Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2364
Peer-review started: February 14, 2020
First decision: April 9, 2020
Revised: April 17, 2020
Accepted: May 13, 2020
Article in press: May 13, 2020
Published online: June 6, 2020
Processing time: 115 Days and 2.4 Hours
Haemophagocytic syndrome (HPS) is rarely seen in patients with acute pancreatitis (AP). HPS as a complication of AP in patients without any previous history has not been elucidated.
A 46-year-old man was admitted for symptom of persistent abdominal pain, nausea, and vomiting for 2 d after heavy drinking. During hospital stay, he suddenly developed skin rash and a secondary fever. The laboratory findings revealed progressive pancytopenia, abnormal hepatic tests, and elevation of serum triglyceride, ferritin, and lactate dehydrogenase levels. However, apparent bacterial or viral infections were not detected. He was also possibly related to autoimmune diseases because of positive expression of various autoimmune antibodies and no remarkable past history. Finally, the bone marrow examination showed a histiocytic reactive growth and prominent hemophagocytosis, which resulted in a diagnosis of HPS. Unexpectedly, the patient responded well to the immunosuppressive therapy.
HPS is a very rare extrapancreatic manifestation of AP. The diagnosis relies on bone marrow examination and immunosuppressive therapy is effective. For AP with skin changes, the possibility of HPS should be considered during clinical work.
Core tip: Haemophagocytic syndrome is a rare extrapancreatic manifestation of acute pancreatitis. We herein report the first such case to share our experience with its diagnosis and treatment. The patient responded well to the immunosuppressive therapy. Early diagnosis and treatment are crucial.
