Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2392
Peer-review started: January 9, 2020
First decision: April 8, 2020
Revised: April 26, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: June 6, 2020
Processing time: 150 Days and 12.1 Hours
The traditional definition of late postpartum hemorrhage is a massive uterine hemorrhage from 24 h after delivery to the puerperal period. Here, we report a case of late postpartum hemorrhage that occurred 3 mo after cesarean section and endangered the patient's life. The cause of the case we are reporting was poor incision healing. By reporting this case, we hope to make doctors aware that late postpartum hemorrhage due to poor incision healing may happen as late as 3 mo after cesarean section.
A 31-year-old woman complained of acute, severe vaginal bleeding for 1 h; the patient had a history of cesarean section 3 mo prior. After receiving anti-inflammatory treatment, fluid supplementation, blood transfusion, oxytocin administration, and hemostatic treatment, the vaginal bleeding ceased, and the patient’s clinical status improved. Unfortunately, she experienced recurrent massive vaginal bleeding, and uterine contractile agents did not decrease the persistent bleeding. To save the patient’s life, she was admitted for emergency laparotomy. At exploratory laparotomy, dehiscence and necrosis of the previous cesarean section scar were noted; the dehiscence penetrated through the entire thickness of the uterine muscle wall and extended to the left uterine artery. Ultimately, we performed a total hysterectomy.
Late postpartum hemorrhage due to poor incision healing after cesarean section may occur in the 3 mo after cesarean section or even later. Therefore, obstetricians-gynecologists should monitor for this potential complication in all patients post–cesarean section. Such hemorrhages can be severe enough to endanger the patient's life.
Core tip: The definition of late postpartum hemorrhage is a massive uterine hemorrhage from 24 h after delivery to the puerperal period. But in this case, massive vaginal bleeding because of cesarean section incision infection, ulcer, and poor healing, appeared at 3 mo after cesarean section, and this is at odds with the traditional concept. Since the bleeding was associated with cesarean delivery operation, we think that it should also be classified as late postpartum hemorrhage, and cause enough attention in clinic, because it is serious enough to endanger the patient's life.
- Citation: Zhang Y, Ma NY, Pang XA. Uterine incision dehiscence 3 mo after cesarean section causing massive bleeding: A case report. World J Clin Cases 2020; 8(11): 2392-2398
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2392.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2392
The traditional definition of late postpartum hemorrhage is a massive uterine hemorrhage from 24 h after delivery to the puerperal period. The onset was most common at 1 to 2 wk postpartum, which could also be delayed to approximately 2 mo postpartum[1]. The American College of Obstetricians and Gynecologists issued postpartum bleeding guidelines, stating that postpartum hemorrhage will typically occur within 24 h to 6 to 12 wk after giving birth, the latter of which is referred to as advanced postpartum hemorrhage[2]. These guidelines indicate that the United States has a much longer time frame for late postpartum hemorrhage. However, we experienced a case of massive postpartum hemorrhage due to cesarean scar dehiscence at 3 mo after delivery.
A 31-year-old woman with a history of cesarean section more than 3 mo prior presented with heavy vaginal bleeding of 1-h duration.
A 31-year-old patient (gravida 4, para 2, abortion 2) was admitted to our hospital with acute, severe vaginal bleeding. She underwent an emergency lower segment cesarean section 5 years ago followed by an elective lower segment cesarean section on December 6, 2018, more than 3 mo before her current presentation. The patient had an unremarkable postoperative course with normal lochia 20 d after cesarean section. The newborn baby was bottle-fed; as a result, 2 mo after cesarean section, menstruation resumed to normal on February 20, 2019. On March 22, 2019, vaginal bleeding recurred, which was thought initially to be normal menstruation, with a typical menstrual volume. The menstrual fluid had a bright, red color, and the patient had no abdominal pain. On the third day of this menstruation, 3 d before presenting to our hospital, massive vaginal bleeding occurred suddenly and was about 3 times that of the previous menstrual volume based on self-reported visual measurement. Therefore, the patient went to the local hospital for treatment. The transvaginal ultrasound result was negative; therefore, the patient received an anti-inflammatory treatment, fluid supplementation, oxytocin, and hemostatic treatment for 2 d. When the vaginal bleeding stopped, she was permitted to be discharged. Massive vaginal bleeding occurred again on the next day after discharge, with a loss of approximately 500 mL in 1 h based upon self-reported visual measurement, and she was admitted to the emergency department of our hospital for diagnosis and treatment. The patient presented with dizziness, confusion, palpitations, and fatigue. She did not have fever, abdominal pain, headache, diarrhea, nausea, or vomiting. The patient stated that she had no changes in diet, bowel movements, or weight, but admitted to poor sleeping habits.
