNP are markers of heart injury and are released into the blood when heart injury occurs. Hence, they are hopeful to be used to predict early heart injury, which is consistent with the studies of Srikanthan et al[24] and Tian et al[25]. ROC curve analysis results showed that although T4 cTnI and T4 Pro-BNP had the potential to predict cardiac toxicity, their accuracy was not high (AUC between 0.7-0.8). In addition, cTnI and Pro-BNP had the value of predicting cardiotoxicity only in the 4th chemotherapy cycle. At that time, the four courses of chemotherapy have ended, so they cannot guide the choice of chemotherapy dose in the course of chemotherapy.
RT3DE is capable of assessing the LAV, and has a high correlation with the evaluation results of CT and MRI[26-29]. Therefore, it is expected to be used as a method for detecting cardiac toxicity. Hatipoglu et al[12] found that in evaluating cardiac function, the systolic RT3DE indicators were more sensitive than the diastolic indicators. This is because the indicators of the systole reveal the heart volume during systole. They are more indicative of the degree of compensatory dilatation of the heart than diastolic indicators. Similarly, this study also showed that RT3DE indicators (LAVmin and LAVprep) were more advantageous in assessing the degree of left atrial damage. Repeated measures analysis of variance showed that LAVmin and LAVprep showed an increase trend during the chemotherapy cycle, indicating that the left ventricular diastolic function decreased due to the cardiotoxic effects of chemotherapy drugs. LAVmin and LAVprep were different between the groups in this study. LAVmin and LAVprep at T3 in the toxic group were higher than those in the non-toxic group, and the difference appeared earlier than the traditional 2D ultrasound indicators, which suggests that LAVmin and LAVprep could be used for predicting heart injury earlier. Multivariate logistic regression analysis found that T3 LAVmin, T4 LAVmin, T3 LAVprep, and T4 LAVprep could independently predict the occurrence of cardiac toxicity. Although LAVmin and LAVprep showed differences at T3, the ROC curve analysis results showed that the AUCs of T3 LAVprep and T3 LAVmin were not high (0.808 and 0.753), which indicated that the RT3DE indicators in the 3rd chemotherapy cycle could only be used for approximate evaluation, and it was difficult to accurately predict cardiotoxicity. The results of this study showed that only T4 LAVmin and T4 LAVprep could accurately predict cardiac toxicity, because T4 LAVmin had the highest AUC for predicting cardiac toxicity (AUC = 0.947), followed by T4 LAVprep (AUC = 0.899), which were significantly better than traditional serological indicators (T4 cTnI and T4 Pro-BNP).
There were some shortcomings in this study. First of all, this study did not follow the delayed effects of cardiotoxicity for a long time. Cardiotoxic symptoms may be delayed and omitted in some patients. Second, the sample size of this study is small, and a larger, multi-center study is needed to provide useful information for the evaluation of the cardiotoxicity caused by chemotherapy drugs.
In conclusion, the RT3DE LAV indicators (LAVmin and LAVprep) of patients with cardiac toxicity in the early stage of chemotherapy show more significant changes compared to traditional detection indicators of the heart. LAV detected by RT3DE is the most accurate method for predicting cardiotoxicity caused by chemotherapy, and it is expected to be used to guide clinical adjustment of the dosage and schedule in time.
Anthracycline chemotherapy drugs in breast cancer can increase the risk of cardiac toxicity. In order to prevent the occurrence of cardiac toxicity, early and accurate prediction of cardiac toxicity is helpful for adjusting the dosage during treatment. Current methods for detecting cardiotoxicity include echocardiographic indicators, two-dimensional ultrasound speckle tracking imaging, and serum natriuretic peptide. However, these methods have some limitations. New methods need to be developed for early detection of cardiotoxicity caused by chemotherapy.
Increased left atrial volume is an early manifestation of cardiac toxicity. Real-time three-dimensional echocardiography (RT3DE) has made it possible to measure left atrial volume precisely. This method does not rely on the geometry of the left atrium, and can dynamically observe the movement and volume changes of the left atrium. But so far, most studies have only explored the relationship between left atrial volume and left ventricular diastolic function. Few studies measure left atrial volume to predict early cardiac toxicity by RT3DE.
To evaluate the RT3DE indicators during chemotherapy to observe changes in left atrium volume in breast cancer patients undergoing chemotherapy, and to assess whether such indicators can help early predict the occurrence of cardiac toxicity.