- Citation: Han CQ, Xie XR, Zhang Q, Ding Z, Hou XH. Hemophagocytic syndrome as a complication of acute pancreatitis: A case report. World J Clin Cases 2020; 8(11): 2364-2373
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2364.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2364
Haemophagocytic syndrome (HPS) is an uncommon disorder that may be characterized by fever, peripheral blood pancytopenia, liver dysfunction, coagulation abnormalities, and hepatosplenomegaly[1,2]. It may be triggered by genetic disorders, malignant neoplasms, viral infections, and autoimmune disorders[3-5]. Acute pancreatitis (AP) and HPS are sporadically observed in the course of systemic lupus erythematosus (SLE) or fulminant ulcerative colitis secondary to autoimmune diseases[2,6,7]. We herein report such a case to share our experience with its diagnosis and treatment.
A 46-year-old man was admitted for symptom of persistent abdominal pain, nausea, and vomiting for 2 d after heavy drinking and then transferred to our hospital.
The patient’s symptoms started with mild abdominal pain, which had worsened in the last 2 h.
He had no previous history except for smoking.
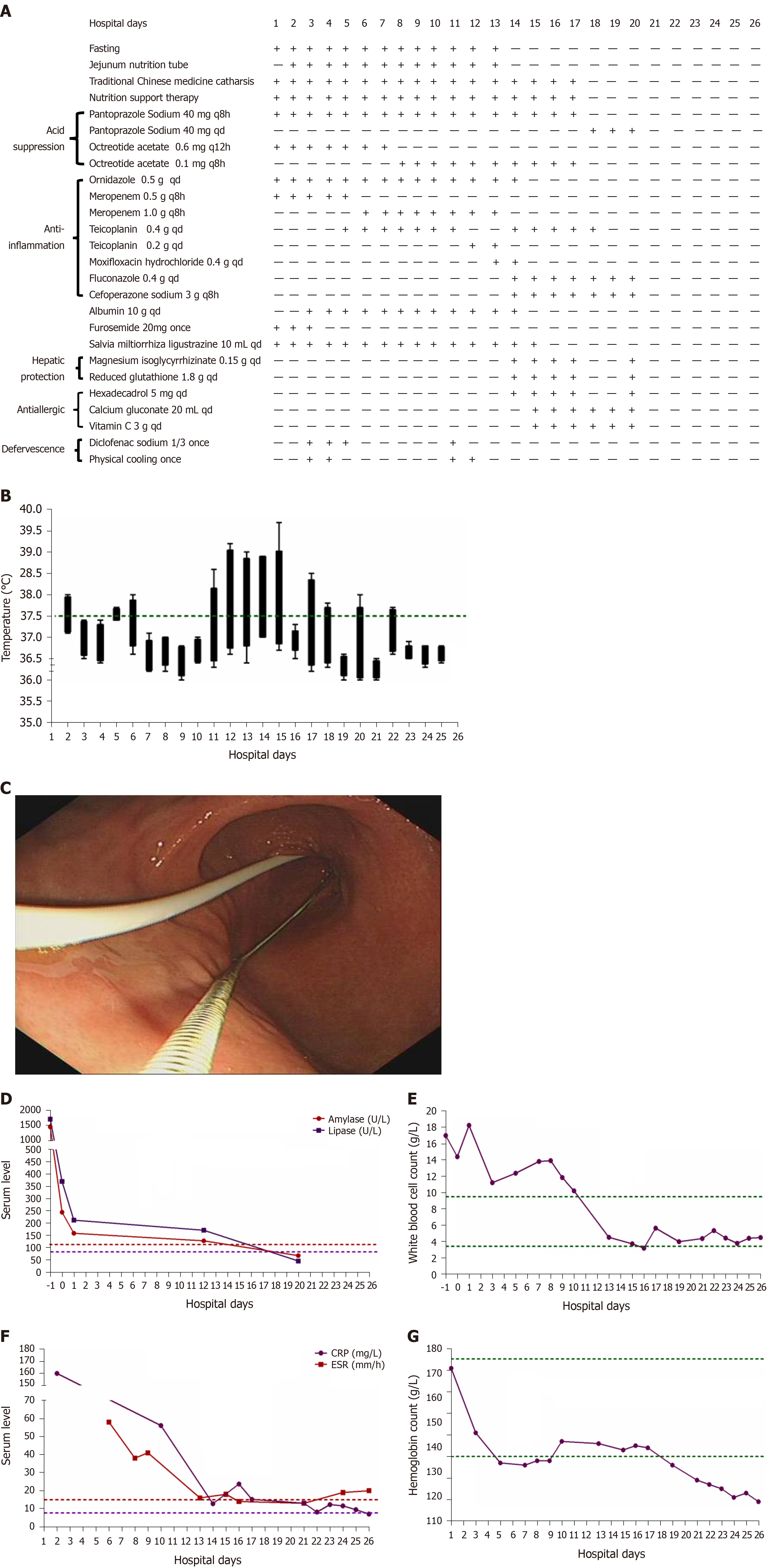
The physical examination on admission revealed a discontinuous fever (38 °C) (Figure 1B) and upper abdomen tenderness. His skin was normal.