The patient had no significant history of past illness.
The patient does not smoke or drink, and she denied a history of drug allergy.
The patient appeared to be in hemorrhagic shock on presentation with a pulse of 110 beats per minute and a blood pressure of 83/40 mmHg. Gynecological examination revealed normal vulvar development, smooth vaginal walls, and cervical hypertrophy with grade II erosion-like changes. Fresh blood of the amount seen with normal menstruation, was seen within the uterine cavity. There was no trauma or active bleeding in the vulva, vagina, or cervix.
On examination, the patient’s hemoglobin concentration on admission was 6.6 g/dL. Her white cell count, platelets, coagulation screen, and liver and kidney function tests were all within normal limits. A test for human chorionic gonadotropin was negative.
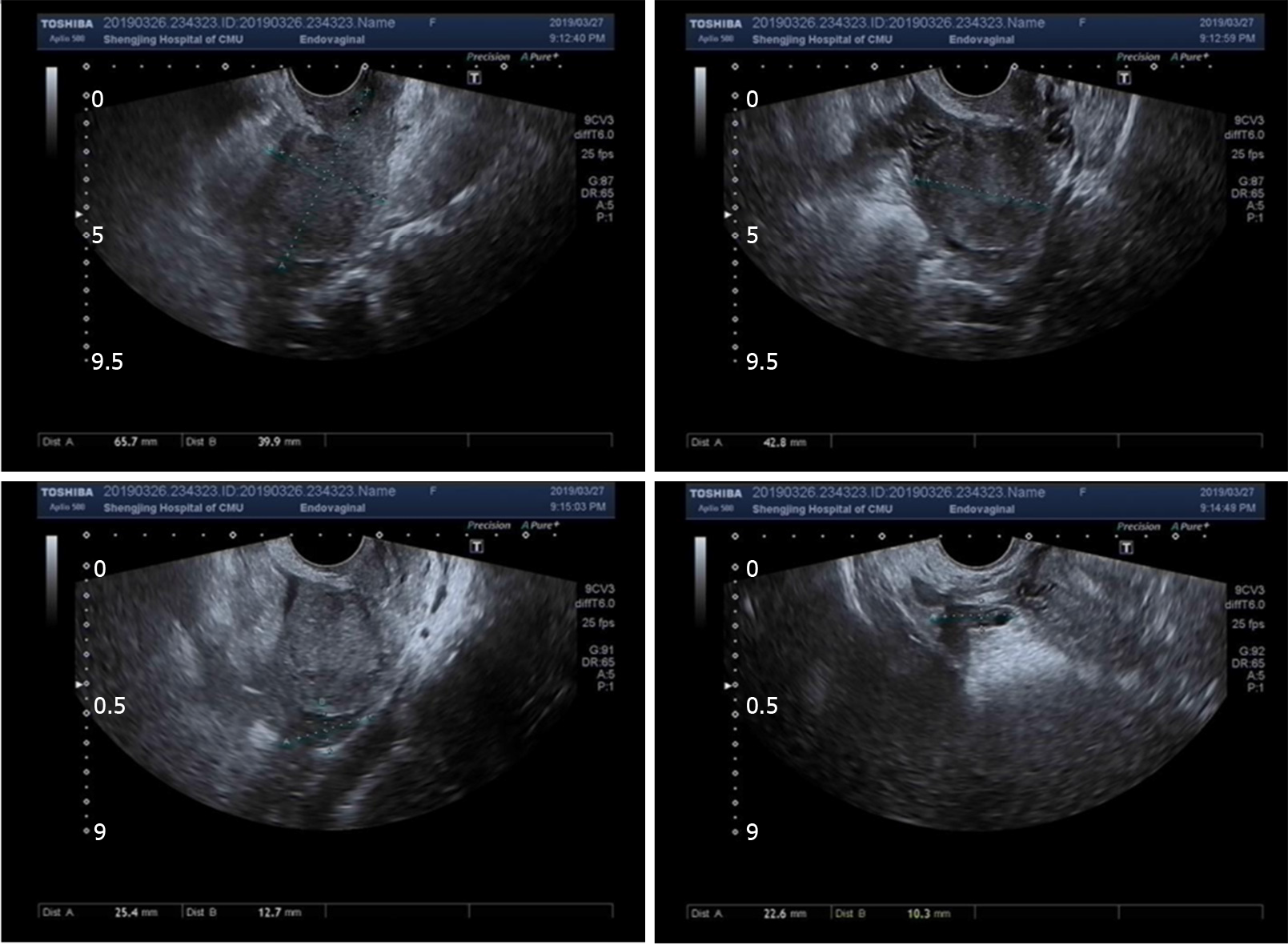
Routine transvaginal ultrasound showed the endometrium to be approximately 0.7 mm thick with slightly uneven echo and no other abnormalities were found (Figure 1).
Uterine incision dehiscence 3 mo after cesarean section causing massive bleeding.
She was given an intravenous oxytocin drip immediately. The patient was resuscitated with crystalloids and transfused with 4 U of packed red blood cells and 400 mL of frozen fresh plasma. After close observation for 48 h, the vaginal bleeding ceased, and the clinical presentation improved. We thought that this vaginal bleeding was due to postpartum ovulation dysfunction caused by abnormal uterine bleeding. Therefore, we planned to administer hormones to regulate the menstrual cycle and prevent further vaginal bleeding. Unfortunately, she experienced recurrent vaginal bleeding on the third day of this hospitalization. She had lost an estimated 2000 mL of blood in no more than 2 h measured by the clinicians. Uterine contractile agents did not decrease the persistent bleeding, including the oxytocin (continued on intravenous drip) and two methyl carprost suppositories administered to the vagina every hour. She became pale and was hemodynamically unstable with a systolic blood pressure below 60 mmHg, diastolic blood pressure below 30 mmHg, and tachycardia above 120 beats per minute. By this time, the patient’s Hb was 66 g/L. Because the interventional therapy was not immediately available, the patient was prepared for emergency laparotomy. At exploratory laparotomy, scar dehiscence and necrosis from a previous cesarean section penetrated the entire thickness of the uterine muscle wall, and the lesion extended to the left uterine artery. Profuse bleeding also was observed. Because the tissues around the lesion were brittle and the suturing process induced profuse bleeding, a decision was made to perform a hysterectomy in order to save the patient's life.
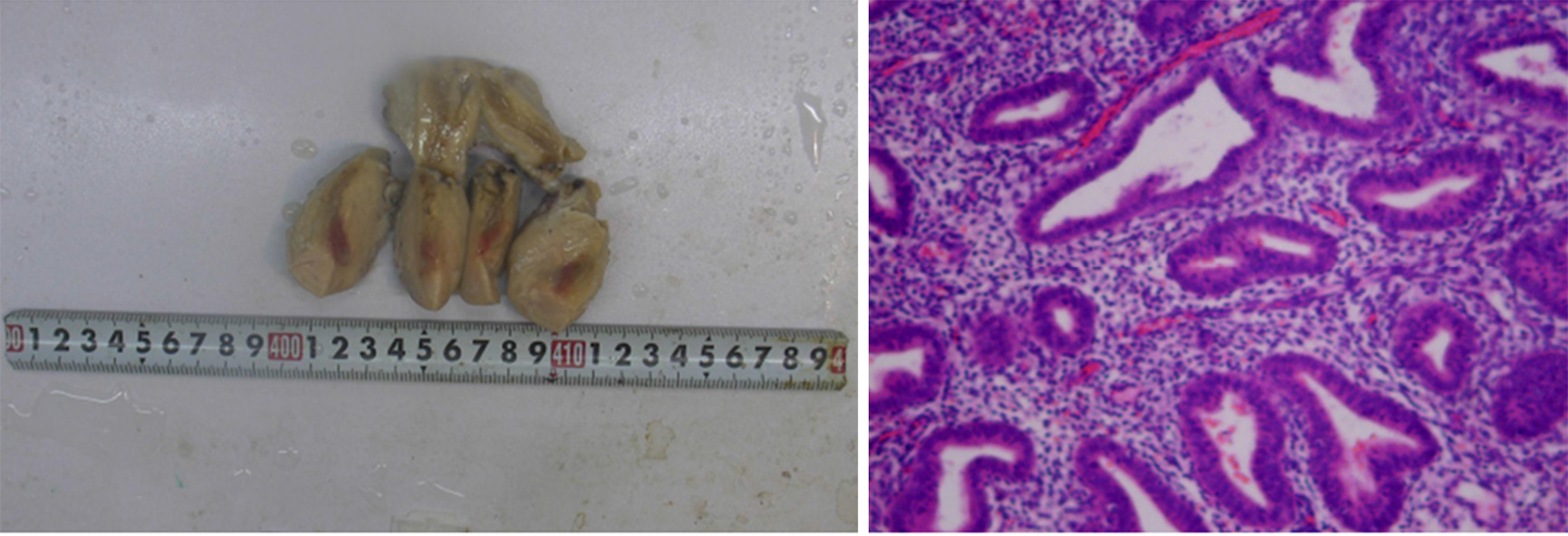
The patient recovered well after the operation with no signs of sepsis. She was discharged on the seventh day after the operation. The paraffin pathology of the uterus showed fibrosis, eosinophilic infiltration, and inflammatory changes in the isthmus (Figure 2).
In the past, the definition of late postpartum hemorrhage was a massive uterine hemorrhage anywhere from 24 h after delivery to the puerperal period, with the most common time being 1 to 2 wk postpartum (although this could be delayed up to 2 mo postpartum)[1]. In 2006, the American College of Obstetricians and Gynecologists issued postpartum bleeding guidelines, which stated that most postpartum hemorrhages occur within 24 h to 2 to 6 wk after delivery, the latter of which was referred to as advanced postpartum hemorrhage[2]. These guidelines indicate that the United States has a much longer time frame for late postpartum hemorrhage. However, in our patient, massive vaginal bleeding as a result of cesarean section incision infection, ulceration, and poor wound healing occurred at 3 mo after cesarean section; this is at odds with the traditional concept. Because postpartum bleeding can be associated with a cesarean delivery, we think that this should also be classified as late postpartum hemorrhage. Further, postcesarean bleeding is serious enough to warrant particular vigilance among obstetricians-gynecologists when caring for these patients. In this case, because the bleeding occurred 3 mo after cesarean section with the start of normal menses 1 mo prior, health care professionals failed to recognize that this could be a case of late postpartum hemorrhage. Instead, the patient was diagnosed with anovulatory dysfunctional uterine bleeding. Based on this wrong diagnosis, we decided to regulate the patient’s menstrual cycle using hormone treatment after achieving hemostasis. Unfortunately, we were unable to do this because shortly after stabilization, the patient rebled. An emergency laparotomy revealed a poorly healed, infected, ulcerated, previous cesarean wound extending to the left uterine artery.
The main causes of postpartum hemorrhage after cesarean section include poor wound healing, uterine involution, residual placenta decidua, and endometritis. Rare causes include pseudo-aneurysm of a uterine vessel, arteriovenous malformation, and choriocarcinoma[3]. Among them, undesirable healing of a uterine incision is the most important cause, accounting for 42.7% of patients with a cesarean delivery[4]. The main factors are as follows: (1) Anatomical; (2) Improper incision location; (3) Suture technology; and (4) Infection.