All breast cancer patients underwent surgical treatment, and four cycles of chemotherapy (T1-T4) were routinely performed after surgery. Patients underwent two-dimensional, RT3DE, or serological examinations before and after each cycle of chemotherapy. Patients were included in either a toxic group (ΔLVEF > 10%) or a non-toxic group (ΔLVEF ≤ 10%). Repeated measurement analysis of variance was used to compare changes in conventional echocardiographic indicators, serological indicators, and RT3DE indicators before and after treatment. Multivariate logistic regression was used to find independent influencing factors on cardiac toxicity. Receiver-operating characteristics (ROC) curve analysis was performed to analyze the predictive value for cardiotoxicity caused by chemotherapy.
The present study found that T4 cTnI, T4 Pro-BNP, T3 LAVmin, T4 LAVmin, T3 LAVprep, and T4 LAVprep had significant effects on cardiac toxicity, suggesting that LAVmin and LAVprep of RT3DE could be used to earlier predict the cardiotoxicity than other indicators. ROC curve analysis results showed that T4 LAVmin had the highest AUC for diagnosing cardiotoxicity (AUC = 0.947), followed by T4LAVprep (AUC = 0.899). In addition, T4 LAVmin and LAVprep were more accurate in predicting cardiac toxicity than T3 LAVmin and T3 LAVprep. These results indicated that the RT3DE index in the third chemotherapy cycle can only early alert the occurrence of cardiac toxicity, and it needs to be tested in the fourth cycle to accurately predict cardiac toxicity.
The left atrial volume detected by RT3DE is the most accurate method for predicting cardiotoxicity caused by chemotherapy, and it is expected to be used to guide clinical adjustment of the dosage and schedule in time.
Since the follow-up time is too short to accurately determine the patients with cardiotoxicity, cardiotoxic symptoms may be delayed and omitted in some patients. A larger, multi-center study should be conducted to provide useful information for the evaluation of the cardiotoxicity caused by chemotherapy drugs in the further study.
We wish to thank Professor Zhong Xiang who provided help for this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bouvier AM, Nagao M S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Rahmani H, Shahriary A, Sheikhi MA, Ebadi A, Davoodzadeh H. Applications of cardiotoxicity in breast cancer: a meta-analysis. Panminerva Med. 2017;59:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Martel S, Maurer C, Lambertini M, Pondé N, De Azambuja E. Breast cancer treatment-induced cardiotoxicity. Expert Opin Drug Saf. 2017;16:1021-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Khosrow-Khavar F, Filion KB, Al-Qurashi S, Torabi N, Bouganim N, Suissa S, Azoulay L. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2017;28:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 4. | Zymliński R, Sokolski M, Siwolowski P, Biegus J, Nawrocka S, Jankowska EA, Todd J, Yerramilli R, Estis J, Banasiak W, Ponikowski P. Elevated troponin I level assessed by a new high-sensitive assay and the risk of poor outcomes in patients with acute heart failure. Int J Cardiol. 2017;230:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Yuksel MA, Alici Davutoglu E, Temel Yuksel I, Kucur M, Ekmekci H, Balci Ekmekci O, Uludag S, Uludag S, Madazli R. Maternal serum atrial natriuretic peptide (ANP) and brain-type natriuretic peptide (BNP) levels in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2016;29:2527-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJ, Mant J; NICE Guideline Development Group for Acute Heart Failure. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ. 2015;350:h910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 7. | Fudim M, Ambrosy AP, Sun JL, Anstrom KJ, Bart BA, Butler J, AbouEzzeddine O, Greene SJ, Mentz RJ, Redfield MM, Reddy YNV, Vaduganathan M, Braunwald E, Hernandez AF, Borlaug BA, Felker GM. High-Sensitivity Troponin I in Hospitalized and Ambulatory Patients With Heart Failure With Preserved Ejection Fraction: Insights From the Heart Failure Clinical Research Network. J Am Heart Assoc. 2018;7:e010364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3897] [Cited by in RCA: 5780] [Article Influence: 525.5] [Reference Citation Analysis (0)] |