The laboratory data showed significantly increased serum levels of amylase (1454.9 U/L), lipase (1705.28 U/L), white blood cell count (17 G/L), positive C-reactive protein (4.8 mg/mL), and erythrocyte sedimentation rate (58 mm/h) (Figure 1D-F), slightly elevated glucose (7.71 mmol/L) and D-dimer (1.63 mg/L), slightly reduced calcium (1.88 mmol/L), and hypoalbuminemia (31.0 g/L). The coagulation, hepatic, and renal functions were all within normal limits. The detailed laboratory values are described in Table 1.
| Day | Index | Value | Range | P/N |
| 1 | Calcium | 1.88 | 2.03-2.54 mmol/L | |
| 1 | Glucose | 7.71 | 3.9-6.1 mmol/L | |
| 1 | Albumin | 31.0 | 35-55 g/L | |
| 1 | Creatinine | 86.6 | 44.0-133.0 μmol/L | |
| 1 | D-Dimer | 1.63 | < 0.5 mg/L | |
| 6 | PCT | 0.28 | < 0.5 ng/mL | |
| 7 | CA125 | 320.0 | < 35.0 U/mL | |
| 7 | CA153 | 8.2 | < 31.3 U/mL | |
| 7 | CA199 | 6.5 | < 37.0 U/mL | |
| 14 | Anti-ANA | 1:1000 | < 1:100 | |
| 14 | IgG | 15.80 | 7.51-15.60 g/L | |
| 14 | IgA | 2.93 | 0.82-4.53 g/L | |
| 14 | IgM | 0.634 | 0.46-3.04 g/L | |
| 15 | Anti-TPO | 384.63 | < 5.61 IU/mL | |
| 15 | Anti-TG | 427.53 | < 4.11 IU/mL | |
| 15 | FT3 | 2.0 | 2.63-5.7 pmol/L | |
| 16 | C3 | 0.395 | 0.79-1.52 g/L | |
| 16 | C4 | 0.052 | 0.16-0.38 g/L | |
| 16 | ASO | 49.7 | < 200.0 IU/mL | |
| 16 | IgG4 | 0.892 | 0.03-2.01 g/L | |
| 19 | IgE | 471.17 | 1-190 IU/mL | |
| 8 | T-SPOT.TB | + | ||
| 14 | Anti-nRNP | - | ||
| 14 | Anti-SSA | + | ||
| 14 | Anti-SSB | - | ||
| 14 | ADA | - | ||
| 14 | DE | - | ||
| 14 | RA-54 | - | ||
| 14 | ACA | - | ||
| 14 | AHA | - | ||
| 14 | Ro-52-Ab | + | ||
| 15 | CoxB3-IgM | - | ||
| 15 | CoxB5-IgM | - | ||
| 15 | Evs-RNA | - | ||
| 15 | CMV-IgM | - | ||
| 15 | pANCA | - | ||
| 15 | cANCA | - | ||
| 15 | GBM-Ab | - | ||
| 15 | EB-DNA | - | ||
| 17 | HBV | - | ||
| 17 | HCV | - | ||
| 17 | HIV | - | ||
| 20 | Type A influenza virus | - | ||
| 20 | Type B influenza virus | - | ||
| 20 | Respiratory syncytial virus | - | ||
| 20 | Adenovirus | - | ||
| 20 | Type 1 parainfluenza virus | - | ||
| 20 | Type 2 parainfluenza virus | - | ||
| 20 | Type 3 parainfluenza virus | - |
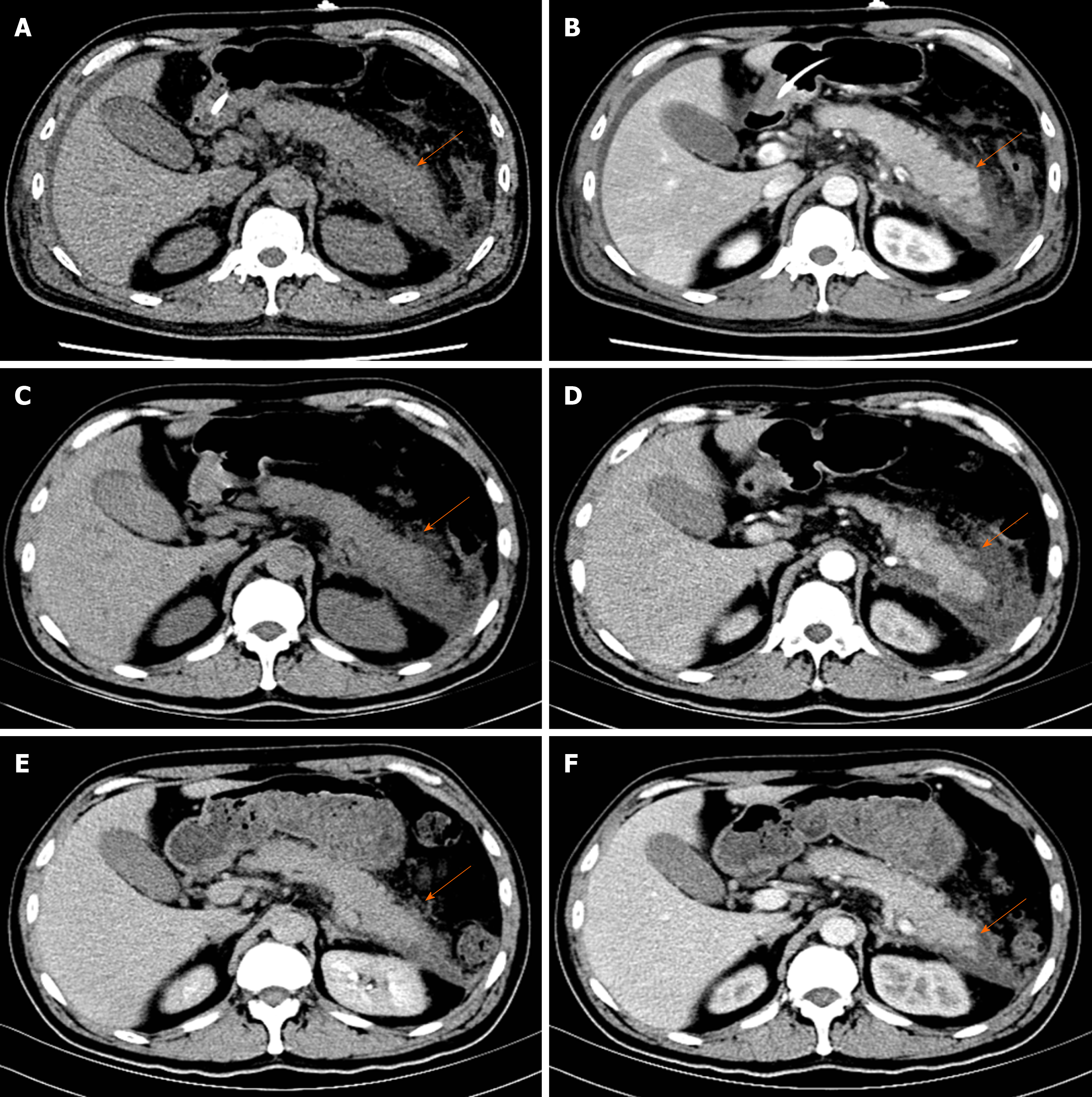
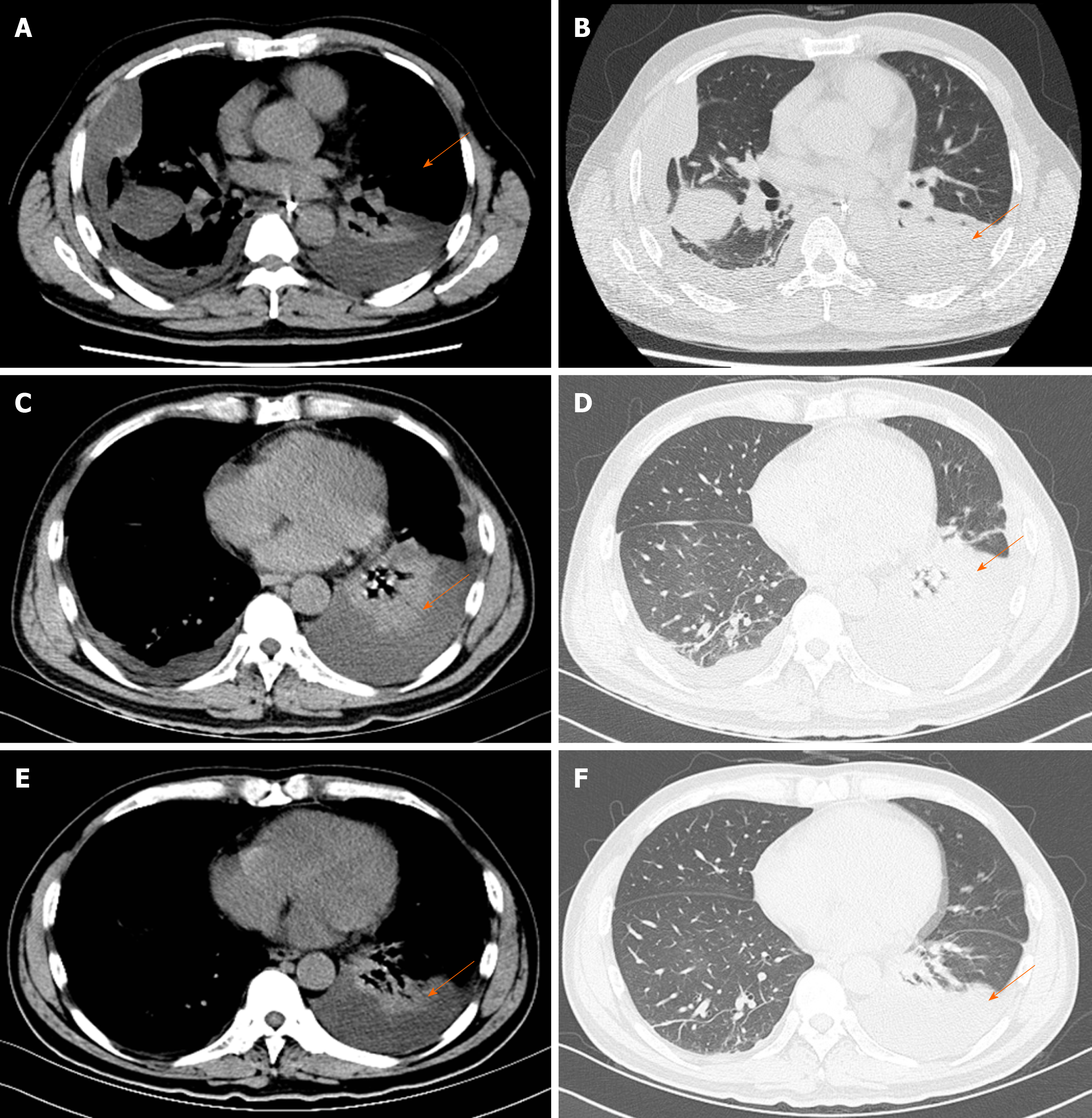
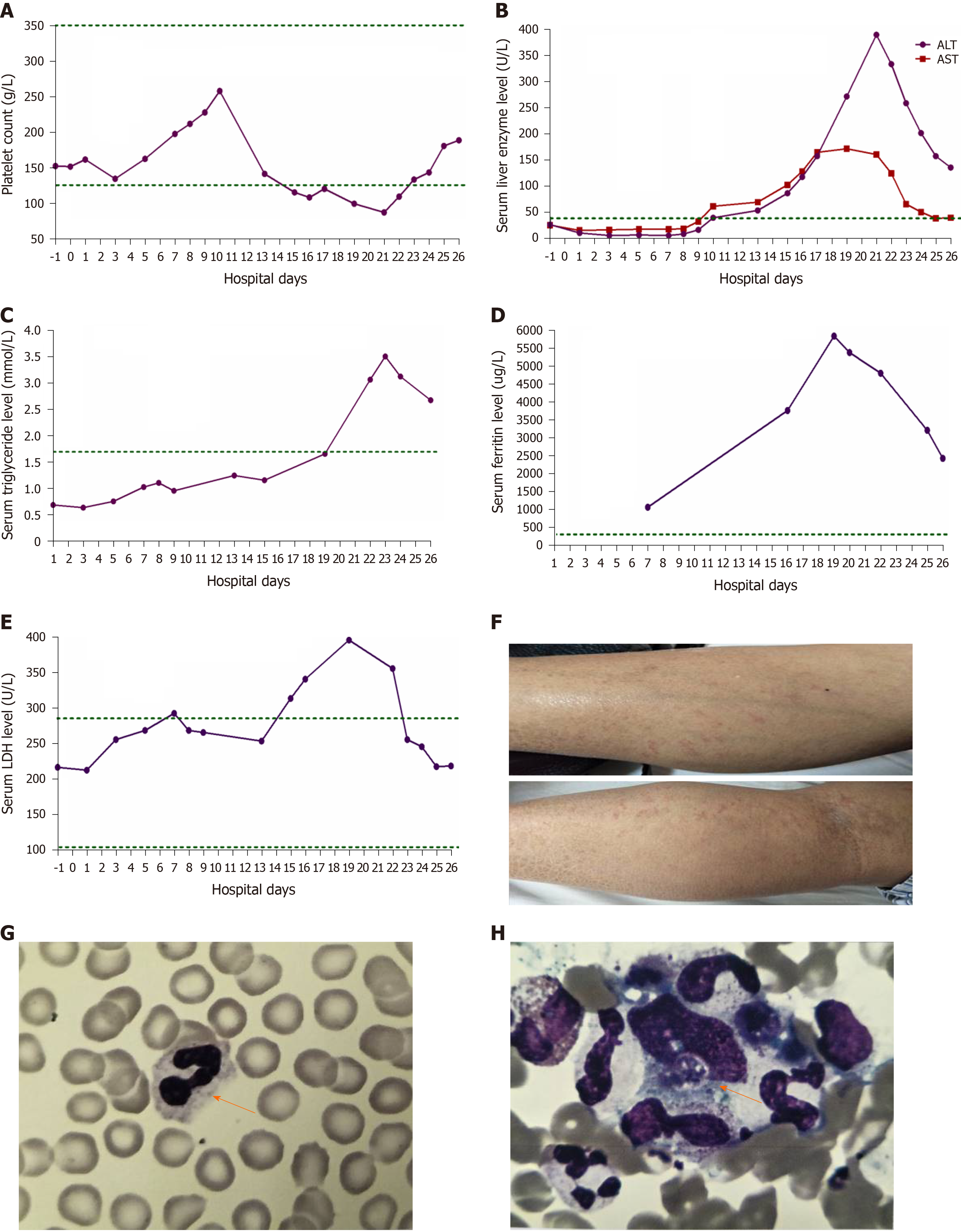
Pancreatic enlargement and peripancreatic seepage on computerized tomography confirmed the diagnosis of AP (Figure 2). Then, the patient was given enteral nutrition via a jejunal nutrition tube (Figure 1C) and treated with pantoprazole sodium (40 mg per 8 h), octreotide aetate (0.6 mg/q12h), and anti-inflammatory drugs (ornidazole 0.5 g/q8h, meropenem 0.5 g/q8h, etc.). Traditional Chinese medicine catharsis was performed for the prevention of intestinal function failure (Figure 1A). The symptoms of the patient gradually disappeared. He had no febrile and each index was close to the baseline level (Figure 1D-F). Bilateral pleural effusion was absorbed better than before (Figure 3). Surprisingly, from the hospital days 11 to 17, the patient suddenly developed a secondary fever and the highest temperature reached 39.7 °C (Figure 1B). We strengthened the antibiotic treatment, including antifungal therapy, although blood culture did not find any evidence of bacterial or fungal infection. Unfortunately, although the temperature was controlled, his general condition deteriorated. On day 16 after hospitalization, he developed a rash on the trunk, upper limbs, and cruses (Figure 4F), then the indexes of autoimmune disease (e.g., SLE), such as anti-ANA (1:1000), anti-SSA (+), Ro-52-Ab (+), IgG (15.80 g/L), and IgE (471.1 IU/mL), were evaluated. Additionally, he also developed pancytopenia (3.18 g/L of white blood cells, 109 g/L of hemoglobin, and 88 g/L of platelets), hepatic dysfunction (alanine aminotransferase [ALT], 390 U/L; aspartate amino transferase [AST], 172 U/L), and a marked elevation of triglyceride (3.51 mmol/L), ferritin (5850 μg/L), and serum lactic acid dehydrogenase (LDH, 396 U/L) (Figure 4A-E), but his coagulation function was normal without significant abdominal ultrasonography findings.
Based on the above findings, a diagnosis of HPS was highly suspected and a peripheral blood smear and bone marrow examination were planned due to the complicated symptoms. Hemophagocytic cells were found in peripheral blood smears (Figure 4G) and the bone marrow examination showed a histiocytic reactive growth and prominent hemophagocytosis (Figure 4H). Thus, HPS as a complication of AP was finally diagnosed.
The patient was then treated with liver-protecting drugs, antiallergic drugs, and hexadecadrol 5 mg/d for 4 consecutive days. By day 20, his symptoms of pancytopenia, liver function, and LDH and ferritin elevations were improved. On the day that the patient left the hospital (day 26), the laboratory parameters were largely close to the baseline levels again.
At the 1-mo follow-up visit after discharge, the patient did not take any drugs and had no symptoms or signs without any recurrence.
Other abnormal clinical and laboratory findings consistent with the diagnosis of HPS include cerebromeningeal symptoms, jaundice, edema, lymph node enlargement, skin rash, hepatic enzyme abnormalities, hypoproteinemia, and hyponatremia[8]. In the present case, the symptoms of patient gradually recovered after effective treatment. However, the results of laboratory re-examination were incomprehensibly deteriorative. He developed a secondary fever, rash, pancytopenia, hepatic dysfunction, hyperferritinemia, and elevation of serum triglyceride and LDH levels. The bone marrow examination proved that he had concurrent HPS[9].
He had no remarkable past medical history, therefore we first considered if HPS was bacteria or virus-associated or malignancy-associated. However, no active infections with viruses as coxsackievirus, enterovirus, cytomegalovirus, hepatitis B virus, Epstein-Barr virus and other respiratory tract infective viruses were observed. Neither prominent hemophagocytosis nor hematological malignancies were detected. There were also no abnormal findings on abdominal CT and tumor markers analysis except slightly elevated CA125. We also suspected concurrent autoimmune diseases because HPS was reported more commonly in patients with pre-existing SLE. However, besides some autoimmune antibodies showing positive expression, no evidence suggested an underlying autoimmune disease.
There was another possibility that HPS was the side effect of pharmaceutical treatment. We indeed used some drugs that could cause rash, pancytopenia, and hepatorenal dysfunction, e.g., antibiotics such as cefoperazone sodium, fluconazole, or teicoplanin and other acid suppression drugs such as pantoprazole sodium. However, all of these are generally well tolerated and therapeutic measures were appropriate[10-13]. Additionally, some common adverse reactions of these drugs, such as headache, nausea, vomiting, diarrhea, and pruritus did not occur[14-17]. Therefore, the possibility that it was the side effect of pharmaceutical treatment was ruled out.
HPS is a rare extrapancreatic manifestation of AP. A ambiguous association between AP and HPS was first mentioned in 1998 by Kanaji et al[2], who found HPS associated with fulminant ulcerative colitis and concurrent AP. So far, there have been just two reports about HPS associated with AP in patients with SLE disease.
We have described the first case of HPS as a complication of AP, which was successfully treated with immunosuppressive therapy. HPS is a severe condition which requires both early diagnosis and treatment, and HPS as one of the extrapancreatic manifestations of AP should be considered in the future.
The authors are grateful to the patient for participating in the study.
| 1. | Reiner AP, Spivak JL. Hematophagic histiocytosis. A report of 23 new patients and a review of the literature. Medicine (Baltimore). 1988;67:369-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 235] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Kanaji S, Okuma K, Tokumitsu Y, Yoshizawa S, Nakamura M, Niho Y. Hemophagocytic syndrome associated with fulminant ulcerative colitis and presumed acute pancreatitis. Am J Gastroenterol. 1998;93:1956-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Ishii E, Ohga S, Imashuku S, Kimura N, Ueda I, Morimoto A, Yamamoto K, Yasukawa M. Review of hemophagocytic lymphohistiocytosis (HLH) in children with focus on Japanese experiences. Crit Rev Oncol Hematol. 2005;53:209-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Haque WM, Shuvo ME, Rahim MA, Mitra P, Samad T, Haque JA. Haemophagocytic syndrome in an adult suffering from pyrexia of unknown origin: an uncommon presentation of tuberculosis: a case report. BMC Res Notes. 2017;10:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Janka GE, Lehmberg K. Hemophagocytic syndromes--an update. Blood Rev. 2014;28:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 6. | Abdallah M, B'Chir Hamzaoui S, Bouslama K, Mestiri H, Harmel A, Ennafaa M, M'Rad S, Ben Dridi M. [Acute pancreatitis associated with hemophagocytic syndrome in systemic lupus erythematous: a case report]. Gastroenterol Clin Biol. 2005;29:1054-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Elqatni M, Mekouar F, Sekkach Y, Elomri N, Fatihi J, Amezyane T, Abouzahir A, Ghafir D. [Haemophagocytic syndrome as a complication of acute pancreatitis during systemic lupus erythematosus]. Ann Dermatol Venereol. 2012;139:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3781] [Article Influence: 199.0] [Reference Citation Analysis (1)] |
| 9. | Oh HS, Kim M, Lee JO, Kim H, Kim ES, Park KU, Kim HB, Song KH. Hemophagocytic lymphohistiocytosis associated with SFTS virus infection: A case report with literature review. Medicine (Baltimore). 2016;95:e4476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Baltatzis M, Jegatheeswaran S, O'Reilly DA, Siriwardena AK. Antibiotic use in acute pancreatitis: Global overview of compliance with international guidelines. Pancreatology. 2016;16:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Mourad MM, Evans R, Kalidindi V, Navaratnam R, Dvorkin L, Bramhall SR. Prophylactic antibiotics in acute pancreatitis: endless debate. Ann R Coll Surg Engl. 2017;99:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Rada G, Peña J. Is antibiotic prophylaxis beneficial in acute pancreatitis?--First update. Medwave. 2015;15:e6125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Choi HM, Choi MH, Yang YW. A Case of Teicoplanin-Induced Pancytopenia Caused by Excessive Dosing. Am J Ther. 2016;23:e307-e310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Ren X, Liu D, Ding N, Huang K, Xiong Y, Du G, Zeng F. Safety evaluation of cephalosporins based on utilization and adverse drug events: analysis of two databases in China. Expert Opin Drug Saf. 2012;11:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Xie JX, Wei JF, Meng L. Teicoplanin-induced hepatocellular liver injury: a case report and literature review of 17 cases in mainland China. Int J Clin Pharmacol Ther. 2014;52:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Holloway WJ, Palmer D. Clinical applications of a new parenteral antibiotic in the treatment of severe bacterial infections. Am J Med. 1996;100:52S-59S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Schiller D, Maieron A, Schöfl R, Donnerer J. Drug fever due to a single dose of pantoprazole. Pharmacology. 2014;94:78-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Can G, Schietroma M S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Qi LL