In cesarean section, the desired choice for type and location of uterine incision is a transverse incision in the lower uterine segment. Because the isthmus uteri of the bow artery are shorter and smaller than the body branch, a transverse incision of the lower uterine segment can result in unintended incision of the descending uterine artery branch. This branch provides less blood supply than the body branch, resulting in inadequate blood supply to the area of the incision.
The cervix is mainly composed of connective tissue, with less muscle fibers and blood vessels. If the labor process is longer, prolonged dilation of the lower part of the uterus results in it being longer and thinner. When the incisions are placed too low on the uterine wall, this can easily lead to ischemic necrosis because of poor healing ability. In addition, the uterus of late pregnancy tends to be situated right and laterally, and incisions of the lower segment often deviate to the left and inadvertently cut off the branch of the left uterine artery. This results in ischemia and necrosis of the lateral horn of the incision.
Performing repeated blind suture hemostasis during active uterine incision bleeding results in the ligation of more small arteries. This can lead to insufficient blood supply to the tissues. On the other hand, suturing too loosely can result in easy hematoma formation, which can lead to poor incision healing.
There are many reports that multiple preoperative vaginal and anal examinations in the second stage of labor or multiple previous cesarean sections can easily induce incision inflammation and ulceration[5].
In our patient, during open surgical exploration, visible on scope was 1.5 cm of necrotic tissue on the left side of the lower uterine segment. This necrotic tissue completely penetrated the entire muscle wall and extended to the left uterine artery. Further, the surrounding tissue was very brittle. Having reviewed the patient’s cesarean section history, we speculated that the incision in the previous cesarean section was selected at the scar site of the initial cesarean section. This incision site was composed of scar connective tissue with few muscle fibers and few blood vessels, which resulted in poor healing ability. In addition, uterine rotation occurred in the third trimester, and the incision was skewed to the left side, easily cutting off the left uterine artery branches. If repeated blind suturing is performed to achieve hemostasis in the setting of incision bleeding, this will result in insufficient blood supply to the tissues and may increase the possibility of poor wound healing.
For patients with poor healing of a uterine incision, vaginal ultrasound can be used as a primary auxiliary examination method[6]. Ultrasound images can indicate if the incision site is protruding outward, if there is a heterogeneous mass, or if the inner wound edge is irregularly shaped. Poor wound healing should be considered if there is no blood flow signal in the mass and edge[7]. However, in this case, there was still the possibility of no abnormal changes on ultrasound. If the patient's general condition permits her surgeons to explore the cause of the massive hemorrhage, further investigation may be necessary, including pelvic angiography, computed tomographic imaging, and magnetic resonance imaging, all of which have a higher specificity in the identification of vascular abnormalities[8]. Borges et al[9] also recommended that hysteroscopy is the preferred diagnostic option for abnormal uterine bleeding after a cesarean section.
For the treatment of uterine bleeding caused by poor wound healing after cesarean section, several methods should be employed. First, for patients with less bleeding and no shock, uterine contraceptive agents, antibiotics, and oral estrogen can be applied to promote endometrial hyperplasia and uterine wound healing[8,10]. Second, interventional embolization can be performed via percutaneous uterine artery or iliac artery angiography, which can accurately pinpoint the location of the pelvic arterial bleeding. Vascular embolization has a high success rate, especially for young patients, and can avoid a hysterectomy (which can result in patient anxiety and pain) and retain reproductive function of the patient[9,11]. However, attention should be paid to the possibility of uterine necrosis and infection after embolization, and there is still a possibility of failure of interventional embolization. After reviewing this case, if our patient was in stable condition, iliac artery angiography may have resulted in an accurate understanding of the origin of the pelvic artery bleeding, a clear diagnosis would have been made, and timely arterial embolization would have been performed, all of which might have avoided late hysterectomy. Third, surgical treatment, namely, laparotomy, should be performed for patients with a large amount of blood loss, rapid bleeding, and suspected uterine incision dehiscence. If the patient has no children or still has fertility requirements, if the intraoperative tissue necrosis range is not large, and if the inflammatory reaction is not serious, debridement or ligation of the uterine or internal iliac artery can be performed and allow for retainment of the uterus. Otherwise, the uterus must be removed. In our case, the patient suffered from hemorrhagic shock due to a large and rapid amount of blood loss in a short time. Emergency laparotomy was performed to save the patient's life. The intraoperative findings exhibited ischemic and necrotic left myometrial tissue of approximately 1.5 cm × 1.0 cm in size located in the anterior inferior segment of uterine anterior wall and the full thickness of the left myometrial wall extending to the left uterine artery. The surrounding tissue was crisp over a range of 4 cm × 3 cm. Our patient did not wish to have more children. Therefore, after a discussion with the patient’s family members, we performed a complete hysterectomy. Postoperative pathological examination showed that the endometrium exhibited proliferative changes, and there were multiple focal lymphocytic infiltrations in the interstitium. Some lymphocytes were mixed with plasma cells. In the red area of the uterine isthmus, the interstitial small blood vessels were dilated, and their walls were thickened with hyaline degeneration. The pathological diagnosis was local fibrous hyperplasia and inflammatory changes in the uterine isthmus. Blood vessels of the superficial myometrium of uterus were widely transparent and sclerotic.
In summary, this is the case of a patient who presented with hemorrhagic shock that resulted from massive vaginal bleeding 3 mo after a previous cesarean section. The patient had resumed menstruation before this presentation, which represents a rare clinical scenario. The main purpose of our report is to improve the current understanding of what constitutes late postpartum hemorrhage, which can present as massive vaginal bleeding occurring more than 12 wk after cesarean section. We should be alert to the possibility of secondary postpartum hemorrhage and keep in mind the possibility of nonunion of the uterine incision. Secondary postpartum hemorrhage due to nonunion of the uterine incision is an unusual cause of postpartum hemorrhage. If not treated in time, it may result in the loss of fertility or even the loss of life in these young patients.
| 1. | Xie X, Kong B, Duan T. Obstetrics and gynecology. 9th Edition. Beijing: People's Medical Publishing House, 2018: 221-223. |
| 2. | American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists Number 76, October 2006: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 358] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 3. | He J, Hu WS, Xu D. Late postpartum hemorrhage after cesarean section:clinical analysis of 46 cases. Zhonghua Shiyong Neike Zazhi. 2003;19:411-412. |
| 4. | Zeng Z, Sun X. Diagnosis and treatment of advanced postpartum hemorrhage. Zhonghua Yixue Zazhi. 2017;20:143-145. [DOI] [Full Text] |
| 5. | Xu WM, Xu XX, Chen JH, Yang J. Monitoring and analysis of related factors of uterine incision healing in 360 cases after cesarean section. Wuhan Daxue Yixue Xuebao. 1999;20:141-142. [DOI] [Full Text] |
| 6. | Baba T, Morishita M, Nagata M, Yamakawa Y, Mizunuma M. Delayed postpartum hemorrhage due to cesarean scar dehiscence. Arch Gynecol Obstet. 2005;272:82-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Du YL, Yang MZ, Li Q. The Application of Ultrasonography in Diseases of Vaginal Bleeding of Obstetrics and Gynecology. Zhonghua Yixue Yingxiang Jishu. 2001;17:1093-1094. |
| 8. | Nasu K, Fujisawa K, Yoshimatsu J, Miyakawa I. Uterine arteriovenous malformation: ultrasonographic, magnetic resonance and radiological findings. Gynecol Obstet Invest. 2002;53:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Borges LM, Scapinelli A, de Baptista Depes D, Lippi UG, Coelho Lopes RG. Findings in patients with postmenstrual spotting with prior cesarean section. J Minim Invasive Gynecol. 2010;17:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Li CD, Han LY, Li HL, Zhang WY. Analysis of 13 cases of late postpartum hemorrhage caused by incision dehiscence after cesarean section. Zhongguo Fuyoubaojian. 2004;19:42-43. [DOI] [Full Text] |
| 11. | Descargues G, Mauger Tinlot F, Douvrin F, Clavier E, Lemoine JP, Marpeau L. Menses, fertility and pregnancy after arterial embolization for the control of postpartum haemorrhage. Hum Reprod. 2004;19:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khajehei M S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Ma YJ