| 9. | Yang L, Ma L, Li Y, Mu Y, Liu L. Real-time three-dimensional echocardiography of left atrial volume and function in patients with severe multi-vessel coronary artery disease. J Med Ultrason (2001). 2017;44:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Li Y, Wang Y, Zhai Z, Guo X, Yang Y, Lu X. Real-Time Three-Dimensional Echocardiography to Assess Right Ventricle Function in Patients with Pulmonary Hypertension. PLoS One. 2015;10:e0129557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Deng Y, Guo SL, Su HY, Wang Q, Tan Z, Wu J, Zhang D. Left atrial asynchrony and mechanical function in patients with mitral stenosis before and immediately after percutaneous balloon mitral valvuloplasty: a real time three-dimensional echocardiography study. Echocardiography. 2015;32:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Hatipoglu S, Ozdemir N, Babur Guler G, Omaygenc MO, Bakal RB, Kahveci G, Unkun T, Sahin G, Kaymaz C. Left atrial expansion index is an independent predictor of diastolic dysfunction in patients with preserved left ventricular systolic function: a three dimensional echocardiography study. Int J Cardiovasc Imaging. 2014;30:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Yamano M, Yamano T, Iwamura Y, Nakamura T, Shiraishi H, Shirayama T, Matoba S. Impact of Left Ventricular Diastolic Property on Left Atrial Function from Simultaneous Left Atrial and Ventricular Three-Dimensional Echocardiographic Volume Measurement. Am J Cardiol. 2017;119:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Francis R, Lewis C. Myocardial biopsy: techniques and indications. Heart. 2018;104:950-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Frikha Z, Girerd N, Huttin O, Courand PY, Bozec E, Olivier A, Lamiral Z, Zannad F, Rossignol P. Reproducibility in echocardiographic assessment of diastolic function in a population based study (the STANISLAS Cohort study). PLoS One. 2015;10:e0122336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Christenson ES, James T, Agrawal V, Park BH. Use of biomarkers for the assessment of chemotherapy-induced cardiac toxicity. Clin Biochem. 2015;48:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Cao L, Zhu W, Wagar EA, Meng QH. Biomarkers for monitoring chemotherapy-induced cardiotoxicity. Crit Rev Clin Lab Sci. 2017;54:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 881] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 19. | Saito K, Okura H, Watanabe N, Hayashida A, Obase K, Imai K, Maehama T, Kawamoto T, Neishi Y, Yoshida K. Comprehensive evaluation of left ventricular strain using speckle tracking echocardiography in normal adults: comparison of three-dimensional and two-dimensional approaches. J Am Soc Echocardiogr. 2009;22:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Wu VC, Takeuchi M, Kuwaki H, Iwataki M, Nagata Y, Otani K, Haruki N, Yoshitani H, Tamura M, Abe H, Negishi K, Lin FC, Otsuji Y. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc Imaging. 2013;6:1025-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Yaylali YT, Saricopur A, Yurtdas M, Senol H, Gokoz-Dogu G. Atrial Function in Patients with Breast Cancer After Treatment with Anthracyclines. Arq Bras Cardiol. 2016;107:411-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Abecasis J, Dourado R, Ferreira A, Saraiva C, Cavaco D, Santos KR, Morgado FB, Adragão P, Silva A. Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace. 2009;11:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Lotrionte M, Cavarretta E, Abbate A, Mezzaroma E, De Marco E, Di Persio S, Loperfido F, Biondi-Zoccai G, Frati G, Palazzoni G. Temporal changes in standard and tissue Doppler imaging echocardiographic parameters after anthracycline chemotherapy in women with breast cancer. Am J Cardiol. 2013;112:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Srikanthan K, Klug R, Tirona M, Thompson E, Visweshwar H, Puri N, Shapiro J, Sodhi K. Creating a Biomarker Panel for Early Detection of Chemotherapy Related Cardiac Dysfunction in Breast Cancer Patients. J Clin Exp Cardiolog. 2017;8:507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Tian S, Hirshfield KM, Jabbour SK, Toppmeyer D, Haffty BG, Khan AJ, Goyal S. Serum biomarkers for the detection of cardiac toxicity after chemotherapy and radiation therapy in breast cancer patients. Front Oncol. 2014;4:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Artang R, Migrino RQ, Harmann L, Bowers M, Woods TD. Left atrial volume measurement with automated border detection by 3-dimensional echocardiography: comparison with Magnetic Resonance Imaging. Cardiovasc Ultrasound. 2009;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Miyasaka Y, Tsujimoto S, Maeba H, Yuasa F, Takehana K, Dote K, Iwasaka T. Left atrial volume by real-time three-dimensional echocardiography: validation by 64-slice multidetector computed tomography. J Am Soc Echocardiogr. 2011;24:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Mor-Avi V, Yodwut C, Jenkins C, Kühl H, Nesser HJ, Marwick TH, Franke A, Weinert L, Niel J, Steringer-Mascherbauer R, Freed BH, Sugeng L, Lang RM. Real-time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging. 2012;5:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 29. | Rohner A, Brinkert M, Kawel N, Buechel RR, Leibundgut G, Grize L, Kühne M, Bremerich J, Kaufmann BA, Zellweger MJ, Buser P, Osswald S, Handke M. Functional assessment of the left atrium by real-time three-dimensional echocardiography using a novel dedicated analysis tool: initial validation studies in comparison with computed tomography. Eur J Echocardiogr. 2011;12:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |